Abstract
Recent gene expression profiling analyses and gain- and loss-of-function studies performed with distinct prostate cancer (PC) cell models indicated that the alterations in specific gene products and molecular pathways often occur in PC stem/progenitor cells and their progenies during prostate carcinogenesis and metastases at distant sites, including bones. Particularly, the sustained activation of epidermal growth factor receptor (EGFR), hedgehog, Wnt/β-catenin, Notch, hyaluronan (HA)/CD44 and stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) during the epithelial-mesenchymal transition (EMT) process may provide critical functions for PC progression to locally invasive, metastatic and androgen-independent disease states and treatment resistance. Moreover, an enhanced glycolytic metabolism in PC stem/progenitor cells and their progenies concomitant with the changes in their local microenvironment, including the induction of tumor hypoxia and release of diverse soluble factors by tumor myofibroblasts, also may promote the tumor growth, angiogenesis and metastases. More particularly, these molecular transforming events may cooperate to upregulate Akt, nuclear factor (NF)-κB, hypoxia-inducible factors (HIFs) and stemness gene products such as Oct3/4, Sox2, Nanog and Bmi-1 in PC cells that contribute to their acquisition of high self-renewal, tumorigenic and invasive capacities and survival advantages during PC progression. Consequently, the molecular targeting of these deregulated gene products in the PC- and metastasis-initiating cells and their progenies represent new promising therapeutic strategies of great clinical interest for eradicating the total PC cell mass and improving current antihormonal treatments and docetaxel-based chemotherapies, thereby preventing disease relapse and the death of PC patients.
Similar content being viewed by others
Introduction
Prostate cancer (PC) is among the most commonly diagnosed malignancies and is the second leading cause of cancer-related deaths in men (1–6). Although progress in developing early detection tests has led to improved clinical treatments of patients diagnosed with low-grade and organ-confined PCs by radical prostatectomy and radiotherapy, the progression to locally advanced, invasive and metastatic castration-resistant prostate cancers (CRPCs) usually leads to disease relapse (1,2,5,7–9). In fact, despite the fact that the patients with locally advanced PCs initially respond to androgen deprivation by surgical or chemical castration, androgen-independent (AI) lesions may eventually develop and progress despite low levels of circulating androgens (9–11). The CRPCs are refractory to conventional treatments by antihormonal therapy, radiotherapy and chemotherapy (1–5,7–9,12). More specifically, the first-line systemic docetaxel-based chemotherapies used as care for the patients with high-risk or metastatic CRPCs are only palliative and typically culminate in the death of patients after about 12–19 months (1–3,5,8,13).
Numerous investigations have been made to define the molecular transforming events occurring in prostatic epithelial cells and their local microenvironment that may contribute to PC initiation and progression to locally invasive and metastatic disease stages as well as their acquisition of an AI phenotype in humans. It has been shown that the sustained activation of epidermal growth factor receptor (EGFR), hedgehog, Wnt/β-catenin, hyaluronan (HA)/CD44, transforming growth factor (TGF)-β/TGF-βR receptors and stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) frequently occurs during PC progression to locally invasive and metastatic CRPCs (5,13–29). These tumorigenic cascades can account for the sustained growth, survival, invasion, metastases and treatment resistance of PC cells. Moreover, the alterations leading to an enhanced expression and/or hypersensibility of androgen receptor (AR) also may occur in PC cells (9–11). The majority of PC patients also express diverse fusion genes resulting from the chromosomal rearrangements of the 5′-untranslated region of the androgen-regulated gene TMPRSS2 and v-ets avian erythroblastosis virus E26 transformation-specific (Ets) family genes including Erg, Etv1 or Etv4 (30–38). These fusion genes encode for oncoproteins that can provide key roles for PC progression and treatment resistance (30–42). More specifically, it has been shown that the overexpression of a truncated form of transcriptional regulator ERG from the TMPRSS2-ERG fusion gene, which occurs in up to approximately 40% of PCs but is not detected in the normal prostate, may contribute to PC development (33,35–41). The truncated ERG oncoprotein can cooperate with the PTEN (phosphatase tensin homolog deleted on chromosome 10) downregulation-induced phosphatidylinositol 3-kinase (PI3K)/Akt activation and induce the PC cell invasion and angiogenesis-like wild-type oncogenic ERG transcription factor (33,35–41). In addition, the changes in the tumor reactive stroma, including the release of different growth factors by activated myofibroblasts, typically take place during PC progression under normoxic and hypoxic conditions and may promote the malignant transformation of PC cells and neoangiogenesis (5,11,13,43–47).
In addition, a growing body of experimental evidence has also revealed that the accumulation of genetic and/or epigenetic alterations occurring in prostatic stem/progenitor cells and changes in their local microenvironment during the lifespan may result in their malignant transformation into highly tumorigenic and migrating PC stem/progenitor cells, also designated as PC- and metastasis-initiating cells, that provide critical functions for tumor formation and metastases (Figure 1) (5,13,48–77). More particularly, the acquisition of a more malignant behavior by tumorigenic PC stem/progenitor cells during disease progression, including a migratory ability during the epithelial-mesenchymal transition (EMT) program, may lead to their invasion, dissemination through the peripheral circulation and metastases at distant sites, including bones, treatment resistance and disease relapse (Figure 1) (5),13, 46, 47, 50–52, 56–60, 63–65,67,68,72–78). In this matter, we review the most recent advancements on the establishment of the cellular origin of PCs and key signal transduction elements that can cooperate for the acquisition of more malignant phenotypes by PC stem/progenitor cells and their progenies during prostate carcinogenesis, metastases at bones and other distant sites and treatment resistance. The provided information should help to design novel effective multitargeted approaches for improving the current antihormonal treatments and docetaxel-based chemotherapies to treat the PC patients at early and late stages, including patients with a high risk of disease recurrence or relapse after treatment initiation.
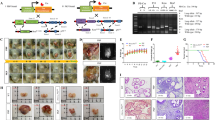
Molecular oncogenic events associated with PC initiation and progression to a locally invasive disease stage and bone metastasis and novel targeting therapies. The scheme shows the PC initiation through the accumulation of genetic and epigenetic alterations in prostate-resident adult stem cells resulting in their malignant transformation into tumorigenic PC stem/progenitor cells also designated as PC-initiating cells. The transformation of tumorigenic PC stem/progenitor cells into migrating PC stem/progenitor cells, which may be induced through the sustained activation of distinct growth factor signaling pathways during the EMT program and PC progression, is also shown. Furthermore, the possible invasion of certain tumorigenic and migrating PC stem/progenitor cells in the activated stroma, which may lead to their dissemination through the peripheral circulation at distant sites including bones along chemoattractant ligand gradient systems such as SDF-1/CXCR4, is illustrated. The activation of metastasis-initiating cells under specific microenvironmental conditions prevalent at bone induced via the release of diverse paracrine growth factors and cytokines by fibroblasts and bone cells, and that is associated with the formation of secondary tumor formation at bone, is also illustrated. The release of growth factors and cytokines such as SHH, TGF-α, TGF-β and MIC-1 by PC cells that can induce in a paracrine manner the differentiation of either osteoblast or osteoclast precursors into osteoblasts or osteoclasts, thereby causing the osteoblastic lesion (new bone formation) or osteolytic lesion (bone destruction) in certain cases, is also indicated. In addition, the molecular targeting of distinct gene products deregulated in PC- and metastasis-initiating cells, which might constitute a potential strategy to improve current treatments, eradicate the total PC cell mass and prevent disease relapse, is also indicated.
Molecular Transforming Events During Prostate Carcinogenesis and Metastases
Cellular Origin and Heterogeneity of PC
PC is a complex, multifactorial and heterogenous disease. Among the factors predisposing to PC development, there are intense oxidative stress, inflammatory atrophies and fibrosis associated with severe tissue injuries, hormonal deregulation, and, more particularly, advancing age (1,10,13,44,79–83). Although the cellular origin for different PC subtypes remains not precisely established, a growing body of evidence suggests that the accumulation of distinct genetic and/or epigenetic alterations in prostatic stem/progenitor cells, and more particularly during chronological aging and intense tissue injuries, may result in their malignant transformation into highly tumorigenic PC stem/progenitor cells (5,13,48–77). It has been proposed on the basis of the cell lineage markers that PCs may originate from the malignant transformation of CD133/CD44/α2β1-integrinhigh prostatic stem/progenitor cells or Sca+ immature cells in mouse localized in the basal epithelial compartment or their early progenies with an intermediate phenotype endowed with stem cell-like properties (5,13,48–77). In fact, in analogy with the normal prostate regeneration process, basal PC stem cells could generate moderately differentiated transit amplifying (TA)/intermediate progenitor cells with a malignant phenotype that can contribute to PC progression (Figure 1) (54),58,60–63,84–96). Alternatively, the specific alterations in basal prostatic stem cells could be insufficient for their malignant transformation but result in the generation of distinct highly proliferative TA progenitor cells that accumulate in the suprabasal or luminal compartment and acquire a malignant behavior and that are the cell types of origin for particular PC subtypes (55,97–100). Hence, the highly tumorigenic PC stem/progenitor cells with a basal or intermediate phenotype, which are endowed with a high self-renewal capacity and aberrant differentiation potential, could act as PC- and metastasis-initiating cells and provide critical functions for primary and secondary tumor growth (Figure 1). In support with this model of prostate carcinogenesis, a subpopulation of human PC stem/progenitor cells expressing stem cell-like markers such as telomerase, CD133, CD44high, α2β1-integrinhigh, nestin, aldehyde dehydrogenase (ALDHhigh), ATP binding cassette (ABC) multidrug transporter ABCG2high, Oct3/4, Sox2 and/or Nanog, but a low or undetectable AR level comprising about 0.1–3% of the total tumor PC cell mass has also been detected in malignant prostatic adeno-carcinomas and metastatic neoplasms (48–53,64–69,81). Importantly, the PC stem/progenitor cells were able to give rise in vitro and in vivo to the bulk mass of differentiated PC cells expressing secretory luminal phenotypes, including AR and prostatic acid phosphatase, and reconstitute the tumors in vivo with a histological architecture of a Gleason grade comparable to the patient’s original tumors (49),51–53,64,65,101). For instance, CD133/α2β1-integrinhigh PC stem cells isolated from primary PC (P4E6), when orthotopically grafted in a matrigel plug containing human prostatic stroma, were able to form multiple intraprostatic tumors in nude mice in vivo, showing a histology such as the original Gleason 4 grade PC (49). Furthermore, it has been shown that certain established human PC cell lines, including a new IGR-CaP1 cell line derived from primary prostatic epithelial neoplasm as well as metastatic and AI PC3 and DU145 cell lines, may represent a heterogeneous population of PC cells. The presence of a subpopulation of highly tumorigenic and migrating PC stem/progenitor cells expressing stem cell-like markers such as CD133, CD44high, ALDHhigh and/or CXCR4 may be responsible for their capacity to form tumors and metastasize in animal models in vivo with a high incidence (56–58,60,65,70,102).
In addition, it has also been observed that the malignant transformation of prostatic epithelial cells with an intermediate phenotype localized in the suprabasal or luminal compartment, which can express the stem cell-like, basal (CK5/14 and p63) and luminal (CK8/18 and AR) markers and persist after the degradation of the basal epithelial cell layer and castration, may contribute to PC development (51),54,55,61,62,87,100,101,103–106). For instance, it was reported that the hedgehog signaling elements, receptor Patched 1 (PTCH1) and glioma-associated oncogene homolog 1 (GLI) transcription factor, were frequently colocalized with a p63 basal marker in CD44/CK8/14-expressing prostatic hyperplasia basal cells and PC cells but were rarely detected in normal basal cells (55). These data suggest that the activation of the hedgehog pathway may induce a transitory differentiation of prostatic stem/progenitor cells into CD44+/p63−/+ hyperplasia basal cells with an intermediate phenotype (CK8/14), and this early transforming event may culminate in tumorigenesis by giving rise to CD44+ PC cells expressing PTCH1 and GLI (55). Moreover, it has also been reported that a rare subpopulation of self-renewing castration-resistant prostatic epithelial cells (CARNs) expressing the homeodomain-containing transcription factor Nkx3.1 and found in the luminal compartment after castration in a mouse model could reconstitute prostate ducts in renal grafts (100). The inducible conditional deletion of the phosphatase tensin homolog deleted on chromosome 10 (PTENflox/ftox) and Nkx3.1CreERT2 tumor suppressor gene in CARNs in castrated male mice was also accompanied by the formation of PCs with evidence of microinvasion, suggesting that the malignant transformation of CARNs localized in the luminal compartment may contribute to PC development in this mouse model (100). Further studies, however, are necessary to more precisely establish the hierarchical organization and specific functions of PC stem/progenitor cells with the basal or intermediate phenotype in prostate initiation and progression as well as after castration in different human PC subtypes and animal models of PC. In this regard, we review frequent molecular transforming events associated with PC initiation and progression to locally invasive, metastatic CRPCs, treatment resistance and disease relapse.
Frequent Gene Products and Molecular Pathways Altered in PC-Initiating Cells and Their Progenies during Prostate Carcinogenesis and the Epithelial-Mesenchymal Transition Process
Recent progress has led to the identification of gene products and molecular pathways that are often deregulated in PC-initiating cells with stem cell-like properties and their progenies during human prostate carcinogenesis and that may contribute to their malignant transformation. More particularly, the immunohistochemical and gene-profiling analyses of nonmalignant and malignant prostatic tissues from patients combined with gain- and loss-of-function studies and development of PC cells and transgenic mouse models have revealed that a downregulation of distinct tumor suppressor proteins and upregulation of different oncogenic products in PC stem/progenitor cells and their progenies may provide critical roles for PC etiopatho-genesis and progression (Figure 2) (29),46,47,57,58,60,63,70–74,76,77,97–99,102,107–119). For instance, the characterization of transgenic mouse models relevant to prostate carcinogenesis has indicated that the downregulation of specific tumor suppressor proteins, including PTEN, Nkx3.1, cyclin-dependent kinase inhibitor p27KIP1, p53 and retinoblastoma (pRb), as well as the overexpression of oncogenes such as TMPRSS2-ERG fusion in PC cells, may cooperate for PC initiation and progression (35),41,63,97–99,109–119). More specifically, the studies using the cell lineage markers have indicated that the Sca-1+ PC stem/progenitor cells in the basal compartment or their more cell lineage-committed progenies with an intermediate phenotype can act as the PC-initiating cells in a prostate-conditional probasin (PB)-Cre4 PTENT-/- transgenic mouse model of PC (97,98). Moreover, although the PB-TMPRSS2:ERG transgenic mice engineered to express the TMPRSS2-ERG fusion in adult prostate did not develop preneoplastic prostatic intraepithelial neoplasm (PIN) lesions, it was observed that the crossing of these mice with germline PTEN+/- or prostate-specific PB-Akt-1+/+ transgenic mice resulted in the development of PIN lesions more rapidly compared with germline PTEN+/- or PB-Akt-1+/+ transgenic mice used as littermate controls (35). In the same way, the prostate-specific ERG overexpression in the germline PTEN+/- transgenic mouse was also accompanied by a marked acceleration of the progression of high-grade PINs to invasive prostatic adenocarcinomas relative to PB-ERG or germline PTEN+/- transgenic mice used as littermate controls (41). Interestingly, it has also been noticed that the expression of murine ERG was markedly increased at the mRNA level in tumors formed in prostate-conditional compound PTEN-/-; p53-/- transgenic mice compared with PTEN-/-;p53+/+ transgenic mice and wild-type mice (41). Moreover, the ERG overexpression in benign prostate hyperplasia (BPH)-1 and PC3 cell lines also promoted their migration in vitro without affecting their proliferation, at least in part by upregulating CXCR4 and a disintegrin and metalloproteinase with thrombospondin motif protein (ADAMTS1) (41). Together, these data suggest that the TMPRSS2-ERG fusion overexpression may cooperate with the PTEN downregulation-induced PI3K/Akt stimulation at early and late stages of the prostate carcinogenesis. Moreover, the loss of PTEN combined with p53-induced enhanced expression of ERG at a late stage of PC might promote the transition to invasive disease states, at least in part by upregulating CXCR4 expression. However, further studies are essential to more precisely establish the implication of the chromosomal rearrangements, including TMPRSS2-ERG gene fusion, in the acquisition of a more malignant behavior by PC stem/progenitor cells and their progenies as well as the molecular mechanisms at the basis of their cooperative interactions with other oncogenic events during human PC progression and metastases.
Frequent oncogenic pathways involved in the malignant transformation of PC stem/progenitor cells and their progenies during PC progression and metastases. The activation of the EGFR, sonic hedgehog SHH/PTCH/GLI, Wnt/β-catenin, HA/CD44, TGFβ/TGFβR, ECM component/β1-integrin and SDF-1/CXCR4 signaling pathways, which may contribute to the sustained growth, survival and migration of PC stem/progenitor cells and their progenies and possible interactions between these signaling cascades, are shown. The activation of the downstream signaling elements including PI3K/Akt, mitogen-activated protein kinases, NF-κB and focal adhesion kinase (FAK), which in turn contribute to the upregulation of the expression of different target genes involved in the malignant transformation of PC stem/progenitor cells and their progenies during the EMT process and treatment resistance, are indicated. More specifically, the inhibition of p21CIP1 and p27KIPI inhibitors of cyclin-dependent kinases induced through these growth factor cascades may cooperate to promote the cell cycle progression and growth of PC cells, while the enhanced expression of antiapoptotic factors such as Bcl-2, Bcl-xL and inhibitor of apoptosis protein (IAP) and phosphorylation of Bad may promote their survival. In addition, the potential stimulation of HIFs via the activation of mTOR and under hypoxic conditions, which may contribute to the enhanced glycolysis and acquisition of a more malignant behavior and chemoresistance of PC cells and tumor angiogenesis, is also illustrated.
In addition, a gene expression analysis has identified a gene signature composed of 66 genes that characterizes the tumorigenic PC cells from patient tumors that express stem cell-like markers and that are able to form the prostaspheres ex vivo (71). This gene signature comprises a subset of genes encoding for diverse growth factors (neuropilin-1 [NRP-1], growth differentiation factor-1 [GDF-1] and jagged 1 ligand for the receptor Notch), the proteins that are implicated in cell adhesion and cytoskeletal maintenance, transcriptional regulators (c-myc binding protein [MYCBP], v-myb avian myeloblastosis viral oncogene homolog-like 1 [MYBLI], DNA-binding protein inhibitors ID1 and ID3, FBJ murine osteosarcoma viral (v-fos) oncogene homolog (FOS), E74-like factors ELF3 and ELF4 and Krueppel-like factors 2 and 5) and factors involved in protein biosynthesis and metabolism (71). It was also observed that an increase of expression levels and/or activities of telomerase and diverse signaling elements of growth factor pathways often occurs during PC etiopathogenesis and progression (Figure 2) (29),46,47,57,58,60,71–74,76,77,102, 107,108). These growth factor cascades include EGFR, hedgehog, Wnt/β-catenin, extracellular matrix (ECM) components/integrins, HA/CD44, interleukin (IL)-6/IL-6R and/or SDF-1/CXCR4 as well as their downstream effectors including PI3K/Akt/mammalian target of rapamycin (mTOR), nuclear factor (NF)-κB, focal adhesion kinase (FAK), hypoxia-inducible factors (HIFs) and Myc (29),46,47,57,58,60,71–74,76,77,102,107, 108). The integration of these oncogenic pathways may cooperate for the sustained growth, survival and acquisition of a migratory phenotype by tumorigenic PC stem/progenitor cells and their progenies during the EMT process as well as their resistance to current antihormonal treatments, radiotherapy and chemotherapy (Figures 1 and 2) (5),11,13,17,44,51,58,120–128).
Consistently, the analyses of differently expressed gene patterns of CD133+/α2β1-integrinhigh PC stem/progenitor cells versus committed and differentiated CD133−/α2β1-integrin-/low PC cells as well as normal prostatic CD133+/α2β1-integrinhigh stem cells from malignant and benign tissues of patients have revealed that multiple genes associated with inflammation, such as NF-κB and IL-6, cellular adhesion (focal adhesion signaling) and metastases, were overexpressed in CD133+/α2β1-integrinhigh PC stem/progenitor cells (76). The treatment with Wnt3a ligand of dissociated C4.2b PC cells derived from spheres has also been observed to enhance their ability to form prostaspheres and sphere size, which was associated with a significant increase of CD133−, CD44−, nuclear β-catenin-positive PC cells detected within prostaspheres (108). In the same pathway, the activation of PI3K/Akt/Fox03a signaling components has also been shown to contribute to the prostasphere formation and maintenance of PTEN+ DU145 and PTEN- PC3 cells (129). Moreover, it was reported that the AR− IGR-CaP1 cell line and its clonally derived subclones showing mutated p53 and high telomerase activity and expressing high levels of different stem cell-like markers (such as CD133, CD44 and CXCR4), basal epithelial markers including cytokeratins CK5/CK14 and hedgehog signaling elements rapidly formed subcutaneous or intraprostatic xenografts in nude mice (70).
The detection of elevated expression levels of different embryonic stem cell-like transcription factors such as Oct3/4, Nanog, Sox2 and/or Polycomb group protein Bmi-1 in PC-initiating cells has also been observed to contribute to their high self-renewal capacity and tumorigenic potential and confer them with survival advantages and an invasive capacity (72–74,77). For instance, the PC cells with an EMT phenotype and stem cell-like features, including an increased expression of Notch1, Oct3/4, Nanog, Sox2 and/or Lin28B, exhibited enhanced clonogenic and prostasphere-forming ability and tumorigenicity in mice that were associated with a decreased expression of microRNAs, miR-200 and/or let-7 family member (73). The PC cell subpopulations expressing the stem cell-like marker CD44 and high levels of Nanog and hedgehog signaling element Bmi-1 from both primary and established PC cell lines also were able to invade Matrigel in vitro through induction of the EMT program, whereas CD44− PC cell fractions were noninvasive (72). It was also noticed that the invasive PC cells with stem cell-like phenotypes from DU145 and primary PC cells were more tumorigenic than noninvasive PC cells in nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice (72). Moreover, the analyses of the differentially methylated genes in invasive PC cell subpopulations with stem cell-like properties from LNCaP and DU145 cell lines indicated that the epigenetic changes were detected in bone marrow X-linked (BMX) nonreceptor tyrosine kinase, transcription factor Sox1 and IL-6/signal transducer and activator of transcription 3 (STAT3) pathway in these invasive PC cells (77).
All together, these data suggest that the intrinsic properties of immature PC stem/progenitor cells as well as their acquisition of a high self-renewal capacity and migratory ability through the enhanced expression of different growth factor pathways and stemness gene products during the EMT process may confer them with greater tumorigenic and invasive properties compared with the differentiated PC cells (Figures 1 and 2). In this review, we are reporting the changes that often occur in the local microenvironment of PC-initiating cells and their progenies, and that they may promote their acquisition of a more aggressive behavior.
Modulation of the Malignant Behavior of PC-Initiating Cells and Their Progenies by the Local Tumor Microenvironment
The PC progression is typically associated with the degradation of basal membrane, loss of the basal epithelial cell layer and cell adhesion, intense remodeling changes that occur in the components of tumor-reactive stroma and interactive cross-talks between the PC-initiating cells and their progenies with stromal cells (Figures 1 and 3) (5,43,44,75,76,130–133). More specifically, an intense remodeling of diverse ECM components, including an upregulation of integrin receptor ligands, peptidoglycans such as perlecan, and secreted proteolytic enzymes including matrix metalloproteinases (MMPs), urokinase-type plasminogen activator (uPA), matriptase and hepsin as well as a decreased expression of decorin often occurs during PC progression (Figure 2) (5,43,44,134). The accumulation of the perlecan in the ECM and its interaction with sonic hedgehog (SHH) ligand molecules can sustain the activation of hedgehog cascade in PC cells (Figure 2) (43,135,136). Moreover, the downregulation of the decorin as well as the upregulation of the ectodomain shedding of membrane precursors of EGFR ligands induced by MMPs may promote the EGFR activation (Figure 2) (137,138). On the other hand, the development of resistance to the cell detachment-induced apoptosis, also designated as anoikis, is an important process that maintains the anchorage-independent growth and survival of PC cells (134,139,140). Interestingly, it was reported that PC3 cells may trigger a constitutive production of reactive oxygen species mediated through a sustained activation of 5-lipoxygenase that leads to the Src oxidation and activation in the absence of cell adhesion and ligand-independent phosphorylation of EGFR (140). The persistent activation of EGFR in turn may culminate to a chronic activation of prosurvival signals and degradation of the proapoptotic protein Bim and thereby promote the PC3 cell survival in the absence of cell adhesion (140).
Novel therapeutic strategies against aggressive, invasive and metastatic PC cancers by targeting distinct growth factor signaling cascades and drug resistance-associated molecules in prostatic cancer stem/progenitor cells and their progenies. The possible antiproliferative, antiinvasive and/or apoptotic effects induced by a specific inhibitor of tyrosine kinase activity of EGFR (gefitinib and erlotinib), receptor tyrosine kinase (RTK) activity, smoothened (SMO) hedgehog signaling element (cyclopamine and GDC-0449), Notch (γ-secretase inhibitor) and Wnt/β-catenin (sFRP2) as well as TGFβR antagonist or CXCR4 antagonist (AM3100) and monoclonal antibody (mAb) directed against SHH ligand, CD44, Wnt ligand or CXCR4 are indicated. Moreover, the inhibitory effect induced by different pharmacological agents on the downstream signaling effectors induced through these growth factor cascades and under hypoxic conditions such as PI3K/Akt/mTOR, NF-κB and HIFs as well as an inhibitor of glycolytic metabolism (2-DG) and 2-DG-induced autophagy (metformin) is also indicated. In addition, the potent inhibitory effect mediated by a specific inhibitor of EGFR or hedgehog on ABCG2-multidrug efflux pump and whose event may lead to the intracellular accumulation of chemotherapeutic drugs is also illustrated.
In addition, the induction of tumor hypoxia and vascularization and a decrease of extracellular pH in local tumor microenvironment of PC stem/progenitor cells and their progenies also may alter their metabolic and survival pathways and promote their invasion and metastasis (75),76,130–133,141). In fact, PC stem/progenitor cells and their progenies can adapt to the persistent oxidative stress and inflammatory and hypoxic conditions prevalent in primary neoplasm by acquiring more malignant phenotypes through the activation of NF-κB and HIFs (Figure 2) (75),76,131–133,141). NF-κB, HIF-1α and/or HIF-2β may induce the expression of different target gene products such as glycolytic enzymes, macrophage-inhibitory cytokine-1 (MIC-1), IL-6, cyclooxygenase-2 (COX-2), vascular endothelial growth factor (VEGF), P-glycoprotein (P-gp) (also designated as multidrug resistance 1 [MDR-1 or ABCB1]), Bcl-2 and/or Bcl-xL in PC cells under normoxic and hypoxic conditions (Figure 2) (75),76,130–132,141). These gene products in turn may play critical roles in PC progression by upregulating the glycolysis, angiogenic switch, survival pathways and chemoresistance of PC stem/progenitor cells and their progenies (Figure 2) (75),76,130–132, 141–144). In fact, an adaptive switch from mitochondrial respiration (oxidative phosphorylation) to an enhanced glycolytic metabolism, known as the Warburg effect, may occur in PC stem/progenitor cells and their progenies through an upregulated expression of glycolytic enzymes such as phosphoglycerate kinase 1 (Pgk1) that breaks down glucose (141,145–149). The enhanced glycolysis may contribute to provide the energy and nutrients necessary for the sustained proliferation and the biosynthesis of new cellular components, including proteins and lipids, in rapidly dividing PC cells (141,145–149).
In addition, the host stromal cells, including fibroblasts, infiltrating immune cells such as macrophages and endothelial cells and the recruitment of bone marrow-derived circulating endothelial progenitor cells, which may release diverse soluble growth factors and chemokines in interstitial stroma, can also influence the remodeling of the ECM components, malignant behavior of PC stem/progenitor cells and their progenies and the tumor angiogenic process (Figure 1) (5,11,46,47,134,135). For instance, the upregulated expression and release of different soluble factors such as TGF-β, fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor and MMPs by myofibroblasts can participate through the autocrine and paracrine loops to promote the PC development and tumor neo-angiogenic process (Figure 1) (11,135). Importantly, it was shown that IL-6, TGF-β1 or conditioned medium from PC3 cells can activate the cancer-associated fibroblasts, which in turn may induce, in a paracrine manner, the EMT program and stem cell-like phenotypes in PC cells (46,47). More specifically, it was shown that cancer-associated fibroblasts may enhance the expression of EMT-associated molecules such as snail and twist in PC3 cells and the number of PC3 cells expressing stem cell-like markers such as CD133+ or CD44high/CD24− as well as their ability to form prostaspheres and to self-renew, and thereby promote, their invasive ability and metastatic spread (46,47). The cancer-associated fibroblast-induced EMT program and stem cell-like features in PC3 cells may be mediated through the secretion of MMPs by cancer-associated fibroblasts that, in turn, may upregulate Rac-1, NF-κB, COX-2-induced reactive oxygen species production and HIF-1α in PC cells (46,47).
Hence, all these molecular transforming events in PC-initiating cells and their progenies as well as the tumor reactive stroma may cooperate for the PC development, neo-angiogenesis and transition to locally invasive and metastatic CRPCs. In this matter, we are reporting the gene products that are often deregulated in invasive and metastatic PC cells at primary and secondary neoplasms and their local microenvironment and that can contribute to their metastatic spread and metastases at distant tissues, including bones.
Frequent Gene Products and Molecular Pathways Altered in Metastasis-Initiating PC Cells and Their Local Microenvironment
PCs are known to metastasize near lymph nodes and different distant tissues and organs, including bones, lungs, liver, brain and the adrenal gland (Figure 1) (150,151). The molecular mechanisms and transforming events that dictate the selective metastatic spread of PC cells from primary tumor to specific distant tissues are not precisely understood. Metastasis is a multistep process that implicates the stromal invasion of only a small subset of PC cells in primary tumors. The spread of metastasis-initiating cells through the lymphatic and blood circulatory systems followed PC cell migration engraftment, and homing to specific distant tissues and formation of secondary neoplasms (Figure 1). Recent accumulating lines of evidence suggest that the metastatic spread of a small number of tumorigenic and migrating PC stem/progenitor cells might contribute to drive tumor growth at distant metastatic sites by giving rise to the total tumor cell mass (Figure 1) (22),50,56–58, 60,65,102). Consistent with this model of metastasis, it has been shown that the PC stem/progenitor cells expressing CD133, CD44high and/or ALDHhigh stem cell-like markers from metastatic tissues of patients and metastatic PC cells lines displayed a high self-renewal ability and capacity to form tumor and metastasize at distant sites (22),50,56–58,60,65,102). For instance, it was observed that ALDH isoforms are expressed in human PC cells and clinical specimens from primary prostate tumors with matched bone metastases, and the ALDHhigh/CD44high PC cell fraction from the metastatic PC3M-Pro4 PC cell line orthotopically implanted in mouse prostate formed the tumors at primary and distant metastatic sites in an animal model in vivo (65).
In addition, it has also been reported that the overexpression of SHH ligand by using pCX-SHH-IG vector in mice led to the malignant transformation of p63+ prostatic stem cells localized in the basal compartment of the prostate into PC stem cells concomitant with the development of PINs that subsequently progressed in invasive and metastatic PC within 3 months (152). Importantly, p63+ PC stem cells detected in metastatic loci within lymph nodes, kidneys and lungs were able to give rise to p63+/AR−, CK14/AR−, CD44+/AR− and AR+ progenies and form primitive prostate-like glandular structures (152). These data suggest that the sustained activation of the hedgehog cascade in p63+ PC stem cells may play critical functions for PC formation as well as for the invasion and metastatic spread of these immature PC cells and formation of secondary tumors in this mouse model (152). Regardless of this important advance, few studies have defined the specific gene products deregulated in metastasis-initiating cells with respect to their stem cell-like properties. Therefore, we will discuss here information that has been obtained about the transforming events that may modulate the metastasis-initiating cell behavior without discriminating against their stem cell-like phenotypes.
Numerous gene expression profiling analyses have allowed researchers to identify specific molecular signatures that may be associated with a high potential of PC cells detected at primary tumors to undergo metastatic spread and establish their homing at specific distant tissues as well as the molecular changes that may occur at the predestinated metastatic sites (23,153,154). In particular, a loss of PTEN, p53 and breast cancer type 1 (BRCA1) combined with an upregulation of EGFR, hedgehog, TGF-β/TGF-βR receptor, ECM components/integrins and SDF-1/CXCR4 and downstream effectors such as PI3K/Akt, small GTPase Rac-1, mitogen-activated protein kinases, NF-κB, MIC-1 and Rho are often detected in metastatic PC cells (11,135,153,155–166). It has also been shown that the migration and engraftment of metastatic PC cells in bones, the most common site of PC metastasis, as well as other distant tissues may be mediated in part through the formation of chemoattractant gradients such as SDF-1 released by host endothelial cells and fibroblasts at distant tissues that specifically attract the metastatic PC cells overexpressing CXCR4 (Figure 1) (23,122,167–169).
Several observations have also indicated that the skeletal sites enriched in bone marrow cellularity and under active turnover, including the spine, pelvis, ribs and proximal metaphyses of long bones, may be the driving force that mediates a preferential migration and homing of metastasis-initiating PC cells in the skeleton and secondary tumor formation (157,170–172). The secretion of different growth factors and cytokines such as SHH, EGF, TGF-α, basic fibroblast growth factor, hepatocyte growth factor, insulin-like growth factor, parathyroid hormone-related protein (PTH-rp), MIC-1 and endothelin by metastasis-initiating PC cells and/or bone cells may promote bone metastasis and osteoblastic and/or osteolytic reactions (Figure 1) (11),135,160–166,173). More specifically, the secretion of SHH, TGF-α, TGF-β, MIC-1 and/or Pgk1 by PC cells has been reported to play critical roles by promoting the ECM remodeling in bones, osteoblast and osteoclast differentiation and induction of osteoblastic and/or osteolytic lesions, which are associated with severe pain in PC patients (Figure 1) (11,162,163,173–176). Moreover, it has been proposed that the metastasis-initiating PC cells that preferentially establish their homing at bones must possess the osteomimetic properties and be able to acquire the mesenchymal cell-like phenotypes as the bone cells (161,177). The data from a study that consisted of performing a heterotypic coculture of metastatic PC3 cells and bone marrow-derived stromal cells have revealed that a specific subset of genes were altered only after their physical contact (178). The altered gene products include collagen types III, IV, X and XII; α1- and α2-integrins, MMP-2, MMP-9, uPA, biglycan, osteopontin and Raf-1 in PC3 cells as well as collagen types VIII and IX, bone morphogenic protein-6, TGF-β1, homolog of the Drosophila gene (SMAD6) and twist in bone marrow-derived stromal cells (178). Similarly, the data from a recent study revealed that metastatic PC3 and DU145 cells may downregulate the expression of ECM components and up-regulate the membrane type 1 metalloprotease (MT1-MMP), vimentin and α5β1-integrin in stromal cells as well as their migratory phenotype (176). Also, the changes in diverse signaling pathways, including chaperone-mediated mitochondrial homeostasis, integrin-dependent cell behavior and runt-related transcription factor 2 (Runx2)-regulated osteoblastic gene expression, such as a1 type I collagen, bone sialoprotein, osteopontin and osteocalcin in the osteoblasts, can occur in the metastatic bone microenvironment (179). These molecular changes in bone cells may promote the formation of secondary tumors and osteoblastic and osteolytic lesions (Figure 1). Hence, these data suggest that metastasis-initiating PC cells may alter their novel local microenvironment at metastatic sites by modulating the ECM components and phenotypic and functional features of stromal cells. Reciprocally, the stromal cells, in turn, may also influence the malignant behavior of PC cells and secondary tumor formation.
Collectively, these recent studies have led to the identification of novel gene products that are often altered in PC stem/progenitor cells and their progenies as well as their local microenvironment during PC progression and metastases at distant sites, including bones, and that can contribute to their aggressive phenotypes. Hence, the simultaneous targeting of these deregulated gene products could be exploited to reverse treatment resistance and develop a novel combination therapy. Consistent with this, we report recent data indicating that PC stem/progenitor cells can provide critical functions in treatment resistance, and the molecular targeting of distinct oncogenic products in these immature PC cells and their progenies constitute a potential therapeutic strategy to eradicate the total PC cell mass (Figures 1 and 3).
Critical Implication of PC- and Metastasis-Initiating Cells and Their Progenies in Treatment Resistance
Recent lines of experimental evidence have revealed that the PC stem/progenitor cells, including side population (SP) of PC cells isolated by using the Hoechst dye efflux technique, exhibiting an AI phenotype and expressing high levels of ATP-binding cassette (ABC) multidrug transporters such as ABCG2, may be more resistant than their differentiated progenies and non-SP cells to the antihormonal and chemotherapeutic treatments (67–69,180). For example, it was observed that the nonadherent suspension culture of metastatic and AI PC3 cells under the form of prostaspheres resulted in an enrichment of CD133+/CD44+ PC cells that are more resistance to cisplatin than adherent cells (68). Similarly, the highly tumorigenic CD133+/CD117high/ABCG2high/nestin+ PC cell subpopulation coexpressing Oct3/4, Nanog and Sox2 from the PC cell line 22RV1 was also more resistant to treatment with a variety of chemotherapeutic drugs such as cisplatin, paclitaxel, adriamycin, and methotrexate than the CD133−/ABCG2low 22RV1 cell fraction (67). Moreover, the CD133+ SP cells endowed with stem cell-like properties isolated from the highly invasive WPE1-NB26 PC cell line were less sensitive to the antiproliferative and apoptotic effects induced by docetaxel treatment than the CD133− non-SP cell fraction (69). In addition, it has also been observed that an enrichment of Sca+ PC stem/progenitor cells occurred after androgen-deprivation or docetaxel treatment in transgenic adenocarcinoma of the mouse prostate (TRAMP) and PTEN knockdown transgenic mouse models of PC and led to tumor regrowth and metastases (63,98,181–184). These data suggest that the immature Sca+ PC stem/progenitor cells may provide critical functions in treatment resistance and disease relapse in these transgenic mouse models of PC.
Together, these observations underline the critical importance to target chemo-resistant and AI PC stem/progenitor cells and their progenies for counteracting the disease progression and overcoming the resistance to current antihormonal and chemotherapies, including first-line docetaxel-based chemotherapeutic regimens that are used in the clinics for treating patients diagnosed with locally invasive and metastatic CRPCs.
Novel Strategies for Preventing PC Progression and Overcoming Treatment Resistance
The development of novel chemopreventive and chemotherapeutic strategies that may prevent the evolution of normal prostatic epithelium to premalignant PIN lesions and prostate carcinogenesis or counteract PC transition to locally invasive, AI and metastatic disease stages is of great clinical interest in considering the long time generally associated with the PC progression. Numerous preclinical studies aiming to develop novel therapies for preventing or treating PCs have led to the identification of a variety of potential chemopreventive and chemotherapeutic agents, including natural and dietary compounds and pharmacological agents or gene therapies, to eradicate the total tumor cell mass, including PC stem/progenitor cells and their progenies (76,137,185–198).
Chemopreventive and Chemotherapeutic Effects of Diverse Dietary Compounds
Some preclinical investigations aiming to develop novel strategies to prevent PC formation or disease progression have aimed to establish the chemopreventive and anticarcinogenic effects induced by diverse dietary compounds using TRAMP and PTEN knockdown transgenic mouse models of PC (137),187–194, 198). In fact, the TRAMP and PTEN knockdown transgenic mouse models of PC, which are driven by PC stem/progenitor cells endowed with stem cell-like properties, represent useful animal models to estimate the chemopreventive and chemotherapeutic effects induced by the dietary substances on total PC cell mass and their local microenvironment as well as their potential to reverse the treatment resistance (63),97,98,181–184,199,200). Of therapeutic interest, it has been shown that different dietary compounds, including curcumin, lycopene, silibinin, feeding of dibenzoylmethane, green tea polyphenols, genistein, α-difluoromethylornithine, toremifene, R-flurbiprofen, celecoxib and sulindax, or their chemical derivatives, significantly decreased the incidence of PIN lesions and PC formation and/or delayed the disease progression in the TRAMP or PTEN knockdown transgenic mouse models of PC (137),187–194,198). The anticarcinogenic effects of these dietary agents, alone or in combination, were mediated at least in part through downregulation of diverse growth factor cascades, including EGFR and sonic hedgehog and their downstream signaling elements such as PI3K/Akt and NF-κB in cancer cells (137),187–194,198). For instance, several botanic compounds such as genistein, apigenin, baicalein, curcumin, epigallocatechin 3-gallate, quercetin and resveratrol have been reported to inhibit the hedgehog cascade and GLI-1 expression and suppress the in vitro growth of PC cell lines such as androgen-dependent LNCaP and AI PC3 cells and disease progression in the TRAMP model of PC (191).
More recently, the natural dietary compounds have also been shown to induce the apoptotic death on PC stem/progenitor cells expressing the stem cell-like markers and their progenies in vitro and in vivo (76,195–197). For instance, it was reported that a sesquiter-pene lactone from the plant parthenolide induced the cytotoxic effects on parental and CD44high and CD44−/low PC cell fractions isolated from DU145, PC3, VCAP and LAPC4 cell lines and primary PC cells from patient samples in vitro through an inhibition of NF-κB and Src-related signaling components (195). Parthenolide was also effective at inhibiting the tumor growth of CD44high DU145 cell xenograft models in vivo (195). Importantly, parthenolide also induced the cytotoxic effects on the CD133+ primary prostate tumor cells, while the CD133+ normal cells from benign prostate hyperplasia were insensitive to this treatment type in vitro (76). Moreover, another natural bioactive phytochemical compound produced by cotton plants (gossypol) was observed to induce the cytotoxic effects on LAPC4, PC3 and DU145 PC cell lines in vitro and inhibit the PC-initiating cell-driven tumor growth in a NOD-SCID xenograft model through an increase of DNA damage and the induction of mitochondria- and p53-mediated apoptotic cell death (196).
Hence, the use of these dietary substances, alone or in combination therapy, constitutes promising strategies to eradicate PC-initiating cells and their progenies, thereby preventing PC development or counteracting its progression to aggressive and metastatic CRPCs. In this regard, we review other pharmacological agents that have been shown to target distinct deregulated signaling elements involved in the malignant transformation and treatment resistance of PC stem/progenitor cells and their progenies, and that represent potential therapeutic tools to develop a multitargeted therapy for improving current treatment of PC patients.
Other Anticancer Agents Targeting PC- and Metastasis-Initiating Cells and Their Progenies
Recent studies have led to the identification of different tumorigenic pathways initiated by diverse growth factors and chemokines and drug resistance-associated molecules that provide critical functions for the growth, survival and treatment resistance of PC- and metastasis-initiating cells with stem cell-like properties and their progenies and that constitute potential molecular targets for eradicating the total PC cell mass. These signaling elements include telomerase, ALDH, CD44, EGFR, hedgehog, Wnt/β-catenin, Notch, integrins, IL-6/IL-6R and SDF-1/CXCR4 and downstream signaling elements such as miRNAs, Myc, PI3K/Akt, NF-κB, HIFs, MIC-1, Nrf2 and ABC multidrug transporters such as P-gp and ABCG2 (Figures 2 and 3) (22),47,51,57,58,60,69,72,73,76,108,129,195, 196,201–207). The data from several preclinical studies have indicated that the targeting of these deregulated gene products and oncogenic pathways by RNA silencing or using specific inhibitory agents induced the antiproliferative, antiinvasive, antimetastatic and cytotoxic effects on PC stem/progenitor cells and their progenies in vitro and in vivo and/or reversed chemoresistance (Figure 3) (22),47,51,57,58,60,69,72,73,76, 108,129,195,196,201–207). More specifically, the telomerase represents an attractive target for eliminating the total PC cell mass because this enzyme is expressed at a high level in the majority of PC cells, including PC stem/progenitor cells, and the normal tissue-resident adult stem/progenitor cells typically have longer telomeres than PC cells, thereby minimizing the systemic toxicity associated with the use of telomerase inhibitors (13,82). In support of this result, it has been shown that the synthetic 13 mer-thio-phosphoramidate oligonucleotide inhibitor of telomerase designated as desimetelstat sodium (GRN163L), which is currently in phase I/II clinical trials for the treatment of diverse cancers, inhibited the enzymatic activity of telomerase and induced the telomere shortening in parental and PC cell fractions expressing CD133, CD44 and/or α2β1-integrin isolated from diverse PC3, DU145, C4-2 and LNCaP cell lines in vitro (Figure 3) (202,208).
Numerous studies also revealed that the targeting of distinct growth factor pathways may be effective for eradicating the invasive and metastatic PC cells with stem cell-like features and their progenies and improving the efficacy of current antihormonal treatments and chemotherapies. Particularly, the reexpression of miR-200 in EMT-type PC cells has been shown to reduce the expression of Notch1 and Lin28B and inhibit their prostasphere-forming ability (73). Importantly, it has also been observed that the blockade of canonical Wnt/β-catenin pathway by using the Wnt inhibitor Dickkopf-related protein-1 (DKK1) or secreted Frizzled related protein-2 (sFRP2) reduced the formation and size of self-renewing prostaspheres derived from nonadherent suspension culture of metastatic LNCaP and C4-2B PC cell lines (Figure 3) (108). In contrast, the treatment with the AR antagonist bi-calutamide, also designated as casodex, reduced the prostasphere size and prostate serum antigen expression but did not inhibit the LNCaP and C4-2B prostasphere formation (108). These observations, which are consistent with a reduction of the bulk mass of proliferative androgen sensitive PC cells, while AI PC cells with stem cell-like features can persist after AR inhibition, underline the importance of using casodex in combination with other cytotoxic drugs for eradicating the total PC cell mass (108). Of particular interest, the blockade of EGFR and/or hedgehog cascades by using a specific inhibitor of EGFR tyrosine kinase activity (such as gefitinib, erlotinib or lapitinib), smoothened (SMO) hedgehog coreceptor inhibitor, cyclopamine or anti-SHH antibody has also been shown to induce the antiproliferative, antiinvasive and cytotoxic effects on PC stem/progenitor cells with stem cell-like properties and their progenies in vitro and in vivo (Figure 3) (14),15,21,22,69,203,209,210). For instance, it was reported that the cotargeting of EGFR and hedgehog pathways by using gefitinib and cyclopamine improved the cytotoxic effects induced by mitox-antrone on parental AI PC3 and DU145 cells and CD44high cell fractions enriched from these metastatic PC cell lines (203). Furthermore, a combination of low concentrations of gefitinib and cyclopamine plus docetaxel also induced greater antiproliferative and apoptotic effects on parental PC3 cells as well as on the CD133+ SP subpopulation and CD133− non-SP cell fractions from highly invasive WPE1-NB26 PC cells than individual drugs or two drug combinations (15,69). In addition, since the chemoattractant gradient mediated by SDF-1 can regulate the proliferation, migration and metastatic spread of CXCR4+ PC cells, the inhibition of the SDF-1/CXCR4 axis also may constitute a potential therapeutic approach to prevent their invasion and metastases at distant sites, including bones and disease relapse (Figures 1–3) (51,167–169). Consistently, it has been shown that the treatment with an anti-CXCR4 antibody of CD133+/CD44+ PC cells from immortalized malignant RC-92a/hTERT cells, which have been derived from a human primary PC, inhibited the SDF-1-induced migration of these immature cells with stem cell-like properties (51).
In addition, other promising therapeutic strategies for eradicating the highly aggressive and chemoresistant PC cells may consist of targeting the deregulated metabolic pathways and specific signaling elements such as PI3K/Akt/mTOR, NF-κB, HIF-1a and 2a and MIC-1. These elements are induced under normoxic or hypoxic conditions and may provide critical functions for PC cell survival, invasion and metastasis; angiogenesis; and/or treatment resistance (Figure 3) (29),46,47,129,133,140,146–148,211–218).
Consistent with this finding, the inhibition of glycolysis by using 2-deoxy-D-glucose (2-DG), alone or in combination with other anticancer agents such as pioglitazone, a microtubule disruptor, 2-methoxyoestradiol-3,17-O,O-bis-sulphamate (STX140) or metformin, which acts at least in part by inhibiting 2-DG-induced autophagy, has been shown to induce cytotoxic effects on the highly proliferative PC cells, including multicellular tumor spheroids from metastatic PC cells, and inhibit tumor growth in vivo (Figure 3) (147,148,212,213). It has also been noted that the inhibition of glycolysis by either 2-DG or iodoacetate downregulated P-glycoprotein expression and inhibited the efflux of doxorubicin in multicellular tumor spheroids generated from metastatic and AI DU145 PC cells, suggesting that this therapeutic strategy may be effective for reversing the multidrug resistance phenotype of PC cells (146). In this regard, the downregulation of COX-2, HIF-1α and/or HIF-2β expression levels or activities also constitute other potential strategies to inhibit glycolysis, tumor angiogenesis and eradicate invasive and metastatic PC cells (Figure 3) (29,46,47,133,140,214–216). Consistently, it has been reported that the targeting of HIF-1α and/or HIF-2α in PC cells by RNA silencing or using a specific inhibitor of HIF-1α, PX-478 (S-2-amino-3-[4′-N,N,-bis(2-chloroethyl)amino]phenyl propionic acid N-oxide dihydrochloride), or inhibitor of the proteasomal degradation of HIF-1α and/or HIF-2α such as ascorbic acid or zinc inhibited their invasive ability in vitro and tumor formation and lung metastases in vivo (Figure 3) (47,206,219). In addition, the inhibition of PI3K/Akt/mTOR by using a specific inhibitor of PI3K (LY294002), mTOR (ra-pamycin, RAD-001, also designated as 40-O-(2-hydroxyethyl)-rapamycin or CCl-779) or dual PI3K/mTOR inhibitor (PI-103 or NVP-BEZ235) also constitutes another strategy for inhibiting HIF-1α-induced target genes including glycolytic enzymes and eradicating the total PC cell mass (Figure 3) (129,217,218). In support of this finding, the treatment of highly metastatic and AI DU145 and PC3 cell lines with LY294002 or NVP-BEZ235 was observed to induce growth inhibition and cytotoxic effects on the CD133+/CD44+ cell fraction and the bulk PC cell mass detected by cytometric analyses (129). However, additional investigations are necessary to establish the benefit to target these metabolic and survival pathways, alone or in combination, for eradicating the total PC cell mass and improving current antihormonal treatments and docetaxel-based chemotherapies. In this regard, an enhanced expression of the MIC-1 level was observed during PC progression to CRPCs and after chemotherapy and was associated with a shorter patient survival after docetaxel treatment (207,220). Of clinical interest, it has also been shown that the MIC-1 expression level was upregulated in metastatic and AI PC3-Rx cells made resistant to docetaxel relative to parental PC3 cells and the downregulation of endogenous MIC-1 level by RNA silencing sensibilized the PC3-Rx cells to the cytotoxic effects induced by docetaxel (207). However, future studies are essential to establish the functions of secreted MIC-1 cytokines in PC cells with stem cell-like properties and the interest of its targeting to eradicate the total PC cell mass.
Conclusions and Future Directions
Taken together, these recent advances in the last few years on establishment of the molecular mechanisms at the basis of prostate carcinogenesis and metastases have revealed that the alterations of a specific subset of gene products may occur in PC- and metastasis-initiating cells endowed with stem cell-like properties versus their progenies during PC etiopathogenesis and transition to locally invasive, metastatic and recurrent disease stages. More particularly, it appears that the intrinsic properties of highly tumorigenic and migrating PC stem/progenitor cells and their acquisition of more malignant and multidrug resistance phenotypes during PC progression may contribute to their persistence at primary and secondary neoplasms, tumor regrowth and disease relapse after treatment initiation. Importantly, it was also shown that the molecular targeting of distinct oncogenic and drug resistance-associated gene products that are frequently deregulated in PC stem/progenitor cells and their progenies, including EGFR, hedgehog, Wnt/β-catenin and Notch and their downstream signaling elements Akt, NF-κB and HIFs, constitute promising therapeutic strategies of great clinical interest to eradicate the total PC cell mass and reverse treatment resistance.
However, future investigations are necessary to further establish the cellular origin of different PC subtypes and molecular pathways and drug resistance-associated molecules that may contribute to the acquisition of more aggressive behavior by PC- and metastasis-initiating cells as well as their resistance to current therapies. It will be especially important to more precisely establish the implication of basal stem/progenitor cells versus their progenies with an intermediate phenotype found in basal and luminal compartments in PC initiation and progression. Moreover, it will be important to define the gene products and molecular pathways altered during the EMT process and metastases of PC stem/progenitor cells at distant tissues, including bones, and that may contribute to their acquisition of aggressive phenotypes and disease relapse. Additional studies are also required to validate the cytotoxic effects induced by diverse dietary substances and novel combinations of drugs targeting distinct oncogenic signaling elements on isolated PC- and metastasis-initiating cells and their progenies on diverse in vitro PC cells and animal models mimicking the early and late stages of PC.
Hence, together these future investigations should lead to the validation of novel dietary compounds and pharmacological agents that could be used to simultaneously target distinct gene products altered in PC stem/progenitor cells and their progenies during PC progression and metastases. These anticarcinogenic agents could be used to develop an effective multitargeted strategy for eradicating the PC- and metastasis-initiating cells and their progenies, improving the current antihormonal treatments and chemotherapies against aggressive and metastatic CRPCs, thereby preventing the disease relapse and death of PC patients.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Winquist E, et al. (2006) Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systematic review from the Cancer Care Ontario Program in Evidence-based Care’s Genitourinary Cancer Disease Site Group. BMC Cancer. 6:112.
Tannock IF, et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351:1502–12.
Petrylak DP, et al. (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351:1513–20.
Jemal A, et al. (2009) Cancer statistics, 2009. CA Cancer J. Clin. 59:225–49.
Mimeault M, Batra SK. (2006) Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 27:1–22.
Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. (2008) The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can. J. Urol. 15:3866–71.
Freedland SJ. (2011) Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 117:1123–35.
Ye XC, Choueiri M, Tu SM, Lin SH. (2007) Biology and clinical management of prostate cancer bone metastasis. Front. Biosci. 12:3273–86.
Yuan X, Balk SP. (2009) Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 27:36–41.
Feldman BJ, Feldman D. (2001) The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 1:34–45.
Karlou M, Tzelepi V, Efstathiou E. (2010) Therapeutic targeting of the prostate cancer microenvironment. Nat. Rev. Urol. 7:494–509.
Di Lorenzo G, De Placido S. (2006) Hormone refractory prostate cancer (HRPC): present and future approaches of therapy. Int. J. Immunopathol. Pharmacol. 19:11–34.
Mimeault M, Mehta PP, Hauke R, Batra SK. (2008) Functions of normal and malignant pro-static stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr. Rev. 29:234–52.
Mimeault M, et al. (2006) Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int. J. Cancer. 118:1022–31.
Mimeault M, et al. (2007) Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol. Cancer Ther. 6:967–78.
Mimeault M, et al. (2007) Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. Int. J. Cancer. 120:160–9.
Di Lorenzo G, et al. (2002) Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 8:3438–44.
Schafer W, et al. (2006) Intensity of androgen and epidermal growth factor receptor immunoreactivity in samples of radical prostatectomy as prognostic indicator: correlation with clinical data of long-term observations. J. Urol. 176:532–7.
Le Page C, Koumakpayi IH, Lessard L, Mes-Masson AM, Saad F. (2005) EGFR and Her-2 regulate the constitutive activation of NF-kappaB in PC-3 prostate cancer cells. Prostate. 65:130–40.
Hammarsten P, et al. (2010) Low levels of phosphorylated epidermal growth factor receptor in nonmalignant and malignant prostate tissue predict favorable outcome in prostate cancer patients. Clin. Cancer Res. 16:1245–55.
Sheng T, et al. (2004) Activation of the hedgehog pathway in advanced prostate cancer. Mol. Cancer. 3:29.
Karhadkar SS, et al. (2004) Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 431:707–12.
Taichman RS, et al. (2002) Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 62:1832–7.
Ayala G, et al. (2004) High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin. Cancer Res. 10:6572–78.
Shukla S, et al. (2007) Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer. 121:1424–32.
Ross JS, et al. (2004) Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin. Cancer Res. 10:2466–72.
Sweeney C, et al. (2004) Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin. Cancer Res. 10:5501–7.
Koumakpayi IH, Le Page C, Mes-Masson AM, Saad F. (2010) Hierarchical clustering of immunohistochemical analysis of the activated ErbB/PI3K/Akt/NF-kappaB signalling pathway and prognostic significance in prostate cancer. Br. J. Cancer. 102:1163–73.
Chae KS, et al. (2011) Opposite functions of HIF-alpha isoforms in VEGF induction by TGF-beta1 under non-hypoxic conditions. Oncogene. 30:1213–28.
Wang J, Cai Y, Ren C, Ittmann M. (2006) Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 66:8347–51.
Tomlins SA, et al. (2005) Recurrent fusion of TM-PRSS2 and ETS transcription factor genes in prostate cancer. Science. 310:644–8.
Tomlins SA, et al. (2006) TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 66:3396–400.
Perner S, et al. (2007) TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am. J. Surg. Pathol. 31:882–8.
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. (2008) Recurrent gene fusions in prostate cancer. Nat. Rev. Cancer. 8:497–511.
King JC, et al. (2009) Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat. Genet. 41:524–6.
Yoshimoto M, et al. (2008) Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod. Pathol. 21:1451–60.
Liu S, et al. (2011) Detection of ERG gene rearrangements and PTEN deletions in unsuspected prostate cancer of the transition zone. Cancer Biol. Ther. 11:562–6.
Bismar TA, et al. (2011) PTEN genomic deletion is an early event associated with ERG gene rearrangements in prostate cancer. BJU Int. 107:477–85.
Demichelis F, et al. (2007) TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 26:4596–9.
Leong M, et al. (2009) Overexpression of truncated ERG from TMPRSS2-ERG fusion and prostate cancer development. Pathol. Lab. Med. Int. 1:13–21.
Carver BS, et al. (2009) Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet. 41:619–24.
Swanson TA, et al. (2011) TMPRSS2/ERG fusion gene expression alters chemo- and radio-responsiveness in cell culture models of androgen independent prostate cancer. Prostate. 2011 March 10 [Epub ahead of print]
Datta MW, et al. (2006) Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol. Cancer. 5:9.
Ao M, et al. (2007) Cross-talk between paracrineacting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 67:4244–53.
Chung LW, Baseman A, Assikis V, Zhau HE. (2005) Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J. Urol. 173:10–20.
Giannoni E, et al. (2010) Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70:6945–56.
Giannoni E, Bianchini F, Calorini L, Chiarugi P. (2011) Cancer associated fibroblasts exploit reactive oxygen species through a pro-inflammatory signature leading to EMT and stemness. Antioxid. Redox. Signal. 14:2361–71.
Brown MD, et al. (2007) Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. Prostate. 67:1384–96.
Maitland NJ, Bryce SD, Stower MJ, Collins AT. (2006) Prostate cancer stem cells: a target for new therapies. Ernst Schering Found. Symp. Proc. 5:155–79.
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65:10946–51.
Miki J, et al. (2007) Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 67:3153–61.
Rowehl RA, Crawford H, Dufour A, Ju J, Botchkina GI. (2008) Genomic analysis of prostate cancer stem cells isolated from a highly metastatic cell line. Cancer Genomics Proteomics. 5:301–10.
Guzman-Ramirez N, et al. (2009) In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue. Prostate. 69:1683–93.
Xin L, Lawson DA, Witte ON. (2005) The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 102:6942–7.
Chen BY, et al. (2007) Hedgehog is involved in prostate basal cell hyperplasia formation and its progressing towards tumorigenesis. Biochem. Biophys. Res. Commun. 357:1084–9.
Wei C, Guomin W, Yujun L, Ruizhe Q. (2007) Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line? Cancer Biol. Ther. 6:763–8.
Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B. (2006) Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 25:1696–708.
Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. (2007) Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 67:6796–805.
Patrawala L, et al. (2005) Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 65:6207–19.
Tang DG, et al. (2007) Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol. Carcinog. 46:1–14.
Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. (2010) Identification of a cell of origin for human prostate cancer. Science. 329:568–71.
Lawson DA, et al. (2010) Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc. Natl. Acad. Sci. U. S. A. 107:2610–5.
Mimeault M, Batra SK. (2011) Animal models of prostate carcinogenesis underlining the critical implication of prostatic stem progenitor cells. Biochim. Biophys. Acta. 1816:25–37.
Li T, et al. (2009) ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab. Invest. 30:234–44.
van den Hoogen C, et al. (2010) High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 70:5163–73.
Gu G, Yuan J, Wills M, Kasper S. (2007) Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 67:4807–15.
Liu T, et al. (2010) Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol. Cell. Biochem. 340:265–73.
Fan X, Liu S, Su F, Pan Q, Lin T. (2010) Effective enrichment of prostate cancer stem cells from spheres in a suspension culture system. Urol. Oncol. 2010, Sep 13. [Epub ahead of print].
Mimeault M, Johansson SL, Henichart JP, Depreux P, Batra SK. (2010) Cytotoxic effects induced by docetaxel, gefitinib, and cyclopamine on side population and non-side population cell fractions from human invasive prostate cancer cells. Mol. Cancer Ther. 9:617–30.
Chauchereau A, et al. (2011) Stemness markers characterize IGR-CaP1, a new cell line derived from primary epithelial prostate cancer. Exp. Cell. Res. 317:262–75.
Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL. (2010) Genomic profiling of tumor initiating prostatospheres. BMC Genomics. 11:324.
Klarmann GJ, et al. (2009) Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis. 26:433–46.
Kong D, et al. (2010) Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 5:e12445.
Bae KM, et al. (2010) Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J. Urol. 183:2045–53.
Acharya A, Das I, Chandhok D, Saha T. (2010) Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 3:23–34.
Birnie R, et al. (2008) Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 9:R83.
Mathews LA, Hurt EM, Zhang X, Farrar WL. (2010) Epigenetic regulation of CpG promoter methylation in invasive prostate cancer cells. Mol. Cancer. 9:267.
Kasper S, Cookson MS. (2006) Mechanisms leading to the development of hormone-resistant prostate cancer. Urol. Clin. North Am. 33:201–10.
De Marzo AM, et al. (2004) Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J. Cell. Biochem. 91:459–77.
De Marzo AM, et al. (2007) Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7:256–69.
Zenzmaier C, Untergasser G, Berger P. (2008) Aging of the prostate epithelial stem/progenitor cell. Exp. Gerontol. 43:981–5.
Mimeault M, Batra SK. (2009) Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res. Rev. 8:94–112.
Ellem SJ, Wang H, Poutanen M, Risbridger GP. (2009) Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic premalignancy. Am. J. Pathol. 175:1187–99.
Evans GS, Chandler JA. (1987) Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate. 11:339–51.
Robinson EJ, Neal DE, Collins AT. (1998) Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 37:149–60.
Hudson DL, O’Hare M, Watt FM, Masters JR. (2000) Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab. Invest. 80:1243–50.
Wang Y, Hayward S, Cao M, Thayer K, Cunha G. (2001) Cell differentiation lineage in the prostate. Differentiation. 68:270–9.
Collins AT, Habib FK, Maitland NJ, Neal DE. (2001) Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J. Cell. Sci. 114:3865–72.
Tokar EJ, Ancrile BB, Cunha GR, Webber MM. (2005) Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 73:463–73.
Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, et al. (2004) CD133, a novel marker for human prostatic epithelial stem cells. J. Cell. Sci. 117:3539–45.
Burger PE, et al. (2005) Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc. Natl. Acad. Sci. U. S. A. 102:7180–5.
Heer R, Robson CN, Shenton BK, Leung HY. (2007) The role of androgen in determining differentiation and regulation of androgen receptor expression in the human prostatic epithelium transient amplifying population. J. Cell. Physiol. 212:572–8.
Burger PE, et al. (2009) High ALDH activity: a novel functional marker of murine prostate stem/progenitor cells. Stem Cells. 27:2220–8.
Tsujimura A, et al. (2002) Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J. Cell. Biol. 157:1257–65.
Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. (2007) Isolation and functional characterization of murine prostate stem cells. Proc. Natl. Acad. Sci. U. S. A. 104:181–6.
Leong KG, Wang BE, Johnson L, Gao WQ. (2008) Generation of a prostate from a single adult stem cell. Nature. 456:804–8.
Wang S, et al. (2006) Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. U. S. A. 103:1480–5.
Mulholland DJ, et al. (2009) Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 69:8555–62.
Zhou Z, et al. (2006) Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 66:7889–98.
Wang X, et al. (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461:495–500.
Man YG, Gardner WA. (2007) Focal degeneration of basal cells and the resultant auto-immunoreactions: a novel mechanism for prostate tumor progression and invasion. Med. Hypotheses. 70:387–408.
Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. (2008) PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 68:1820–1825.
Hayward SW, et al. (2001) Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 61:8135–42.
van Leenders GJ, Aalders TW, Hulsbergen-van de Kaa CA, Ruiter DJ, Schalken JA. (2001) Expression of basal cell keratins in human prostate cancer metastases and cell lines. J. Pathol. 195:563–70.
van Leenders GJ, et al. (2003) Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am. J. Pathol. 162:1529–37.
Yang XJ, Lecksell K, Gaudin P, Epstein JI. (1999) Rare expression of high-molecular-weight cytokeratin in adenocarcinoma of the prostate gland: a study of 100 cases of metastatic and locally advanced prostate cancer. Am. J. Surg. Pathol. 23:147–52.
Dubrovska A, et al. (2009) The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. U. S. A. 106:268–73.
Bisson I, Prowse DM. (2009) WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 19:683–97.
Wang S, et al. (2003) Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 4:209–21.
Kim MJ, et al. (2002) Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 62:2999–3004.
Kim MJ, et al. (2002) Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 99:2884–9.
Song H, et al. (2009) Loss of Nkx3.1 leads to the activation of discrete downstream target genes during prostate tumorigenesis. Oncogene. 28:3307–19.
Gao H, et al. (2004) A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 101:17204–9.
Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. (2001) Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat. Genet. 27:222–4.
Abate-Shen C, et al. (2003) Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 63:3886–90.
Couto SS, et al. (2009) Simultaneous haploinsufficiency of Pten and Trp53 tumor suppressor genes accelerates tumorigenesis in a mouse model of prostate cancer. Differentiation. 77:103–11.
Chen Z, et al. (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 436:725–30.
Elgavish A, et al. (2004) Transgenic mouse with human mutant p53 expression in the prostate epithelium. Prostate 61:26–34.
Abou-Kheir WG, Hynes PG, Martin PL, Pierce R, Kelly K. (2010) Characterizing the contribution of stem/progenitor cells to tumorigenesis in the Pten(−/−)TP53(−/−) prostate cancer model. Stem Cells. 28:2129–40.
Shah RB, Ghosh D, Elder JT. (2006) Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate 66:1437–44.
Misra S, Toole BP, Ghatak S. (2006) Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J. Biol. Chem. 281:34936–41.
Zhang S, et al. (2008) Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J. Exp. Clin. Cancer Res. 27:62.
Katoh M, Katoh M. (2010) Integrative genomic analyses of CXCR4: transcriptional regulation of CXCR4 based on TGFbeta, Nodal, Activin signaling and POU5F1, FOXA2, FOXC2, FOXH1, SOX17, and GFI1 transcription factors. Int. J. Oncol. 36:415–20.
Mimeault M, Batra SK. (2010) Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacol. Rev. 62:497–524.
Skvortsova I, et al. (2008) Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics. 8:4521–33.
Goel HL, Underwood JM, Nickerson JA, Hsieh CC, Languino LR. (2010) Beta1 integrins mediate cell proliferation in three-dimensional cultures by regulating expression of the sonic hedgehog effector protein, GLI1. J. Cell. Physiol. 224:210–7.
Narita S, et al. (2008) GLI2 knockdown using an antisense oligonucleotide induces apoptosis and chemosensitizes cells to paclitaxel in androgen-independent prostate cancer. Clin. Cancer Res. 14:5769–77.
Mimeault M, Batra SK. (2007) Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 26:203–14.
Dubrovska A, et al. (2009) The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. U. S. A. 106:268–73.
Penet MF, et al. (2009) Noninvasive multiparametric imaging of metastasis-permissive microenvironments in a human prostate cancer xenograft. Cancer Res. 69:8822–9.
Chen N, et al. (2009) BCL-xL is a target gene regulated by hypoxia-inducible factor-1α. J. Biol. Chem. 284:10004–12.
Anderson KM, Guinan P, Rubenstein M. (2011) The effect of normoxia and hypoxia on a prostate (PC-3) CD44/CD41 cell side fraction. Anticancer Res. 31:487–94.
Pidgeon GP, et al. (2007) Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 26:503–24.
Mimeault M, Batra SK. (2007) Interplay of distinct growth factors during epithelial-mes-enchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann. Oncol. 18:1605–19.
Zhao H, Peehl DM. (2009) Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 69:991–1000.
Chen M, et al. (2010) Hedgehog/Gli supports androgen signaling in androgen deprived and androgen independent prostate cancer cells. Mol. Cancer. 9:89.
Hu Y, et al. (2009) Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia. 11:1042–53.
Kasina S, Scherle PA, Hall CL, Macoska JA. (2009) ADAM-mediated amphiregulin shedding and EGFR transactivation. Cell. Prolif. 42:799–812.
Frisch SM, Screaton RA. (2001) Anoikis mechanisms. Curr. Opin. Cell. Biol. 13:555–62.
Giannoni E, Fiaschi T, Ramponi G, Chiarugi P. (2009) Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene. 28:2074–86.
Higgins LH, et al. (2009) Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim. Biophys. Acta 1787:1433–43.
LaTulippe E, et al. (2002) Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 62:4499–506.
Semenza GL, Roth PH, Fang HM, Wang GL. (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269:23757–63.
Daly EB, Wind T, Jiang XM, Sun L, Hogg PJ. (2004) Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim. Biophys. Acta. 1691:17–22.
Warburg O. (1956) On the origin of cancer cells. Science. 123:309–14.
Wartenberg M, et al. (2010) Glycolytic pyruvate regulates P-glycoprotein expression in multicellular tumor spheroids via modulation of the intra-cellular redox state. J. Cell. Biochem. 109:434–46.
Ben Sahra I, et al. (2010) Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 70:2465–75.
Gottfried E, et al. (2011) Pioglitazone modulates tumor cell metabolism and proliferation in multicellular tumor spheroids. Cancer Chemother. Pharmacol. 67:117–26.
Lopez-Lazaro M. (2010) A new view of carcinogenesis and an alternative approach to cancer therapy. Mol. Med. 16:144–53.
Bubendorf L, et al. (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 31:578–83.
Mundy GR. (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2:584–93.
Chang HH, et al. (2011) Hedgehog overexpression leads to the formation of prostate cancer stem cells with metastatic property irrespective of androgen receptor expression in the mouse model. J. Biomed. Sci. 18:6.
Setlur SR, et al. (2007) Integrative microarray analysis of pathways dysregulated in metastatic prostate cancer. Cancer Res. 67:10296–303.
LaTulippe E, et al. (2002) Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 62:4499–506.
Suzuki H, et al. (1998) Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 58:204–9.
Whang YE, et al. (1998) Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl. Acad. Sci. U. S. A. 95:5246–50.
Wu Z, McRoberts KS, Theodorescu D. (2007) The role of PTEN in prostate cancer cell tropism to the bone micro-environment. Carcinogenesis. 28:1393–400.
DeHaan AM, Wolters NM, Keller ET, Ignatoski KM. (2009) EGFR ligand switch in late stage prostate cancer contributes to changes in cell signaling and bone remodeling. Prostate. 69:528–37.
Bednarz N, et al. (2010) BRCA1 loss preexisting in small subpopulations of prostate cancer is associated with advanced disease and metastatic spread to lymph nodes and peripheral blood. Clin. Cancer Res. 16:3340–8.
Morrissey C, Vessella RL. (2007) The role of tumor microenvironment in prostate cancer bone metastasis. J. Cell. Biochem. 101:873–86.
Koeneman KS, Yeung F, Chung LW. (1999) Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 39:246–61.
Festuccia C, et al. (2000) Osteoblast-derived TGF-beta1 modulates matrix degrading protease expression and activity in prostate cancer cells. Int. J. Cancer 85:407–15.
Zunich SM, et al. (2009) Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol. Cancer. 8:12.
Karsdal MA, et al. (2002) Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 277:44061–7.
Chen SJ, et al. (2007) Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells. Prostate. 67:557–71.
Senapati S, et al. (2010) Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene. 9:1293–302.
Sun YX, et al. (2003) Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell. Biochem. 89:462–73.
Sun YX, et al. (2005) Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Miner. Res. 20:318–29.
Engl T, et al. (2006) CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 8:290–301.
Logothetis CJ, Lin SH. (2005) Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 5:21–8.
Schneider A, et al. (2005) Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 146:1727–36.
Sikes RA, et al. (2004) Cellular interactions in the tropism of prostate cancer to bone. Int. J. Cancer. 110:497–503.
Jung Y, et al. (2009) Expression of PGK1 by prostate cancer cells induces bone formation. Mol. Cancer Res. 7:1595–604.
Zhang J, et al. (2004) In vivo real-time imaging of TGF-beta-induced transcriptional activation of the RANK ligand gene promoter in intraosseous prostate cancer. Prostate. 59:360–9.
Wakchoure S, et al. (2009) Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate. 69:652–61.
Coulson-Thomas VJ, et al. (2010) Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-beta, and extracellular matrix down-regulation. Exp. Cell Res. 316:3207–26.
Josson S, Matsuoka Y, Chung LW, Zhau HE, Wang R (2010) Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin. Cell. Dev. Biol. 21:26–32.
Wang J, Levenson AS, Satcher RL Jr. (2006) Identification of a unique set of genes altered during cell-cell contact in an in vitro model of prostate cancer bone metastasis. Int. J. Mol. Med. 17:849–56.
Altieri DC, et al. (2009) Prostate cancer regulatory networks. J. Cell. Biochem. 107:845–52.
Bui M, Reiter RE. (1998) Stem cell genes in androgen-independent prostate cancer. Cancer Metastasis Rev. 17:391–9.
Tang Y, Hamburger AW, Wang L, Khan MA, Hussain A. (2009) Androgen deprivation and stem cell markers in prostate cancers. Int. J. Clin. Exp. Pathol. 3:128–38.
Tang Y, et al. (2009) The relationship of neuroendocrine carcinomas to anti-tumor therapies in TRAMP mice. Prostate. 69:1763–73.
Tang Y, et al. (2008) Divergent effects of castration on prostate cancer in TRAMP mice: possible implications for therapy. Clin. Cancer Res. 14:2936–43.
Banach-Petrosky W, et al. (2007) Prolonged exposure to reduced levels of androgen accelerates prostate cancer progression in Nkx3.1; Pten mutant mice. Cancer Res. 67:9089–96.
Wang J, Eltoum IE, Lamartiniere CA. (2004) Genistein alters growth factor signaling in transgenic prostate model (TRAMP). Mol. Cell. Endocrinol. 219:171–80.
Li Y, et al. (2006) Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 66:4816–25.
Khor TO, et al. (2009) Dietary feeding of diben-zoylmethane inhibits prostate cancer in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 69:7096–102.
Zhang Y, et al. (2010) A novel sulindac derivative lacking cyclooxygenase-inhibitory activities suppresses carcinogenesis in the transgenic ade-nocarcinoma of mouse prostate model. Cancer Prev. Res. (Phila). 3:885–95.
Konijeti R, et al. (2010) Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate. 70:1547–54.
Ghosh R, et al. (2010) Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer Res. 30:857–65.
Slusarz A, et al. (2010) Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 70:3382–90.
Narayanan NK, Nargi D, Randolph C, Narayanan BA. (2009) Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer. 125:1–8.
Raina K, et al. (2008) Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 68:6822–30.
Basu HS, et al. (2009) A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 69:7689–95.
Kawasaki BT, et al. (2009) Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: an integrated molecular profiling approach. Prostate. 69:827–37.
Volate SR, et al. (2010) Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol. Cancer Ther. 9:461–70.
Kallifatidis G, et al. (2011) Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol. Ther. 19:188–95.
Sarkar FH, Li Y, Wang Z, Kong D. (2010) Novel targets for prostate cancer chemoprevention. Endocr. Relat. Cancer. 17:R195–212.
Gingrich JR, et al. (1997) Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 57:4687–91.
Zhang ZX, Xu QQ, Huang XB, Zhu JC, Wang XF. (2009) Early and delayed castrations confer a similar survival advantage in TRAMP mice. Asian J. Androl. 11:291–7.
Koeneman KS. (2006) Prostate cancer stem cells, telomerase biology, epigenetic modifiers, and molecular systemic therapy for the androgen-independent lethal phenotype. Urol. Oncol. 24:119–21.
Marian CO, Wright WE, Shay JW. (2010) The effects of telomerase inhibition on prostate tumor-initiating cells. Int. J. Cancer. 127:321–31.
Mimeault M, et al. (2007) Improvement of cytotoxic effects of mitoxantrone on hormone-refractory metastatic prostate cancer cells by co-targeting epidermal growth factor receptor and hedgehog signaling cascades. Growth Factors. 25:400–16.
Fu Y, et al. (2008) Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc. Natl. Acad. Sci. U. S. A. 105:19444–9.
Singh A, et al. (2010) Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol. Cancer Ther. 9:2365–76.
Nardinocchi L, et al. (2010) Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One. 5:e15048.
Zhao L, et al. (2009) Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling. Cancer Res. 69:7696–703.
Marian CO, Shay JW. (2009) Prostate tumor-initiating cells: a new target for telomerase inhibition therapy? Biochim. Biophys. Acta. 1792:289–96.
Sanchez P, et al. (2004) Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc. Natl. Acad. Sci. U. S. A. 101:12561–6.
Shaw G, Prowse DM. (2008) Inhibition of androgen-independent prostate cancer cell growth is enhanced by combination therapy targeting Hedgehog and ErbB signalling. Cancer Cell Int. 8:3.
Stein M, et al. (2010) Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 70:1388–94.
Tagg SL, et al. (2008) 2-Methoxyoestradiol-3,17-O,O-bis-sulphamate and 2-deoxy-D-glucose in combination: a potential treatment for breast and prostate cancer. Br. J. Cancer. 99:1842–8.
Heyfets A, Flescher E. (2007) Cooperative cytotoxicity of methyl jasmonate with anti-cancer drugs and 2-deoxy-D-glucose. Cancer Lett. 250:300–10.
Lee K, et al. (2010) LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 80:982–9.
Koh MY, et al. (2008) Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 7:90–100.
Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. (2004) Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 3:233–44.
Hudson CC, et al. (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22:7004–14.
Majumder PK, et al. (2004) mTOR inhibition reverses Akt-dependent prostate intraepithelial neo-plasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 10:594–601.
Gao P, et al. (2007) HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 12:230–8.
Huang CY, et al. (2007) Molecular alterations in prostate carcinomas that associate with in vivo exposure to chemotherapy: identification of a cytoprotective mechanism involving growth differentiation factor 15. Clin. Cancer Res. 13:5825–533.
Acknowledgments
This work was supported in part by the U.S. Department of Defense (PC074289) and the National Institutes of Health (R01CA138791) for prostate cancer research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mimeault, M., Batra, S.K. Frequent Gene Products and Molecular Pathways Altered in Prostate Cancer- and Metastasis-Initiating Cells and Their Progenies and Novel Promising Multitargeted Therapies. Mol Med 17, 949–964 (2011). https://doi.org/10.2119/molmed.2011.00115
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2011.00115