Abstract
Background
Essential oils from aerial parts of Achillea wilhelmsii, Tanacetum polycephalum and Teucrium polium were isolated by using Clevenger-type apparatus and tested at different concentrations for their nematicidal activity against the second stage juvenile (J2) of Meloidogyne incognita in vitro condition. The chemical components of the essential oils and seed extracts of each plant (0.2 g) were extracted with maceration with methanol/acetic acid mixture (85:15, v/v). Analysis was done by Gas Chromatography, GC-Mass Spectrometry) and HPLC. Identified chemical components were tested after this on J2 of M. incognitain. Nuclear magnetic resonance spectroscopy was done to investigate the properties of organic molecules by drawing their spectrum using Broker AVANCE AQS-300 MHz.
Results
Significant difference was achieved on nematicidal activity of essential oils based on the plant species and oil concentrations. GC and GC–MS led to identification of 41, 39 and 45 major compounds from T. polium, T. polycephalum and A. wilhelmsii oils, respectively. A number of 10 components with different ranges of percentage were recorded in all of the tested plants oils. Use of HPLC resulted in identification of 4, 3 and 2 chemical compounds in the extracts of A. wilhelmsii, T. polycephalum and T. polium, respectively. The nematicidal activity of commercial polyphenols at the concentration of 1100 ppm showed 58.3, 48.9, 28.2 and 26.8 percentages J2 mortalities by catechin, coumarin, gallic acid and chlorogenic, respectively. Nematotoxicity test of commercial terpenoids showed the highest J2 mortalities (more than 80%), in concentrations of 100 and 200 ppm limonene, β-pinene and α-pinene. However, it was less than 30% of J2 mortality caused by terpinen-4-ol, α-terpineol and linalool.
Conclusions
Compounds such as Limonene, β-pinene and α-pinene were detected in all of the tested plants, A. wilhelmsii, T. polycephalum and T. polium, having an effective nematicidal action versus terpinen-4-ol, α-terpineol and linalool.
Similar content being viewed by others
Background
Plant parasitic nematodes have caused more than $100 billion in annual losses of crops and plants worldwide (Li et al., 2013). Root knot nematode (Meloidogyne incognita) is the predominant plant parasitic nematode in Iran and causes serious problems to many crops (Akhyani et al., 1984). Human health safety and environmental concerns have been discussed due to use of chemical pesticides including different chemical nematicides, since their side effects were observed and repotted (Chitwood, 2002).
Nematicidal activities of many plant essential oils and phytochemicals of plants like Teucrium polium and Achillea wilhelmsii have been reported previously (Chitwood, 2002). Plant essential oils consist of volatile compounds such as alcohols, aldehydes, terpenoids and phenolics. These compounds are ecofriendly and show many biological activities such as insecticidal, antifungal or nematicidal effects (Ohri, & Kaur, 2009). Also, plant secondary metabolites with a nematicidal activity can be released during decomposition of plant biomasses (Chitwood, 2002; Renco et al. 2012; Renco, 2013; Baidoo et al., 2017). Among main components of plants essential oil and also their aquatic extracts, terpenoids and polyphenols are, respectively, known for a large structural and functional diversity, a high nematicidal activity and no adverse effects on plant growth parameters (Echeverrigaray et al., 2010).
The objectives of this study were to identify chemical components from water extracts and essential oils of Achillea wilhelmsii, Tanacetum polycephalum and Teucrium polium, and their nematicidal effects using gas chromatography (GC), gas chromatography coupled with mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC) and HN.M.R.
Methods
Plant materials
In April and May, at the time of flowering, annual grown aerial parts of T. polium, T. polycephalum and A. wilhelmsii were obtained from Kohgyloyeh va Boyrahmad province with geographical coordinates of 51°31′ E and 30°31′ N. Plant materials were washed with distilled water. Their aerial parts were then separated and dried individually in the shade at room temperature (22–25 °C) for 7 days. They were then powdered by a laboratory grinder and used for extraction of essential oil as well as water extracts.
Essential oil extraction
The essential oil of all dried samples (100 g of each plant) was isolated by hydro-distillation for 3 h, using a Clevenger-type apparatus according to the method recommended in British Pharmacopoeia (British pharmacopoeia, 1988; Erskine, 2007). The distillated oils were dried over anhydrous sodium sulfate and stored in tightly closed dark vials at refrigerator (4 °C), until analysis.
Gas chromatography (GC) and gas chromatography-mass spectrometry (GC–MS)
GC analysis was performed using an Agilent gas chromatograph series 7890-A with a flame ionization detector (FID). The analysis was carried out on fused silica capillary HP-5 column (30 m × 0.32 mm i.d.; film thickness 0.25 mm). Temperatures of the injector and the detector were 250 °C and 280 °C, respectively. Nitrogen was used as carrier gas at a flow rate of 1 ml/min; oven temperature program was 60–210 °C at the rate of 4 °C/min and then programmed to 240 °C at the rate of 20 °C/min and finally held isothermally for 8.5 min; split ratio was 1:50. GC–MS analysis was carried out by use of Agilent gas chromatograph equipped with fused silica capillary HP-5MS column (30 m × 0.25 mm i.d.; film thickness 0.25 mm) coupled with 5975-C mass spectrometer. Helium (99.9%) was used as carrier gas with ionization voltage of 70 eV. Ion source and interface temperatures were 230 °C and 280 °C, respectively. Mass range was from 45 to 550 amu. Oven temperature program was the same given above for the GC.
The constituents of the essential oils were identified by calculation of their retention indices under temperature-programmed conditions for n-alkanes (C8–C25) and the oil on a HP-5 column under the same chromatographic conditions. Identification of individual compounds was made by comparison of their mass spectra with those of the internal reference mass spectra library or with authentic compounds and confirmed by comparison of their retention indices with authentic compounds or with those of reported in the literature (Adams, 2007). For quantification purpose, relative area percentages obtained by FID were used without the use of correction factors.
High-performance liquid chromatography (HPLC)
Powdered samples of each plant (0.2 g) were extracted with maceration with methanol/acetic acid mixture (85:15, v/v), with the ratio of raw materials to methanol/acetic acid mixture of 1:10, for 24 h in darkness in a freezer at − 18 °C and subsequently extracted in a ultrasonic bath for 15 min. Solutions then were centrifuged at 10,000 rpm for 20 min in 0 °C. One ml of supernatant was separated and mixed with 1 ml of n-hexane (1:1, v/v). This solution was centrifuged again at 10,000 rpm for 10 min in 0 °C. The below layer was then filtered through 0.2-µm-pore size membrane filters and stored in darkness in a freezer at − 18 °C until analysis (Mandal et al., 1991).
HPLC analysis was performed by using an Agilent 1200 series, equipped with a diode array detector (DAD), Chemstation Software (Agilent Technologies), a quaternary pump an online vacuum degasser, an auto sampler and a thermo-stated column compartment, on an Agilent Zorbax Eclipse XDB-C18, 5 µm (ID), 4.6 × 150 mm (FT) column, at a flow-rate of 1 ml min−1. Solvent gradient was performed by varying the proportion of solvent A (formic acid 1% in deionized water) to solvent B (methanol (v/v) as follows: methanol/formic acid 1%: (10:90), hold time: 0 min; methanol/formic acid 1%: (25:75), hold time: 10 min; methanol/formic acid 1%: (60:40), hold time: 20 min; methanol/formic acid 1%: (70:30), hold time: 30 min; the total running time was 30 min. The column temperature was 30 °C. The injected volume of samples and standards was 20µL, and it was done automatically using an auto-sampler. Chromatograms were plotted at 280 and 320 nm (Mandal et al., 1991).
Nuclear magnetic resonance spectroscopy (HN.M.R)
Two kg of each plant powder was put in a double-layered muslin cloth and macerated in 2000 ml of distilled water for 12 h at 25 °C. The suspensions were then filtered and stored at 28 °C until all liquid materials were evaporated. Dried materials obtained from the bottom of glasses vessels were used for nuclear magnetic resonance spectroscopy to investigate the properties of organic molecules by drawing their spectrum.
A suspension of 0/2 molar of each dried plant unknown material was prepared by adding 5 g of each one in 0/5 ml of D2O. They were then put in the Broker AVANCE AQS-300 MHz tubes separately and drawn their spectrum (Gadian, 1982).
Nematode population
The inoculum of M. incognita was initially isolated from infected tomato roots, identified and then multiplied in a glasshouse (16:8 h L:D, 25–28 °C, 75%–80% RH) in 14-cm-diameter pots containing sterile moist loamy soil (80% sand, 15% silt and 5% clay) for 2 months. Heavily infected tomato roots were washed free of adhering soil and used for preparing the inoculum. Nematode egg masses were carefully separated from the infected roots, put in Petri dishes in distilled water, and stored at 4 °C before use. Then the egg masses were incubated in a growth cabinet at 25 ± 2 °C to encourage emergence of second-stage juveniles (J2). The emerged J2 were collected daily from the Petri dishes for up to 5 days and used for the experiments (Southey, 1986).
Nematotoxicity test of essential oils
Essential oil was diluted in a 1.0% distilled water solution of Tween 80, as to prepare 8000, 4000, 2000, 1000, 500, 250, 125, 62.5 and 0 mg l−1 test solutions. Approximately 200 M. incognita juveniles were added to 5 ml of each test solution in 3 cm diameter Petri dishes, providing three replicates of each treatment. Distilled water and 1.0% Tween 80 water solution were used as controls. Nematodes were incubated at 25 °C for 24 h and then observed under a stereomicroscope. Immobile Juveniles, even after stimulation, were recorded as dead nematodes. Nematode mortality was confirmed by counting again after 24 h permanence in distilled water. The experiment was repeated twice.
Nematotoxicity test of polyphenols
The commercial products (Sigma) of gallic acid, catechin, chlorogenic, and coumarin were purchased from the markets, and their nematicidal effects were assayed according to Kimura et al. (1981). Two milliliters of aqueous solution of each test compound was taken and added in 2 L of distilled water to obtain a final product concentration of 1000 ppm. The mouth of the glass via1 was secured with tissue paper using a rubber band. The pH remained near 7 throughout the experiments. Control wells received 100 μl of tap water. Three replica of each compound were maintained along with control. The vials were then placed in dark at 27–28 °C for 48 h before being inverted in small beakers containing 5 ml of 1000 ppm streptocycline. A small piece of glass was placed under the rim of the via1 to allow nematodes to egress. Nematodes recovered in the beaker after 24 h were counted and percent mortality calculated by the formula B-A / B × 100, where B was the number of nematodes recovered after in the control and A was the number of nematodes recovered in the treatment. Experiments were repeated twice.
Nematotoxicity test of terpenoids
Concentrations of 400 and 200 ppm of different terpenoids (α-pinene, β-pinene, limonene, linalool, terpinen-4-ol and α-terpineol) were prepared in deionized distilled water as basic concentrations. To determine the effect of terpenoids on M. incognita, 1 ml of the each basic concentration (400 and 200 ppm) was put in a separate cavity-slide to which a 1 ml suspension of freshly hatched juveniles (about 200 juveniles/ml) was added. By adding 1 ml of each terpenoid suspension in 1 ml of nematode suspension, they were finally concentrations of 200 and 100 ppm. A glass slide containing 1 ml sterile distilled water served as the control. Each dilution was replicated 3 times at room temperature (25 ± 2 DC). After a 48-h incubation period, the numbers of dead juveniles were counted. Nematodes were considered dead if they did not move when probed with a fine needle (Aoudia et al., 2012).
Statistical analysis
Rate of mortality was calculated according to Abbott’s formula m = 100 × (1 − nt/nc) (Finney, 1978), in which: m = % nematode mortality; nt = number of alive nematodes after the treatment; nc = number of alive nematodes in the control. Data collected as percent of dead nematodes were arc sin transformed and subjected to a one-way analysis of variance (ANOVA) followed by means comparison using Duncan’s multiple range test at P = 0.05.
Results
Identification of components from aquatic extracts
Results of HPLC analysis for T. polium, T. polycephalum and A. wilhelmsii are presented in Table 1. Gallic acid was presented in all tested plants. Catechin and chlorogenic acid were found in T. polycephalum and A. wilhelmsii. Coumarin was found in A. wilhelmsii and T. polium. Other polyphenols such as quercetin and caffeic acid were not found in any of the tested plants.
Identification of components from essential oils
The chemical compositions of the all tested plants essential oils are shown in Table 2. Totally, 39, 45 and 41 major compounds were identified from T. polycephalum, A. wilhelmsii, and T. polium oils, respectively.
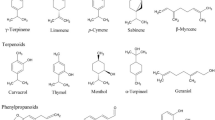
Among the components identified in each plant, the highest amount of chemical component was for trans-thujone (74.483%) in T. polycephalum, 1,8-cineole (15.240%) in A. wilhelmsii and (E)-caryophyllene, (24.410%) in T. polium oils. Ten components (α-pinene, sabinene, β-pinene, p-cymene, limonene, linalool, terpinen-4-ol, α-terpineol, (E)-caryophyllene and caryophyllene oxide) were common and presented in all tested plants within different ranges of the percentage.
Nematotoxicity test of essential oils
Based on the results presented in Tables 3 and 4, the essential oils of all the tested plants were nematicide at the concentrations of 8000, 4000, 2000 and 1000 ppm. No J2 mortality was seen for other tested concentrations. There was no significant difference in J2 mortality between all of the tested essential oils.
Concentrations of 4000 and 8000 ppm of essential oil of all studied plants caused death of J2. Nematode mortality increased significantly (P = 0.05) with increasing plant essential oil content. The least effect on J2 mortality was recorded on T. polium.
Nematotoxicity test of polyphenols
Nematicidal effects of tested polyphenols showed J2 mortality as 58.3, 48.9, 28.2 and 26.8% by catechin, coumarin, gallic acid and chlorogenic, respectively.
Nematotoxicity test of terpenoids
Results of nematotoxicity test of commercial terpenoids are shown in Table 4. The highest J2 mortalities (more than 80%) were observed in concentrations of 100 and 200 ppm of limonene, β-pinene and α-pinene. However, it was less than 30% of J2 mortality caused by other tested terpenoids (terpinen-4-ol, α-terpineol and linalool).
Nuclear magnetic resonance spectroscopy (HN.M.R)
The graphs obtained from HN.M.R showed the abundance of hydroxyl (-OH) and methoxy (OCH3) groups in the all tested plants (Figs. 1, 2, 3).
Discussion
Identification of components from aquatic extracts and their nematicidal effects
In the present study, in comparison with the other tested plants, maximum numbers of polyphenols (gallic acid, catechin, chlorogenic acid and coumarin) were found in A. wilhelmsii. It could conclude that highly nematicidal effects of A. wilhelmsii may be related to the presence of these polyphenols. The present study supported our previous results in which low nematicidal effects was reported by use of T. polium (Ardakani, 2011, 2012; Ardakani & Parhizkar, 2012).
In the present study, gallic acid was presented in all of the tested plants. Nematicidal effects of gallic acid, catechin, chlorogenic acid and coumarin have been reported by different authors (Korayem & Osman, 1992; Rajesh et al., 1985; Ahmed & Davide, 1988). Our results showed lower nematicidal activity of chlorogenic which is in agreement to results obtained by Rajesh et al. (1985).
Sultana et al. (2010) indicated good nematicidal effects of gallic acid against freshly hatched second stage juveniles of M. incognita.
Identification of components from essential oils and their nematicidal effects
Totally, 92 known and 7 unknown components were identified in the essential oils of all tested plants.
Results of nematotoxicity test of commercial terpenoids showed the highest J2 mortalities (more than 80%), in both concentrations (100 and 200 ppm) of limonene, β-pinene and α-pinene. However, it was less than 30% of J2 mortality caused by other tested terpenoids (terpinen-4-ol, α-terpineol and linalool). Among identified components of oils, nematicidal activity of α-pinene, camphene, β-pinene, myrcene, limonene, γ-terpinene, terpinen-4-ol, thymol and carvacrol against pine wood nematode (PWN) has been reported in a previous study (Choi et al., 2007). However, abietic acid, phytol and nerolacted have been reported as attractant, but β-pinene, citronellol, isoborneol, p-cymene and d-camphoric acid exhibited repellent activity (Ohriand Kaur, 2009). Echeverrigaray (2010) reported that borneol, carveol, citral, geraniol and alpha-terpineol showed the highest nematicidal activity. The results suggest that the selected monoterpenoids, and essential oils with high concentration of these compounds, are potential nematicides against M. incognita.
Nuclear magnetic resonance spectroscopy (HN.M.R)
Results of HN.M.R. test indicated that polyphenolic compounds contain large amounts of hydrogen element in their molecular structure. It has been reported that compounds with hydroxyl and carbonyl groups exhibited higher nematicidal activity than other terpenoids (Echeverrigaray, 2010).
Lei et al. (2010) suggested that the existence of a phenolic hydroxyl group in thymol and carvacrol might be responsible for their nematicidal activity. Thymol and carvacrol were very effective against PWN.
Choi et al. (2007) reported that nematicidal activity of compounds with hydroxyl (-OH) or methoxy (OCH3) groups was stronger than those with an acetyl group. This result indicates that the positions of the double bond of the propenyl group as well as position of the substituent in geometrical isomer are very important for nematicidal activity. Among the compounds with a hydroxyl group, nematicidal activities of allylic alcohol and phenolic alcohols, such as geraniol, thymol and carvacrol, were stronger than other alcoholic compounds (Choi et al., 2007).
Conclusions
Some components of A. wilhelmsii, T. polycephalum and T. polium presented in their essential oils and aquatic extracts appear to be useful as natural nematicides against M. incognita. Further study is necessary for the practical use of these components as novel nematicides.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DAD:
-
Diode array detector
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatography
- GC-MS:
-
Gas chromatography coupled with mass spectrometry
- HPLC:
-
High-performance liquid chromatography
- PWN:
-
Pine wood nematode
References
Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Publishing Corporation.
Ahmed, A. O., & Davide, R. V. (1988). Efficacy of biologically active agents as nontraditional nematicides for Meloidogynejavanica. Revue De Nématologie, 2(1), 93–98.
Akhyani, A., Modjtahedi, H., & Naderi, A. (1984). Species and physiological races of root-knot nematodes in Iran. Iranian Journal of Plant Pathology, 20, 57–70.
Aoudia, H., Ntalli, N., Aissani, N., Yahiaoui-Zaidi, R., & Caboni, P. (2012). Nematotoxic phenolic compounds from Melia azedarach against Meloidogyneincognita. Journal of Agricultural and Food Chemistry, 60, 11675–11680.
Ardakani, A. S. (2011). Nematicidal effect of Tanacetumpolycephalum Schultz Bip. (Compositea). In 9th International nematology symposium (p. 7). Institute of Biology of Karelian Research Center, Russian Academy of Sciences.
Ardakani, A. S. (2012). Effects of melia azedarach on meloidogyne incognita in vitro and in vivo conditions. Nematlogia Mediterranea, 40, 55–60.
Ardakani, A. S., & Parhizkar, S. (2012). Inhibitory effects of three medicinal plants, Teucriumpolium L., Artemisia sieberiBesser. and Achilleawilhelmsii C. Koch on Meloidogyneincognita (Kofoid and White) Chitwood (in vitro and under greenhouse conditions). International Journal of Medicinal and Aromatic Plants, 2(4), 596–602.
Baidoo, R., Mengistu, T., McSorley, R., Stamps, R., Brito, J., & Crow, W. T. (2017). Management of Root-knot Nematode (Meloidogyne incognita) on Pittosporum tobira under greenhouse, field, and on-farm conditions in Florida. Journal of Nematology, 49(2), 133–139.
British pharmacopoeia. (1988). British pharmacopoeia (vol. 2, pp. 137–138). London: HMSO.
Chitwood, D. J. (2002). Phytochemical based strategies for nematode control. Annual Review of Phytopathology, 40, 221–249.
Choi, I. H., Kim, J. H., Shin, S. C., & Park, I. K. (2007). Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchusxylophilus). Russian Journal of Nematology, 15, 35–40.
Echeverrigaray, S., Zacaria, J., & Beltrao, R. (2010). Nematicidal activity of monoterpenoids against the root-knot nematode Meloidogyne incognita. Phytopathology, 100(2), 199–203.
Erskine, C. (2007). Extraction process. US: 0128236.
Gadian, D. G. (1982). Nuclear magnetic resonance and its applications to living systems. Clarendon Press.
Korayem, A. M., & Osman, H. A. (1992). Nematicidal potential of the henna plant Lawsonia inermis against the root knot nematode Meloidogyne incognita. Anzeiger Fuer Schaedlingskunde, Pflanzenschutz, Umweltschutz, 65, 14–16.
Lei, J., Leser, M., & Enan, E. (2010). Nematicidalacitivity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditiselegans. Biochemical Pharmacology, 79, 1062–1071.
Li, W., Sun, Y. N., Yan, X. T., Yang, S. Y., Lee, S. J., Byun, H. J., Moon, C. S., Han, B. S., & Kim, Y. H. (2013). Isolation of nematicidal triterpenoid saponins from Pulsatillakoreana root and their activities against Meloidogyne incognita. Molecules, 18, 5306–5316.
Mandal, S., Naqvi, A. A., & Thakur, R. S. (1991). Analysis of some tropane alkaloids in plants by mixd column high performance liquid chromatography a. Journal of Chromatography, 547, 468–471.
Ohri, P., & Kaur, P. S. (2009). Effect of terpenoids on nematodes: A review. Journal of Environmental Research and Development, 4(1), 171–178.
Rajesh, M., Prabhsharan, S., & Krishan, L. B. (1985). Nematicidal activity of some phenolic compounds against Meloidogyne incognita. Revue De Nématologie, 8(2), 161–164.
Renco, M. (2013). Organic amendments of soil as useful tools of plant parasitic nematodes control Review article. Helminthologia, 50, 3–14.
Renco, M., Sasanelli, N., Papajova, I., & Maistrello, L. (2012). The nematicidal effect of chestnut tannin solutions on the potato cyst nematode Globoderarostochiensis (Woll.) Behrens. Helminthologia, 2, 108–114.
Southey, J. F. (1986). Laboratory methods for work with plant parasitic nematodes (6th edn. p. 202). Ministry of agriculture, Fisher and food.
Sultana, N., Akhter, M., & Khatoon, Z. (2010). Nematicidal natural products from the aerial parts of Rubusniveus. Natural Product Research, 24(5), 407–415.
Acknowledgements
The authors would like to thank Agriculture Research Education and Extension Organization (AREEO) for supporting us for this study.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
A.S.A. and S.A.H. designed the experiments. A.S.A. performed the experiments and wrote the paper. S.A.H. edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
This study does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ardakani, A.S., Hosseininejad, S.A. Identification of chemical components from essential oils and aqueous extracts of some medicinal plants and their nematicidal effects on Meloidogyne incognita. JoBAZ 83, 14 (2022). https://doi.org/10.1186/s41936-022-00279-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00279-6