Abstract
Purpose of Review
The current systematic review and meta-analysis was done to evaluate the effects of selenium and probiotic co-supplementation on lipid profile and glycemia indices of the adult population using randomized controlled clinical trials (RCTs).
Recent Findings
Five studies involving 282 participants with a sample size ranging from 38 to 79 were eligible to be enrolled in the current study. Co-supplementation with probiotic and selenium reduced fasting plasma glucose (WMD = −4.02 mg/dL; 95% CI: −5.87 to −2.18; P < 0.001), insulin (WMD = −2.50 mIU/mL; 95% CI: −3.11 to −1.90; P < 0.001), homeostatic model assessment for insulin resistance (WMD = −0.59; 95% CI: −0.74 to −0.43; P < 0.001), quantitative insulin sensitivity check index (WMD = 0.01; 95% CI: 0.01 to 0.02; P < 0.001), total cholesterol (WMD = −12.75 mg/dL; 95% CI: −19.44 to −6.07; P < 0.001), low-density lipoprotein cholesterol (WMD = −7.09 mg/dL; 95% CI: −13.45 to −0.73; P = 0.029), and triglyceride (WMD = −14.38 mg/dL; 95% CI: −23.13 to −5.62; P = 0.001).
Summary
The findings of the current systematic review and meta-analysis suggested that co-supplementation with probiotics and selenium may benefit adults in terms of glycemia indices and lipid profile. However, due to the small number of included studies, further trials are needed to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With nearly one in three deaths, cardiovascular disease (CVD) is most common cause of death in the USA [1]. Most of the CVD risk factors are either modifiable or preventable including cigarette smoking, overweight/obesity, diabetes, dyslipidemia, and high blood pressure [2]. Accordingly, 14% of US adults smoke cigarettes [2], 72% are overweight/obese [3], 14% have diabetes [4], 29% have hypercholesterolemia [3], and 32% have hypertension [5]. Moreover, the American Heart Association predicted an increase in CVD prevalence accompanied by health care costs by 2030 [6]. Therefore, finding effective approaches to prevent or modify CVD risk factors should be prioritized.
It has been reported that gut microbiota, microbes living in the human intestinal tract, may affect CVD pathogenesis and related risk factors [7]. Early studies on the gut microbiome proposed that alteration of the composition of the fecal microbial community is linked with insulin resistance and obesity [8, 9]. Moreover, sequencing studies also suggested an association between gut microbiota and atherosclerosis [10]. Therefore, strategies to improve the composition of gut microbiota are suggested as a complementary approach for preventing CVD through modifying related risk factors.
Probiotics are defined as living microorganisms that exert beneficial health effects when consumed in adequate amounts. It has been suggested that joint selenium and probiotic supplementation are much more effective than single selenium or probiotic supplementation in terms of metabolic profile [11•]. Moreover, co-supplementation of selenium and probiotics in animal studies also suggested a synergistic effect on metabolic profile compared to the selenium or probiotics alone [12, 13]. To date, various clinical trials have been conducted to evaluate the effects of probiotic and selenium co-supplementation on CVD risk factors; however, their findings are contradictory [11•, 14, 15••, 16, 17]. Moreover, their sample size is small which precludes clinicians to reach a firm conclusion in this regard. Therefore, the current systematic review and meta-analysis was done to evaluate the effects of selenium and probiotic co-supplementation on lipid profile and glycemia indices of the adult population using randomized controlled clinical trials (RCTs).
Methods
Search Strategy and Data Source
Selected electronic databases including ISI Web of Science, PubMed, and Scopus were searched systematically from the earliest available date to February 2022 to find relevant studies. Two independent reviewers conducted a database search to identify studies that investigated the effects of selenium and probiotic co-supplementation on lipid profile and glycemia indices using the following keywords: probiotic OR probiotics OR Lactobacillus OR Bifidobacterium OR Streptococcus OR Saccharomyces OR Enterococcus AND selenium (Table 1). The reference list of eligible studies was also screened to minimize the chance of missing relevant studies. Since the studied outcomes in the present study may have been considered a secondary outcome in the primary studies and therefore not mentioned in the abstract, we conducted a systematic search without considering these outcomes (i.e., lipid profile and glycemia indices) and then examined them in the title/abstract and full-text phases. No filtering was made upon the database searching in terms of publication time, study design, and language. The present study was done on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statements [18].
Eligibility Criteria and Study Selection
The PICOS (Population, Intervention, Comparison, Outcome, Study design) framework was used during study selection as follows: P (> 18 years individuals), I (probiotic + selenium supplement), C (placebo), O (lipid profile [triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC)] and glycemia indices [homeostatic model assessment for insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), fasting insulin, and fasting plasma glucose (FPG)]), S (RCTs). All the search results were exported to the EndNote X7 software (Thomson Corporation, Stamford, USA) to be screened by two independent investigators for eligible studies. Inclusion criteria were as follow: original peer-reviewed full-text RCTs recruited > 18 years old subjects with either parallel or cross-over design that implemented co-supplementation of selenium and probiotic and assessed at least one of the outcomes of interest. Exclusion criteria were as follows: non-human studies; recruited < 18 years old individuals; or non-original full-length studies (i.e., review articles, poster abstract, commentary, editorials, and case reports).
Data Extraction
Eligible articles were screened by two independent reviewers for extraction of the data of interest using pre-defined Excel sheets. The extracted data were as follows: first author’s name, year of publication, study location, characteristics of the study population (e.g., sample size, sex, and body mass index [BMI]), study duration, RCT design, the dose of selenium and probiotics, number of probiotic bacteria, and mean and standard deviation (SD) of change in each outcome in the intervention and the control group.
Risk of Bias Assessment
The risk of bias of the included studies was examined using the Cochrane Collaboration’s tool [19]. It consists of seven domains including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting, and other biases. Each domain scored as low risk, high risk, or unclear risk, and the overall risk of bias for each study was stated as good, fair, and poor quality.
Statistical Analysis
Statistical analysis was done using STATA software (version 11.0; Stata Corporation). For each outcome, data was collected as mean ± SD in a similar unit to estimate the pooled effect size. Weighted mean differences (WMDs) with corresponding 95% confidence intervals (CIs) were calculated for each studied outcome using inverse-variance fixed-effect models. Between effect sizes heterogeneity was estimated using the I-squared (I2) index and values equal to 25, 50, or 75% were interpreted as low, moderate, or high heterogeneity, respectively. Visual inspection of funnel plots in combination with Begg’s and Egger’s tests was implemented to assess publication bias. The influence of each study on overall meta-analysis findings was examined via sensitivity analysis. Each time, one study was removed and a meta-analysis was done with the remaining articles to evaluate the robustness of the findings. P values < 0.05 considered statistically significant.
Results
Search Findings
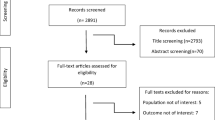
A primary search of the selected electronic databases yielded a total of 2321 articles. After the omission of duplicates, 1706 studies remained to be screened on the basis of title/abstract by two independent investigators. Nineteen articles remained after this phase and subsequently were assessed on the basis of full-text and finally, five documents were selected to be eligible for the current systematic review and meta-analysis. The PRISMA flow diagram of the selection process is shown in Fig. 1.
General Characteristics of the Included Studies
Five studies involving 282 participants with a sample size ranging from 38 to 79 were eligible to be enrolled in the current systematic review and meta-analysis regarding the beneficial role of probiotics and selenium co-supplementation on parameters of glycemia and lipid profile. Participants’ mean age and BMI at baseline ranged from 27.2 to 77.8 years and 21.13 to 30.65 kg/m2. The included studies were conducted between 2005 and 2021 in Iran [11•, 15••, 16, 17] and Slovakia [14]. All of the included studies were double-blind with an intervention duration ranging from 12 to 60 weeks. Enrolled studies administered selenium with a dose ranging from 50 to 200 µg/day in combination with a probiotic supplement with a number of strains ranging from 1 to 4. The general characteristics of the included studies are presented in Table 2.
Risk of Bias of the Included Studies
The findings of the risk of bias of the included studies are shown in Table 2. As can be seen, all of the enrolled studies were low risk in terms of blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. For domains of random sequence generation and allocation concealment, all of the studies were low risk except for the work of Hlivak et al. which was unclear. Overall, four studies [11•, 15••, 16, 17] ranked as high quality and one [14] as fair quality.
Findings from Meta-analysis
The Effect of Probiotic and Selenium Co-supplementation on FPG
Co-supplementation of probiotic and selenium was examined using four datasets [11•, 15••, 16, 17] including 244 subjects. Overall meta-analysis revealed a beneficial role for probiotic and selenium in reducing FPG (WMD = −4.02 mg/dL; 95% CI: −5.87 to −2.18; P < 0.001) with evidence of a significant heterogeneity (I2 = 69.4%, P = 0.020). The overall finding was not sensitive to any individual study. No evidence of publication bias was observed (P = 0.497, Begg’s test, and P = 0.539, Egger’s test) (Fig. 2).
The Effect of Probiotic and Selenium Co-supplementation on Insulin
The analysis of four studies [11•, 15••, 16, 17] regarding the effect of probiotic and selenium co-supplementation in serum levels of insulin proposed a significant reduction (WMD = −2.50 mIU/mL; 95% CI: −3.11 to −1.90; P < 0.001). There was evidence of significant heterogeneity among the included studies (I2 = 66.3%, P = 0.031). The overall result was not changed following sensitivity analysis. No evidence of publication bias was observed (P = 0.174, Begg’s test, and P = 0.486, Egger’s test) (Fig. 3).
The Effect of Probiotic and Selenium Co-supplementation on HOMA-IR
The meta-analysis of four documents [11•, 15••, 16, 17] for the mean differences in HOMA-IR suggested a significant reduction following probiotic and selenium co-supplementation (WMD = −0.59; 95% CI: −0.74 to −0.43; P < 0.001) with evidence of moderate heterogeneity (I2 = 39.5%, P = 0.175). The overall result was not sensitive to any individual study. No evidence of publication bias was observed (P = 0.174, Begg’s test, and P = 0.234, Egger’s test) (Fig. 4).
The Effect of Probiotic and Selenium Co-supplementation on QUICKI
The effect of probiotic and selenium co-supplementation on QUICKI was assessed by four RCTs including 244 individuals [11•, 15••, 16, 17]. Overall meta-analysis revealed that probiotic and selenium co-supplementation significantly improve QUICKI (WMD = 0.01; 95% CI: 0.01 to 0.02; P < 0.001) with evidence of a considerable heterogeneity (I2 = 16.8%, P = 0.307). The overall finding was not sensitive to any individual study. No evidence of publication bias was observed (P = 0.174, Begg’s test, and P = 0.497, Egger’s test) (Fig. 5).
The Effect of Probiotic and Selenium Co-supplementation on TC
Five datasets [11•, 14, 15••, 16, 17] with a total sample size of 282 patients evaluated the effect of probiotic and selenium co-supplementation on serum levels of TC. The overall findings suggested a significant reduction in TC following administration of probiotic and selenium (WMD = −12.75 mg/dL; 95% CI: −19.44 to −6.07; P < 0.001) with no evidence of heterogeneity (I2 = 0.0%, P = 0.520). Sensitivity analysis revealed that no study can influence overall meta-analysis findings. No evidence of publication bias was observed (P = 0.142, Begg’s test, and P = 0.177, Egger’s test) (Fig. 6).
The Effect of Probiotic and Selenium Co-supplementation on LDL-C
The hypothesis of the beneficial role of probiotic and selenium co-supplementation on serum levels of LDL-C was evaluated in five studies [11•, 14, 15••, 16, 17] with a total sample size of 282 individuals. Overall meta-analysis revealed a significant reduction in LDL-C (WMD = −7.09 mg/dL; 95% CI: −13.45 to −0.73; P = 0.029) with no evidence of heterogeneity (I2 = 0.0%, P = 0.789). The overall finding was sensitive to Raygan et al. (WMD = −6.55 mg/dL; 95% CI: −13.53 to 0.43) and Shabani et al. (WMD = −6.99 mg/dL; 95% CI: −14.65 to 0.66) studies. No evidence of publication bias was observed (P = 0.327, Begg’s test, and P = 0.243, Egger’s test) (Fig. 7).
The Effect of Probiotic and Selenium Co-supplementation on HDL-C
Five studies [11•, 14, 15••, 16, 17] consisting of 282 participants reported on the effect of probiotic and selenium co-supplementation on HDL-C. Probiotic and selenium consumption could not improve HDL-C (WMD = 0.55 mg/dL; 95% CI: −0.98 to 2.08; P = 0.481) with no evidence of significant heterogeneity (I2 = 16.8%, P = 0.307). The overall result was not changed following sensitivity analysis. No evidence of publication bias was observed (P = 0.142, Begg’s test, and P = 0.772, Egger’s test) (Fig. 8).
The Effect of Probiotic and Selenium Co-supplementation on TG
The meta-analysis of five datasets [11•, 14, 15••, 16, 17] proposed a significant decrease in serum levels of TG following probiotic and selenium co-supplementation (WMD = −14.38 mg/dL; 95% CI: −23.13 to −5.62; P = 0.001). There was no evidence of significant heterogeneity among the included studies (I2 = 13.2%, P = 0.330). The omission of each study did not change the overall meta-analysis finding. No evidence of publication bias was observed (P = 0.624, Begg’s test, and P = 0.892, Egger’s test) (Fig. 9).
Discussion
The current systematic review and meta-analysis was conducted to reach a firm conclusion on the role of selenium and probiotic co-supplementation on lipid profile and glycemia indices of the adult population. Our findings suggested that probiotic and selenium co-supplementation could significantly improve all the glycemia indices including FPG, insulin, HOMA-IR, QUICKI, and lipid profile parameters including TC, LDL-C, and TG. These findings may imply that co-supplementation with selenium and probiotic can be used as a complementary approach in modifying CVD risk factors; however, between-study heterogeneity should be taken into account during the interpretation of results.
The hypothesis of selenium and probiotic co-supplementation was suggested by previous animal studies that mentioned the superiority of combined probiotic and selenium compared to each of the probiotics or selenium alone [12, 13]. In agreement with these findings, Tamtaji et al. also proposed that selenium and probiotic co-supplementation show greater efficacy in terms of metabolic markers compared to probiotics alone [11•]. Synergistic effects of selenium supplementation on lipid profile and glycemia indices may be explained through its inhibitory properties on the expression of P-selectin and cyclooxygenase-2 and upregulation of some fatty acid enzymes including medium-chain Acyl-CoA dehydrogenase and very-long-chain dehydrogenase [20]. Moreover, it was observed that selenization of lactic acid bacteria strains led to binding selenium to their cells, and subsequently increase their antioxidant capacity compared to the parental strains. Integration of accumulated selenium in bacterial cells with antioxidant enzymes seemed to increase the antioxidant properties of these strains [21]. Moreover, enrichment of probiotic bacteria with selenium can affect their cell surface hydrophobicity. This factor has an important role in the adhesion of bacteria to epithelial cells, resulting in the colonization of intestinal epithelium by beneficial bacteria. The assessment of the hydrophobicity of the selenized probiotic bacteria showed higher hydrophobicity values in comparison to the parental strains [21].
In the present work, probiotic and selenium co-supplementation significantly reduced serum levels of FPG (−4.02 mg/dL), insulin (= −2.50 mIU/mL), HOMA-IR (−0.59), and increased QUICKI (0.01). It was reported among Asia Pacific region that each 18 mg/dL lower FPG was associated with a 23% lower risk of ischemic heart disease and a 21% lower risk of total stroke [22]. A meta-analysis proposed that an increase of 7.19 mIU/mL fasting insulin led to a 18% higher risk of CVD [23]. A more recent meta-analysis also revealed that each 6.26 mIU/mL, 2.23, and 18.9 mg/dL increase in insulin, HOMA-IR, and FPG correspondence to relative risk of 1.04, 1.46, and 1.21 for CVD, respectively [24]. Previous documents are in line with our findings regarding the beneficial role of probiotic supplementation on diabetes health outcomes [25, 26]. Moreover, another meta-analysis suggested that pro-/synbiotic supplementation for a duration ≥ 12 weeks can improve glycemia indices. Other meta-analyses also proposed a beneficial effect of probiotic consumption on glycemia indices [27,28,29,30]; however, uncertainties still remain regarding the best form (supplement/food) and species of probiotics [31]. Reduction in inflammatory signals and increase in T-cell receptors and hepatic natural killer receptors are among the other suggested mechanisms regarding the role of probiotics on insulin resistance [32, 33]. Also, augmented gut permeability can lead to translocation of bacterial products (inflammatory lipopolysaccharides (LPS)), leading to insulin resistance [34]. Probiotic consumption can diminish gut permeability leading to improve insulin sensitivity, as evidenced by reduced zonulin and calprotectin levels following probiotic supplementation [35, 36]. Probiotics can improve the gut microbiome that plays an important role in metabolizing bile acid subsequently leading to a reduction in insulin resistance and inflammation [37].
Results of our meta-analysis revealed a significant reduction in TC (−12.75 mg/dL), LDL-C (−7.09 mg/dL), and TG (−14.38 mg/dL) but not in HDL-C (0.55 mg/dL) following selenium and probiotic co-supplementation. A meta-analysis reported that a 1 mmol/l (38.67 mg/dL) reduction in LDL-C level linked with a 19% reduction in CVD-related mortality and a 12% reduction in all-cause mortality [38]. Our findings were in line with previous work [39,40,41] regarding the beneficial role of probiotics on lipid parameters’ improvement. Probiotics can reduce the reabsorption of bile cholesterol and diminish dietary cholesterol absorption by incorporating cholesterol in their cellular membrane [42]. Also, some species of probiotics can produce hydrolases leading to lower cholesterol absorption via higher bile salt excretion [43, 44]. Production of short-chain fatty acids (SCFA) (i.e., butyrate and propionate) increased following probiotic consumption which led to the inhibition of hydroxymethylglutaryl CoA reductase (HMG-CoA reductase), reducing cholesterol synthesis [45]. Moreover, it was suggested that butyrate can improve insulin sensitivity and reduce body fat that subsequently preventing metabolic syndrome, diabetes, and obesity [46].
The strength of our work is that this systematic review and meta-analysis is the first study that comprehensively pooled the results of available literature regarding the role of probiotics and selenium co-supplementation on lipid profile and glycemia indices. Our findings have research and clinical implications and can be used by clinicians and health practitioners. However, some limitations should be considered while interpreting the results. The small number of the included studies (n = 5) may diminish the precision of pooled effect estimates. Substantial heterogeneity should also be considered that can reduce the generalizability of our findings. Participants’ characteristics (i.e., sex, age, ethnicity, genetic profile, and health status), sample size, study duration, dose, and strain of probiotics are among the possible sources of heterogeneity. However, due to the small number of the included studies, we were unable to run a sub-group analysis to find the sources of heterogeneity.
Conclusion
The findings of the current systematic review and meta-analysis suggested that co-supplementation with probiotics and selenium may benefit adults in terms of FPG, insulin, HOMA-IR, QUICKI, TC, LDL-C, and TG. However, due to the small number of included studies, further trials are needed to further investigate this issue. Moreover, further studies are needed with better methodology to compare the synergistic effects of selenium and probiotic co-supplementation to supplementation with probiotic or selenium alone.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
24 February 2023
Missing Open Access funding information has been added in the Funding Note.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135(10):e146-e603. https://doi.org/10.1161/CIR.0000000000000485.
Fryar CD, Fakhouri TH, Carroll MD, Frenk SM, Ogden CL. The association of nativity/length of residence and cardiovascular disease risk factors in the United States. Prev Med 2020;130:105893. https://doi.org/10.1016/j.ypmed.2019.105893.
National Center for Health Statistics (US). Health, United States, 2017: with special feature on mortality [Internet]. Hyattsville (MD): National Center for Health Statistics (US); 2018.
Mendola ND, Chen TC, Gu Q, Eberhardt MS, Saydah S. Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United States, 2013–2016. NCHS Data Brief. 2018;319:1–8.
Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017;289:1–8.
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. https://doi.org/10.1161/CIR.0b013e31820a55f5.
Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–70. https://doi.org/10.1161/CIRCRESAHA.120.316242.
Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, Lin J, Zhang Z. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. 2019;170(1):43–52. https://doi.org/10.1016/j.resmic.2018.09.002.
Haro C, Montes-Borrego M, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, Quintana-Navarro GM, Tinahones FJ, Landa BB, López-Miranda J. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J Clin Endocrinol Metab. 2016;101(1):233–42. https://doi.org/10.1210/jc.2015-3351.
Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA 2011;108 Suppl 1(Suppl 1):4592–8. https://doi.org/10.1073/pnas.1011383107.
• Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, Tajabadi-Ebrahimi M, Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr 2019;38(6):2569–75. https://doi.org/10.1016/j.clnu.2018.11.034. This study aimed to determine the effects of probiotic and selenium co-supplementation on cognitive function and metabolic status among patients with AD and found that probiotic and selenium co-supplementation for 12 weeks to patients with AD improved cognitive function and some metabolic profiles.
Nido SA, Shituleni SA, Mengistu BM, Liu Y, Khan AZ, Gan F, Kumbhar S, Huang K. Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res. 2016;171(2):399–409. https://doi.org/10.1007/s12011-015-0552-8.
Gan F, Chen X, Liao SF, Lv C, Ren F, Ye G, Pan C, Huang D, Shi J, Shi X, Zhou H, Huang K. Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agric Food Chem. 2014;62(20):4502–8. https://doi.org/10.1021/jf501065d.
Hlivak P, Odraska J, Ferencik M, Ebringer L, Jahnova E, Mikes Z. One-year application of probiotic strain Enterococcus faecium M-74 decreases serum cholesterol levels. Bratisl Lek Listy. 2005;106(2):67–72.
•• Jamilian H, Ghaderi A. The effects of probiotic and selenium co-supplementation on clinical and metabolic scales in chronic schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 2021;199(12):4430–8. https://doi.org/10.1007/s12011-020-02572-3. This study evaluated the effects of probiotic and selenium co-supplementation on clinical and metabolic symptoms in patients with chronic schizophrenia and found that probiotic and selenium co-supplementation for 12 weeks to patients with chronic schizophrenia had beneficial effects on the general PANSS score and some metabolic profiles.
Raygan F, Ostadmohammadi V, Asemi Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(4):1594–8. https://doi.org/10.1016/j.clnu.2018.07.017.
Shabani A, Noshadian M, Jamilian M, Chamani M, Mohammadi S, Asemi Z. The effects of a novel combination of selenium and probiotic on weight loss, glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome. J Funct Foods. 2018;46:329–34. https://doi.org/10.1016/j.jff.2018.04.071.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Kim JE, Choi SI, Lee HR, Hwang IS, Lee YJ, An BS, Lee SH, Kim HJ, Kang BC, Hwang DY. Selenium significantly inhibits adipocyte hypertrophy and abdominal fat accumulation in OLETF rats via induction of fatty acid β-oxidation. Biol Trace Elem Res. 2012;150(1):360–70. https://doi.org/10.1007/s12011-012-9519-1.
Krausova G, Kana A, Hyrslova I, Mrvikova I, Kavkova M. Development of selenized lactic acid bacteria and their selenium bioaccummulation capacity. Fermentation. 2020;6(3):91. https://doi.org/10.3390/fermentation6030091.
Collaboration APCS. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27(12):2836–42. https://doi.org/10.2337/diacare.27.12.2836.
Ruige JB, Assendelft WJJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease. Circulation. 1998;97(10):996–1001. https://doi.org/10.1161/01.CIR.97.10.996.
Gast KB, Tjeerdema N, Stijnen T, Smit JWA, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLOS ONE 2012;7(12):e52036. https://doi.org/10.1371/journal.pone.0052036
Shah NJ, Swami OC. Role of probiotics in diabetes: a review of their rationale and efficacy. Diabetes. 2017;5:104–10.
Razmpoosh E, Javadi M, Ejtahed HS, Mirmiran P. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2016;32(2):143–68. https://doi.org/10.1002/dmrr.2665.
Ruan Y, Sun J, He J, Chen F, Chen R, Chen H. Effect of probiotics on glycemic control: a systematic review and meta-analysis of randomized, controlled trials. PloS one 2015;10(7):e0132121. https://doi.org/10.1371/journal.pone.0132121.
Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T. Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Adv Nutr 2021;12(3):722–34. https://doi.org/10.1093/advances/nmaa133.
Jafarabadi MA, Dehghani A, Khalili L, Barzegar A, Mesrizad M, Hassanalilou T. A meta-analysis of randomized controlled trials of the effect of probiotic food or supplement on glycemic response and body mass index in patients with type 2 diabetes, updating the evidence. Curr Diabetes Rev. 2021;17(3):356–64. https://doi.org/10.2174/1573399816666200812151029.
Tabrizi R, Ostadmohammadi V, Akbari M, Lankarani KB, Vakili S, Peymani P, Karamali M, Kolahdooz F, Asemi Z. The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. 2022;14(1):1–14. https://doi.org/10.1007/s12602-019-09559-0.
Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T. The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: a meta-analysis of nine randomized controlled trials. Nutrients. 2019;11(3):671. https://doi.org/10.3390/nu11030671.
Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J Nutr. 2007;137(3):791S-S793. https://doi.org/10.1093/jn/137.3.791S.
Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48(6):945–51. https://doi.org/10.1016/j.jhep.2008.02.015.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. https://doi.org/10.2337/db07-1403. (Epub 2008 Feb 27).
Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915(1):214–22. https://doi.org/10.1111/j.1749-6632.2000.tb05244.x.
Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(24):4435–40. https://doi.org/10.1242/jcs.113.24.4435.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91. https://doi.org/10.1152/physrev.00010.2008.
Unit ES. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. https://doi.org/10.1016/S0140-6736(05)67394-1.
Zarezadeh M, Musazadeh V, Faghfouri AH, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr 2021:1–14. https://doi.org/10.1080/10408398.2021.2004578.
Hadi A, Arab A, Khalesi S, Rafie N, Kafeshani M, Kazemi M. Effects of probiotic supplementation on anthropometric and metabolic characteristics in adults with metabolic syndrome: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr. 2021;40(7):4662–73. https://doi.org/10.1016/j.clnu.2021.05.027.
Hadi A, Ghaedi E, Khalesi S, Pourmasoumi M, Arab A. Effects of synbiotic consumption on lipid profile: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. 2020;59(7):2857–74. https://doi.org/10.1007/s00394-020-02248-7.
Kimoto H, Ohmomo S, Okamoto T. Cholesterol removal from media by lactococci. J Dairy Sci. 2002;85(12):3182–8. https://doi.org/10.3168/jds.S0022-0302(02)74406-8.
Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72(3):1729–38. https://doi.org/10.1128/AEM.72.3.1729-1738.2006.
Patel AK, Singhania RR, Pandey A, Chincholkar SB. Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol. 2010;162(1):166–80. https://doi.org/10.1007/s12010-009-8738-1.
Boschetti E. Biochemical and molecular aspects of the nutritional regulation of cholesterol biosynthesis. 2011.
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Metabolism and metabolic disorders and the microbiome: The intestinal microbiota associated with obesity, lipid metabolism, and metabolic health—pathophysiology and therapeutic strategies. Gastroenterology 2021;160(2):573–99. https://doi.org/10.1053/j.gastro.2020.10.057.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Vida Mohammadparast: conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft, writing—reviewing and editing, visualization, supervision, project administration; Tanin Mohammadi: methodology, validation, formal analysis, investigation, resources, data curation, writing—reviewing and editing, project administration; Parisa Karimi: validation, formal analysis, investigation, data curation, writing—reviewing and editing; Beth L. Mallard: writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammadparast, V., Mohammadi, T., Karimi, E. et al. Effects of Probiotic and Selenium Co-supplementation on Lipid Profile and Glycemia Indices: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Curr Nutr Rep 12, 167–180 (2023). https://doi.org/10.1007/s13668-023-00448-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00448-1