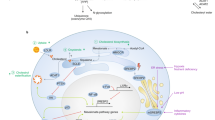
Abstract
Purpose of Review
Since obesity is a major risk factor for many different types of cancer, examining one of the most closely associated comorbidities, such as hypercholesterolemia, is crucial to understanding how obesity causes cancer. Hypercholesterolemia is usually associated with many cardiovascular complications such as hypertension, angina, and atherosclerosis. In addition, cholesterol may be a major factor in increasing cancer risk. Cancer patients who received statins, an anti-hypercholesteremic medicine, demonstrated improved prognosis possibly through its effect on tumor proliferation, apoptosis, and oxidative stress. Cholesterol could also aid in tumor progression through reprogramming tumor immunological architecture and mediators. This review focuses on the immunomodulatory role of cholesterol on cellular and molecular levels, which may explain its oncogenic driving activity. We look at how cholesterol modulates tumor immune cells like dendritic cells, T cells, Tregs, and neutrophils. Further, this study sheds light on the modification of the expression pattern of the common cancer-related immune mediators in the tumor immune microenvironment, such as programmed cell death 1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), transforming growth factor-beta (TGF-β), interleukin 12 (IL-12), IL-23, and forkhead box protein P3 (FOXP3).
Recent Findings
We highlight relevant literature demonstrating cholesterol's immunosuppressive role, leading to a worse cancer prognosis. This review invites further research regarding the pathobiological role of cholesterol in many obesity-related cancers such as uterine fibroids, post-menopausal breast, colorectal, endometrial, kidney, esophageal, pancreatic, liver, and gallbladder cancers.
Summary
This review suggests that targeting cholesterol synthesis may be a fruitful approach to cancer targeting, in addition to traditional chemotherapeutics.
Graphical abstract




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021;14(6):101043.
•• Cardoso D, Perucha E. Cholesterol metabolism: a new molecular switch to control inflammation. 2021;135(11):1389–408. This article focuses on how cholesterol metabolism affects different immune cells' function.
Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol Cell. 2021;81(18):3708–30.
Pinzon Grimaldos A, Bini S, Pacella I, Rossi A, Di Costanzo A, Minicocci I, D'Erasmo L, Arca M, Piconese S. The role of lipid metabolism in shaping the expansion and the function of regulatory T cells. Clin Exp Immunol. 2022;208:181–92.
Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019;9(2):219–27.
Matsushita Y, Nakagawa H, Koike K. Lipid metabolism in oncology: why it matters, how to research, and how to treat. Cancers (Basel). 2021;13.
Cho IJ, Shin JH, Jung MH, Kang CY, Hwang J, Kwon CH, Kim W, Kim DH, Lee CJ, Kang SH, Lee JH, Kim HL, Kim HM, Cho I, Lee HY, Chung WJ, Ihm SH, Kim KI, Cho EJ, Sohn IS, Park S, Shin J, Ryu SK, Kim JY, Kang SM, Cho MC, Pyun WB, Sung KC. Antihypertensive drugs and the risk of cancer: a nationwide cohort study. J Clin Med. 2021;10.
Wang S, Xie L, Zhuang J, Qian Y, Zhang G, Quan X, et al. Association between use of antihypertensive drugs and the risk of cancer: a population-based cohort study in Shanghai. BMC Cancer. 2023;23(1):425.
Copland E, Canoy D, Nazarzadeh M, Bidel Z, Ramakrishnan R, Woodward M, et al. Antihypertensive treatment and risk of cancer: an individual participant data meta-analysis. Lancet Oncol. 2021;22(4):558–70.
Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and cancer: a current overview of epidemiology, pathogenesis, outcomes, and management. Cancers (Basel). 2023;15.
Krupa-Kotara K, Dakowska D. Impact of obesity on risk of cancer. Cent Eur J Public Health. 2021;29(1):38–44.
Peltomaa AI, Talala K, Taari K, Tammela TLJ, Auvinen A, Murtola TJ. Inverse association between statin use and cancer mortality relates to cholesterol level. Cancers (Basel). 2022;14.
Narii N, Zha L, Komatsu M, Kitamura T, Sobue T, Ogawa T. Cholesterol and breast cancer risk: a cohort study using health insurance claims and health checkup databases. Breast Cancer Res Treat. 2023;199(2):315–22.
Hartmann P, Trufa DI, Hohenberger K, Tausche P, Trump S, Mittler S, et al. Contribution of serum lipids and cholesterol cellular metabolism in lung cancer development and progression. Sci Rep. 2023;13(1):5662.
da Costa RF, Freire VN, Bezerra EM, Cavada BS, Caetano EW, de Lima Filho JL, et al. Explaining statin inhibition effectiveness of HMG-CoA reductase by quantum biochemistry computations. Phys Chem Chem Phys. 2012;14(4):1389–98.
Abd El-Fattah EE. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20(1):347.
Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: new perspectives and emerging mechanisms. Dev Cell. 2021;56(10):1363–93.
• Afrin S, Ali M, El Sabeh M, Yang Q, Al-Hendy A, Borahay MA. Simvastatin inhibits stem cell proliferation in human leiomyoma via TGF-β3 and Wnt/β-Catenin pathways. 2022;26:1684–98. This article shows how statins (antihypercholesteremic) inhibit leiomyoma cell proliferation.
El Sabeh M, Vincent KL, Afrin S, Motamedi M, Saada J, Yang J, et al. Simvastatin-loaded liposome nanoparticles treatment for uterine leiomyoma in a patient-derived xenograft mouse model: a pilot study. J Obstet Gynaecol. 2022;42(6):2139–43.
Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A, et al. Novel effects of simvastatin on uterine fibroid tumors: in vitro and patient-derived xenograft mouse model study. Am J Obstet Gynecol. 2015;213(2):196.e1–.e8.
Afrin S, El Sabeh M, Islam MS, Miyashita-Ishiwata M, Malik M, Catherino WH, et al. Simvastatin modulates estrogen signaling in uterine leiomyoma via regulating receptor palmitoylation, trafficking and degradation. Pharmacol Res. 2021;172:105856.
El Sabeh M, Saha SK, Afrin S, Borahay MA. Simvastatin inhibits Wnt/β-Catenin pathway in uterine leiomyoma. Endocrinology. 2021;162.
Malik M, Catherino WH, Laknaur A, Ali M, Al-Hendy A, Segars J, et al. Synergistic effects of simvastatin and ulipristal acetate on uterine leiomyoma. Fertility and sterility. 2017;108(3, Supplement):e65.
Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GD, Al-Hendy A, et al. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J Biol Chem. 2014;289(51):35075–86.
Homma Y, Kondo Y, Kaneko M, Kitamura T, Nyou WT, Yanagisawa M, et al. Promotion of carcinogenesis and oxidative stress by dietary cholesterol in rat prostate. Carcinogenesis. 2004;25(6):1011–4.
Rauchbach E, Zeigerman H, Abu-Halaka D, Tirosh O. Cholesterol induces oxidative stress, mitochondrial damage and death in hepatic stellate cells to mitigate liver fibrosis in mice model of NASH. Antioxidants. 2022;11(3):536.
Saad EE, Michel R, Borahay MA. Immunosuppressive tumor microenvironment and uterine fibroids: role in collagen synthesis. Cytokine Growth Factor Rev. 2024;75:93–100.
Abd El-Fattah EE, Zakaria AY. Metformin modulate immune fitness in hepatocellular carcinoma: molecular and cellular approach. Int Immunopharmacol. 2022;109:108889.
Abdelhamid AM, Saber S, Youssef ME, Gaafar AGA, Eissa H, Abd-Eldayem MA, et al. Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: emerging approach for new application. Biomed Pharmacother. 2022;145: 112455.
Jeon H-S, Jen J. TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5(4):417–9.
Youssef ME, Abd El-Fattah EE, Abdelhamid AM, Eissa H, El-Ahwany E, Amin NA, Hetta HF, Mahmoud MH, Batiha GE, Gobba N, Ahmed Gaafar AG, Saber S. Interference with the AMPKα/mTOR/NLRP3 signaling and the IL-23/IL-17 axis effectively protects against the dextran sulfate sodium intoxication in rats: a new paradigm in empagliflozin and metformin reprofiling for the management of ulcerative colitis. Front Pharmacol. 2021;12:719984.
•• Zhou X, Johnston TP, Johansson D, Parini P, Funa K, Svensson J, et al. Hypercholesterolemia leads to elevated TGF-beta1 activity and T helper 3-dependent autoimmune responses in atherosclerotic mice. Atherosclerosis. 2009;204(2):381–7. This article show how cholesterol induce TGF-B1 expression (one of the most potent immunosuppressants).
Xiao A, Brenneman B, Floyd D, Comeau L, Spurio K, Olmez I, et al. Statins affect human glioblastoma and other cancers through TGF-β inhibition. Oncotarget. 2019;10(18).
Abd El-Fattah EE, Selim HM. Reprograming immune microenvironment modulates CD47 cancer stem cells in hepatocellular carcinoma. Int Immunopharmacol. 2022;113:109475.
Macek Jilkova Z, Aspord C, Decaens T. Predictive factors for response to PD-1/PD-L1 checkpoint inhibition in the field of hepatocellular carcinoma: current Status and Challenges. Cancers. 2019;11(10):1554.
Cholesterol modulates immune checkpoint expression in TILs. Cancer Discov. 2019;9:OF15-OF15.
Ni W, Mo H, Liu Y, Xu Y, Qin C, Zhou Y, et al. Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation. Mol Ther. 2021;29(10):2995–3010.
•• Lim W-J, Lee M, Oh Y, Fang X-Q, Lee S, Lim C-H, et al. Statins decrease programmed death-ligand 1 (PD-L1) by inhibiting AKT and β-catenin signaling. Cells. 2021;10(9):2488. This article focuses on how statins (anti-hypercholesterolemic medication) decrease PD-L1 and thus reverse its immunosuppressive effect.
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30(1):143-56.e5.
Yue T, Zheng X, Dou Y, Zheng X, Sun R, Tian Z, et al. Interleukin 12 shows a better curative effect on lung cancer than paclitaxel and cisplatin doublet chemotherapy. BMC Cancer. 2016;16(1):665.
Lo CH, Chang CM, Tang SW, Pan WY, Fang CC, Chen Y, et al. Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma. J Gene Med. 2010;12(5):423–34.
Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84.
Coward WR, Marei A, Yang A, Vasa-Nicotera MM, Chow SC. Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of Caspase-1 and IL-18 secretion in Monocytes1. J Immunol. 2006;176(9):5284–92.
Jia H, Qi H, Gong Z, Yang S, Ren J, Liu Y, et al. The expression of FOXP3 and its role in human cancers. Biochim Biophys Acta. 2019;1871(1):170–8.
El-Ashmawy NE, Salem ML, Abd El-Fattah EE, Khedr EG. Targeting CD166+ lung cancer stem cells: molecular study using murine dendritic cell vaccine. Toxicol Appl Pharmacol. 2021;429:115699.
Abd El-Fattah EE, Abdelhamid AM. Benzo[a]pyrene immunogenetics and immune archetype reprogramming of lung. Toxicology. 2021;463:152994.
Zhang H, Chen Y, Liao W, Wang L, Xie X, Fei R, et al. FOXP3 expression in FOXP3+ tumor cells promotes hepatocellular cells metastasis. Transl Cancer Res. 2020;9(10):5868–81.
Tu J-F, Ding Y-H, Ying X-H, Wu F-Z, Zhou X-M, Zhang D-K, et al. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6(1):35056.
Li F, Guo Z, Lizée G, Yu H, Wang H, Si T. Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin Chem Lab Med. 2014;52(9):1357–65.
Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia induces differentiation of regulatory T cells in the liver. Circ Res. 2017;120(11):1740–53.
Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep. 2017;7(1):15655.
Wen X, Zhao W-H, Chen L-Z, Qu W, Liu H-X, Yan H-Y, et al. Attenuated cholesterol metabolism pathway suppresses regulatory T cell development in prenatal nicotine exposed female mice. Toxicology. 2019;428:152309.
Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569(7756):428–32.
Salem ML, El-Ashmawy NE, Abd El-Fattah EE, Khedr EG. Immunosuppressive role of Benzo[a]pyrene in induction of lung cancer in mice. Chem Biol Interact. 2021;333:109330.
Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers. 2021;13(6):1440.
Okoye I, Namdar A, Xu L, Crux N, Elahi S. Atorvastatin downregulates co-inhibitory receptor expression by targeting Ras-activated mTOR signalling. Oncotarget. 2017;8:98215–32.
Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013;499(7459):485–90.
Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. 2018;10(7):a028530.
Nie W, Yu T, Sang Y, Gao X. Tumor-promoting effect of IL-23 in mammary cancer mediated by infiltration of M2 macrophages and neutrophils in tumor microenvironment. Biochem Biophys Res Commun. 2017;482(4):1400–6.
Leite BF, Morimoto MA, Gomes C, Klemz BNdC, Genaro PDS, Damasceno NRT, et al. Higher bodily adiposity, fat intake, and cholesterol serum levels are associated with higher disease activity in psoriatic arthritis patients: is there a link among fat and skin and joint involvement? Lipids Health Dis. 2020;19(1):21.
Przepiera-Będzak H, Fischer K, Brzosko M. Serum interleukin-23 protects, whereas methotrexate treatment stimulates selected components of the metabolic syndrome in patients with SAPHO syndrome. Arch Med Sci. 2021;17(1):120–6.
Ma X, Liu S, Li T, Yuan H. Intensive statin treatment ameliorate the Th17/Treg functional imbalance in patients with non-ST elevation acute coronary syndrome underwent percutaneous coronary intervention. Clin Cardiol. 2020;43(4):379–85.
Forero-Peña DA, Gutierrez FRS. Statins as modulators of regulatory T-cell biology. Mediators Inflamm. 2013;2013:167086.
Manti S, Leonardi S, Panasiti I, Arrigo T, Salpietro C, Cuppari C. Serum IL-10, IL-17 and IL-23 levels as “bioumoral bridges” between dyslipidemia and atopy. Cytokine. 2017;99:43–9.
Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–67.
Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173-.
Akinyemi AJ, Oboh G, Ademiluyi AO, Boligon AA, Athayde ML. Effect of two ginger varieties on arginase activity in hypercholesterolemic rats. J Acupunct Meridian Stud. 2016;9(2):80–7.
Grzywa TM, Sosnowska A, Matryba P, Rydzynska Z, Jasinski M, Nowis D, et al. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020;11:938.
Guasti L, Marino F, Cosentino M, Maio RC, Rasini E, Ferrari M, et al. Prolonged statin-associated reduction in neutrophil reactive oxygen species and angiotensin II type 1 receptor expression: 1-year follow-up. Eur Heart J. 2008;29(9):1118–26.
Abdelhamid AM, Youssef ME, Abd El-Fattah EE, Gobba NA, Gaafar AGA, Girgis S, et al. Blunting p38 MAPKα and ERK1/2 activities by empagliflozin enhances the antifibrotic effect of metformin and augments its AMPK-induced NF-κB inactivation in mice intoxicated with carbon tetrachloride. Life Sci. 2021;286:120070.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–93.
Picarda E, Ren X, Zang X. Tumor cholesterol up T cells down. Cell Metabol. 2019;30(1):12–3.
Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm. 2012;27(8):495–503.
Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, et al. (2014) Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immun (Baltimore, Md: 1950). 2014;192(6):2920–31.
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16(8):880–6.
Maher BM, Dhonnchu TN, Burke JP, Soo A, Wood AE, Watson RWG. Statins alter neutrophil migration by modulating cellular Rho activity—a potential mechanism for statins-mediated pleotropic effects? J Leukoc Biol. 2008;85(1):186–93.
Lenglet S, Quercioli A, Fabre M, Galan K, Pelli G, Nencioni A, et al. Statin treatment is associated with reduction in serum levels of receptor activator of NF-κB ligand and neutrophil activation in patients with severe carotid stenosis. Mediators Inflamm. 2014;2014:720987.
• Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, Dhungana Y, Chapman NM, Long L, Saravia J, Vogel P, Chi H. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306–11. This article indicates how lipids induce Tregs differentiation in tumors which aids in its progression.
Funding
This work was supported by NIH grant 1R01HD094380.
Author information
Authors and Affiliations
Contributions
Eslam E. Saad: Conceptualization, Methodology, Investigation, Writing- Original draft preparation. Rachel Michel: English editing. Borahay M: Supervision, Reviewing, and Editing. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saad, E.E., Michel, R. & Borahay, M.A. Cholesterol and Immune Microenvironment: Path Towards Tumorigenesis. Curr Nutr Rep (2024). https://doi.org/10.1007/s13668-024-00542-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s13668-024-00542-y