Abstract
The green synthesis of nanopesticides has been recently proposed to improve the efficacy of mosquito control programs. However, limited efforts shed light on the impact of sub-lethal doses of nanopesticides on behavioral traits of mosquito biocontrol agents. We described the synthesis of silver nanoparticles (AgNP) at room temperature using the aqueous extract of Chenopodium ambrosioides, and their high toxicity against the invasive mosquito Aedes albopictus. LC50 calculated on young instars ranged from 13 ppm (first instar larvae) to 19 ppm (pupae). LC50 calculated on adults was 14 ppm. The chemical composition of the C. ambrosioides extract was characterized by GC–MS analysis. The production of AgNP was confirmed by the surface Plasmon resonance band illustrated in UV–Vis, FTIR spectroscopy, EDX, XRD, TEM, and Zeta Potential analyses. In the field, a single treatment of AgNP (10 × LC50) led to complete elimination of larval populations within 72 h. Sub-lethal doses of the reducing extract and AgNP magnify predation rates of Oryzias melastigma fishes against A. albopictus larvae. Overall, this study highlights the concrete potential of C. ambrosioides-synthesized AgNP to develop effective and cheap tools to control young instars and adults of the invasive mosquito A. albopictus.
Similar content being viewed by others
Introduction
Mosquitoes represent a major public health problem, since they act as vectors of serious diseases, including malaria, yellow fever, West Nile virus, filariasis, Japanese encephalitis, dengue and chikungunya [9, 15, 74, 75]. Dengue is an emerging disease, currently considered the most important arbovirus in the world. Aedes mosquitoes mainly vector it. Dengue slyly arrived in the Western Hemisphere over decades, and then its incidence has grown dramatically from the 1990s. The actual numbers of dengue cases are underreported and many cases are misclassified [14, 47, 134]. WHO estimates that dengue infects about 400 million people annually in the part of tropical and subtropical regions [18, 20, 134]. Very recently, mosquito from the Aedes genus also vectored Zika virus to people [135], leading to outbreaks in the Americas, and the Pacific area. Zika symptoms are similar to other arbovirus infections such as dengue, and include fever, skin rashes, conjunctivitis, muscle and joint pain, malaise, and headache. These symptoms normally last for 2–7 days and can be followed by neurological complications and malformations in neonates [37, 135]. Although there are several potential dengue vectors, the field isolation of viruses and epidemiological evidence show that Aedes aegypti and A. albopictus are the main vectors [14]. A. albopictus, also known as the Asian tiger mosquito, originates in Asia and also serves as a vector of chikungunya and many other arboviruses [57, 59, 129].
The use of chemicals insecticides in routine mosquito control operations led to the development of resistance in the targeted vector species [55, 91], as well as to detrimental effects on non-target organisms, with special reference to biological control agents such as larvivorous fishes and other important aquatic predators of Culicidae [26, 96, 105, 112]. Therefore, plant-based insecticides may serve as suitable alternative to synthetic molecules as they are environmentally safe, biodegradable, and are easily available in all parts of the world [4, 10, 16, 53, 124]. In addition, it is worthy to note that the toxicity of botanical-based biopesticides such as plant extracts and essential oils is usually exerted by multiple mechanisms of action, lowering the chances of resistance development in targeted arthropods [99].
Recently, silver nanoparticles (AgNP) gained a focus of intensive research owing to their wide range of applications in areas such as catalysis, optics, antimicrobials, pesticides, biomedical and biomaterial production [7, 39, 64, 126]. The biological synthesis of metal nanoparticles is a research area currently considered more eco-friendly and cost-effective, if compared to other chemical and physical methods [3, 11]. Nano-technology is envisaged to be the next frontier in the ongoing development of cancer therapy [22, 35] as researchers in the biomedical and material engineering fields are working together to discover the possibility of using nano-materials as novel tools for medical sciences. In particular, a number of approaches are available for the synthesis of silver nanoparticles, such as thermal decomposition [93], electrochemical [121], microwave-assisted process [120] and green chemistry [8, 12].
Phytochemicals have a major role in current mosquito control research [9, 10]. Plant extracts have been used as reducing and capping agent for the synthesis of nanoparticles. Indeed, the latter is advantageous over photochemical reduction, heat evaporation, electrochemical reduction, and chemical reduction methods [11]. Because of such a wide range of applications, numerous methods concerning the fabrication of AgNP, as well as various silver-based compounds containing ionic silver (Ag+) or metallic silver (Ag0) have been developed. The synthetic methods used for the preparation of AgNP rely to some toxic chemical used as reducing agents such as NaBH4, citrate or ascorbate. On the other hand, in plant-mediated reducing processes leading to the production of nanoparticles, no chemical reagent or surfactant template is required, which consequently enables the bioprocess with the advantage of being eco-friendly [13, 87–90].
Another important challenge for mosquito control is the successful implementation of biological control programs. Indeed, the natural enemies feeding on mosquito larvae and pupae in aquatic environments play an important role in reducing Culicidae populations (e.g. [65, 131, 136]. Larvivorous fishes are being successfully exploited for control of mosquito vectors aquatic stages in European, Asian, African and Arabian countries [21, 65]. Moreover, the larvivorous fishes provide dual benefits by reducing the mosquito populations and indirectly augmenting the aqua cultural economics [26, 73, 114, 132]; see [17] for a recent review).
Oryzias melastigma [72] (Beloniformes: Adrianichthyidae) [60] is a tiny cyprinodontid fish. It is a carnivorous, surface feeder found in both lentic and lotic waters. This semitransparent and hardy fish can tolerate a wide range of salinity [68], temperature, and many other adverse water qualities. Popularly known as rice fish orminnow [108] or Indian Medaka, or Bechi, it is a sexually dimorphic species [69]. It is found in limited areas of West Bengal, Tamil Nadu, Kerala, Orissa [60, 70] in India and also some riverine areas of Bangladesh.
Chenopodium ambrosioides Linn. (Chenopodiaceae) is widely distributed throughout India. Leaves are useful in the cure of influenza, pneumonia, typhoid and also as vermicide [32, 77]. Chenopodium oil is a mixture ascaridole (55.3 8%), p-cymene (16.2%), alpha-terpinene (9.7%), isoascaridole (4.3%) and limonene (3.8%) [24]. By contrast, little is known about the chemical composition of the polar extracts from C. ambrosioides. Nowadays, this species can be occasionally found also in pathways and near home gardens. It has diverse pharmacological applications in the treatment of influenza, cold or gastrointestinal and respiratory ailments, as well as vomiting, antihelmintic, healing of skin ulceration caused by Leishmania species, anti-inflammatory and antitumor properties [23, 38, 62, 92].
Even if the green synthesis of nanopesticides has been recently proposed to improve the efficacy of mosquito control programs [11], only limited efforts shed light on the potential impact of sub-lethal doses of nanopesticides on behavioral traits of mosquito biological control agents [82–84, 87–89, 127]. Here, we described the synthesis of AgNP at room temperature using the extract of C. ambrosioides as a reducing and capping/stabilizing agent, and their high toxicity against larvae, pupae and adults of the invasive mosquito Ae. albopictus. The chemical composition of the C. ambrosioides extract was characterized by GC–MS analysis. The effective production of AgNP was confirmed UV–Vis and FTIR spectroscopy, EDX, XRD, TEM, and Zeta Potential analysis. In the final experiments, the impact of sub-lethal doses of the reducing extract and AgNPs on predation rates of O. melastigma fishes against A. albopictus larvae was evaluated.
Materials and Methods
Collection of Plant Materials
C. ambrosioides plants used in this study were collected from the villages of The Nilgris, (Western Ghats of South India) Tamil Nadu, India. The plants were authenticated at Botanical Survey of India. Voucher specimens were deposited at Zoology Department, Bharathiar University, Coimbatore, India (Voucher ID n. CHENO-03).
Mosquito Rearing
Eggs of Ae. albopictus were provided by the National Centre for Disease Control (NCDC) field station of Mettuppalayam (Tamil Nadu, India). Eggs were transferred to laboratory conditions [27 ± 2 °C, 75–85% R.H., 14:10 (L:D) photoperiod] and placed in 18 × 13 × 4 cm plastic containers containing 500 mL of tap water, to await larval hatching [41, 128]. Larvae were reared in these containers and fed daily with a mixture of crushed dog biscuits (Pedigree, USA) and hydrolyzed yeast (Sigma-Aldrich, Germany) at a 3:1 ratio (w:w). Water was renewed every 2 days. The breeding medium was checked daily and dead individuals were removed. Breeding containers were kept closed with muslin cloth to prevent contamination by foreign mosquitoes. Pupae were collected daily from culture containers and transferred to glass beakers containing 500 mL of water. Each glass beaker contained about 50 mosquito pupae and was placed in a mosquito-rearing cage (90 × 90 × 90 cm, plastic frames with chiffon walls) until adult emergence. Mosquito adults were continuously provided with 10% (w:v) glucose solution on cotton wicks. The cotton was always kept moist with the solution and changed daily. Five days after emergence, females were supplied with a blood meal which was furnished by means of professional heating blood (lamb blood), at a fixed temperature of 38 °C and enclosed in a membrane of cow gut. After 30 min, the blood meal was removed and a fresh one was introduced [86, 95].
C. ambrosioides-Mediated Synthesis of Silver Nanoparticles
The C. ambrosioides aqueous leaf extract was prepared by adding 10 g of washed and finely cut leaves in a 300-mL Erlenmeyer flask filled with 100 mL of sterilized double distilled water, then boiling the mixture for 5 min before decanting it. The extract was filtered using Whatman filter paper n. 1, was stored at −4 °C and tested within 5 days. The filtrate was treated with aqueous 1 mM AgNO3 (Precision Scientific Co., Coimbatore, India) solution in an Erlenmeyer flask and incubated at room temperature [82]. A dark brown solution indicated the formation of AgNP, as aqueous silver ions were reduced by the C. ambrosioides extract generating stable AgNP in water.
GC–MS Analysis
GC–MS analysis of the plant ethanolic extract was performed using a Perkin Elmer GC Claurus 500 system and Gas Chromatograph interfaced to a Mass Spectrometer (GC/MS) equipped with a Elite-1 fused silica capillary column (30 m × 0.25 mm ID. × 1 μMdf, composed of 100% Dimethyl poly siloxane). The plant ethanolic extract was prepared following the method by [98]. For GC–MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium gas (99.999%) was used as the carrier gas at a constant flow rate of 1 ml/min. and an injection volume of 2 μl was employed (split ratio of 10:1). The injector temperature was 250 °C. The ion-source temperature was 280 °C. The oven temperature was programmed from 110 °C (isothermal for 2 min.), with an increase of 10 °C/min, to 200 °C, then 5 °C/min to 280 °C, ending with a 9 min. isothermal at 280 °C. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 45 to 450 Da. Total GC running time was 36 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. Software adopted to handle mass spectra and chromatograms was a TurboMass Ver 5.2.0 [130].
Characterization of Silver Nanoparticles
C. ambrosioides-synthesized AgNP were characterized by UV–Vis spectrophotometry, FTIR spectroscopy, TEM, EDX, XRD and Zeta potential analysis [85, 107]. In UV–Vis absorbance spectrophotometry, the bio-reduction of AgNO3 in the aqueous medium was monitored by periodic sampling of aliquots (2 mL), measuring the UV–Vis spectrum in 10 mm quartz cuvette with a systronics. We used a UV–Vis spectrophotometer (Hewlett-Packard diode array spectrophotometer, model HP-8452, resolution: 1 nm) operating at 500 and 680 nm with a scanning speed of 1856 nm/min. OD values were recorded until 3 days after biosynthesis at regular intervals. Samples were centrifuged at 42,000 rpm for 10 min; pellets were dried; and the nano-powder obtained was used for further analyses. TEM was performed using a JEOL model 1200 EX instrument operating at an accelerating voltage of 120 kV. Samples were prepared by placing tiny drops of AgNP solutions on carbon-coated TEM grids. The film on the TEM grid was allowed to dry for 5 min under laboratory conditions. XRD analysis of drop-coated films on glass substrates from the AOT-capped AgNP was carried out on a Phillips PW1830 instrument operating at 40 kV and a current of 30 mA with Cu Kα radiation. EDX analyzed the presence of metals in the sample (JEOL-MODEL 6390); the XRD patterns were phase matched using match software version 1.10c Inc. Standard values are obtained from the International Centre for Diffraction Data ICDD. Hkl indices and the mean size of AgNP were calculated using the Debye–Scherer equation by determining the width of (111) and similar Bragg’s reflection parameters [83]. For FTIR measurements, samples were prepared as described for XRD analysis, and measured using a Shimadzu 8400 s with spectral range of 4000–400 cm−1 and resolution of 4 cm−1. FTIR spectra of leaf extracts sampled before and after the biosynthesis of AgNP were compared to examine possible functional groups involved in AgNP formation [41, 126].
Larvicidal Activity Against A. albopictus
Following the methods reported by [128], 25 mosquito larvae (I, II, III or IV instar) or pupae were placed for 24 h in a 500-mL glass beaker filled with dechlorinated water plus C. ambrosioides leaf ethanolic extract (80, 160, 240, 360 and 400 ppm) or C. ambrosioides-synthesized AgNP (10, 20, 30, 40 and 50 ppm). Larval food (0.5 mg) was provided for each tested concentration. Each concentration was replicated 5 times against all instars. In the control, 25 larvae or pupae were transferred to 250 mL of dechlorinated water. No mortality was observed in the control. Percentage mortality was calculated as follows:
Larvicidal Activity in the Field
C. ambrosioides ethanolic extract and C. ambrosioides-synthesized AgNP were applied in six external water storage reservoirs in each of two field sites at the National Institute of Communicable Disease Centre (Coimbatore, India).Treatments were carried out using a knapsack sprayer (Private Limited 2008, Ignition Products, India) [82]. Pre-treatment Aedes larval density was monitored. Post-treatment observations were conducted after 24, 48 and 72 h, using a larval dipper. Toxicity was assessed against third- and fourth instars larvae. Six trials were conducted for each test site with similar weather conditions (28 ± 2 °C; 80% R.H.). The required quantity of mosquitocide was calculated on the basis of the total surface area and volume (i.e. 0.25 m3 and 250 L for all sites). Then, the required concentration was prepared using 10 × LC50 values [80, 128]. Percentage reduction of the larval density was calculated using the formula:
where C is the total number of mosquitoes in the control, and T is the total number of mosquitoes in the treatment [126].
Adulticidal Activity
Adulticidal experiments were performed following the methods reported by the [126, 127, 133]. C. ambrosioides ethanolic leaf extract was tested at 60, 120, 180, 240 and 300 ppm. AgNP were tested at 6, 12, 18, 24 and 30 ppm formulated in 5 mL of aqueous solution. C. ambrosioides aqueous extract and AgNP were applied on Whatman n. 1 filter paper (size 12 × 15 cm) lining a glass holding tube (diameter 30 mm; length 60 mm). In control treatments, filter papers were treated with either the same volume of distilled water plus ethanol or AgNO3 (1 mM) in aqueous solution. In each test, 20 A. albopictus females were gently transferred into another glass holding tube. The mosquitoes were allowed to acclimatize in the tube for 1 h and then exposed to a test tube lined with treated or control paper for 1 h. At the end of exposure period, the mosquitoes were transferred back to the original holding tube, kept for a 24 h recovery period and then mortality was recorded. A pad of cotton soaked with 10% (w:v) glucose solution was placed on the mesh screen at the top of the holding tube [126].
Oryzias melastigma Predation on A. albopictus Larvae
O. melastigma fishes were collected from Tamil Nadu Fisheries Department, Mettur Dam, Salem, and maintained in cement tanks (120 cm diameter and 60 cm depth) containing field collected water at 27 ± 3 °C and external RH 85%. For the assays, the predatory fishes were released in separate transparent containers (14 × 10 cm) containing clean water. The predatory efficiency of O. melastigma was assessed against II and III instar larvae of A. albopictus. In each trial, 200 mosquito larvae were introduced, with 1 adult O. melastigma, in plastic cups (2 L) containing dechlorinated water. For each tested mosquito instar, five replicates were conducted. Control was 2 L of dechlorinated water plus 200 larvae, without O. melastigma. All experimental cups checked after 24 h and the number of dead/preys consumed by predator was recorded. After each checking, the predated mosquito larvae were replaced with new ones. Similarly, five replicates were made for each prey density with predators or without predators (control), before and after the treatment of the leaf extract or AgNP. Using the same fish, the rate of predation was observed for five consecutive days. The prey density was set to same value after every 24 h. The fish predatory efficiency was calculated using the following formula:
Oryzias melastigma Predation on A. albopictus Larvae Post-Treatment with Ag Nanoparticles.
Here, 200 mosquito larvae were introduced, with 1 adult O. melastigma; in plastic cups (2 L) containing dechlorinated water plus 1/3 of the LC50 calculated against III and IV instar larvae of A. albopictus [82, 83]. For each tested mosquito instar, five replicates were conducted. Control was 2 L of AgNP-contaminated water plus 200 larvae, without predator fish (O. melastigma). All experimental cups checked after 24 h and the number of preys consumed by O. melastigma was recorded. After each checking, the predated mosquito larvae were replaced with new ones. Similarly, five replicates were made for each prey density with predators or without predators (control), before and after the treatment of the leaf extract or AgNP. Using the same predator individual, the rate of predation was observed for five consecutive days. The prey density is being set to same value after every 24 h. The fish predatory efficiency was calculated using the above-mentioned formula.
Data Analysis
Mosquito mortality data from laboratory assays were analyzed by probit analysis, calculating LC50 and LC90 following the method by [44]. Mosquito larval density data from field assays were analyzed using a two-way ANOVA with two factors (i.e. the mosquitocidal treatment and the elapsed time from treatment). In all analyses, a probability level of P < 0.05 was used for the significance of differences among values.
O. melastigma predation data were analyzed using a weighted general linear model with two fixed factors:\(y = X\beta \, + \,\varepsilon\), where y is the vector of the observations (the number of consumed preys), X is the incidence matrix, ß is the vector of fixed effects (treatment and targeted mosquito instar), and ε is the vector of the random residual effect. A probability level of P = 0.05 was used for the significance of differences between values.
Results and Discussion
Chemical Composition of C. ambrosioides Leaf Extract
The interpretation on mass spectrum GC–MS was conducted using the database of National Institute Standard and Technology (NIST) having more than 62,000 patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained. A total of 15 components were identified (Fig. 1; Table 1), among them tetradecanoic acid (C14H28O2) was the major component available at a RT of 12.29 min and with a peak area of 22.43%, the second major component was 3-methoxysalicylic acid (C8H8O4) with a RT of 4.88 min and 18.38% peak area. Further components were identified by GC–MS spectral comparison with the database NIST, including bicyclo[4.1.0] heptan-3-ol, 4,7,7-trimethyl-(1α,3α,4α,6α)-(C10H18O) 4.10 min RT, peak area 13.28%, and 5-isopropenyl-2-methyl-7-oxabicyclo[4.1.0]heptan-2-ol (C10H16O2) 6.53 min RT, peak area 9.04% (Table 1). It has been reported that tetracyclic triterpenoids showed activity on entomopathogenic nematodes [6], and tetradecanoic acid acted as a good larvicide and repellent against the dengue and yellow fever vector A. aegypti, while squalene has a variety of health-promoting functions, including tumor-suppressing [1, 94, 104, 119], antibacterial/antifungal [118], and cholesterol-lowering [76] effects. Also, squalene has recently attracted attention as a feasible source of biofuels [109]. The insecticidal properties of essential oils containing these compounds against several pest species, including mosquito vectors, have been reported by [31, 116, 138].
Characterization of Silver Nanoparticles
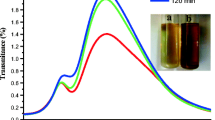
In our experiments, UV–Vis spectrum showed a maximum absorbance peak at 421 nm which increased over time during the incubation of silver nitrate with the C. ambrosioides extract (Fig. 2). When the AgNO3 solution was added to the C. ambrosioides leaf extract, the color changed from light to dark brown, indicating the reduction from Ag+ to Ag0 (Fig. 2a). The formation of AgNP was confirmed through the presence of an absorption peak at 421 nm (Fig. 2). Our UV–Vis results are in agreement with previous research [66, 82, 107, 115, 127, 137]. The main peak detected here indicated a surface Plasmon resonance (SPR), which has been recorded for different metal nanoparticles ranging from 2 to 100 nm in size [56, 106].
TEM observations showed different shapes of C. ambrosioides-synthesized AgNP, including spherical, round and hexagonal ones, with mean size ranging from 25 to 50 nm (Fig. 3). Similarly, the morphological features of green synthesized silver, gold and metal nanoparticles fabricated using extracts from several terrestrial and marine plants, lead to mean nanoparticle sizes ranging from 15 to 70 nm (e.g. [82, 83, 103, 125, 127] Furthermore, C. ambrosioides-synthesized AgNP did not show direct contact within aggregates, allowing us to argue that their stabilization occurred through capping agents.
The EDX spectrum recorded from C. ambrosioides synthesized AgNP revealed a distinct signal and high atomic percent values for Ag (Fig. 4). EDX analysis confirmed the presence of elemental Ag. The presence of oxygen (O) and silver (Ag) indicates that the extracellular organic compounds were adsorbed on the surface of AgNP (Fig. 4). The present finding corroborates previous reports on AgNP biosynthesis using botanical and microbial products [3, 43, 50–52, 67]. XRD patterns showed intense peaks corresponding to the (111), (200), (220), (311) and (222) sets of lattice planes (Fig. 5). The XRD patterns showed that the AgNP formed by the reduction of AgNO3 using C. ambrosioides leaf extract were crystalline in nature (Fig. 5). The XRD pattern observed in this study was consistent with previous reports [5, 48]. For instance, [111] reported diffraction peaks at 44.50°, 52.20°, and 76.7° = 2θ, which correspond to the (111), (200), and (220) facets of the face-centered cubic crystal structure.
FTIR spectroscopy was carried out to identify the possible biomolecules in the C. ambrosioides extract, which may be responsible for synthesis and stabilization of AgNP (Fig. 6). FTIR spectrum of AgNP prepared using the C. ambrosioides leaf extract showed peaks at 3431.36, 2362.80, 2063.83, 1633.71, 1514.12, 1456.26, and 418.55 cm−1 (Fig. 6). The peak located at about 2,362.80 cm−1 can be attributed to the N–H stretching vibrations or the C=O stretching vibrations. The sharp absorption peak at 1633.71 cm−1 may be assigned to C=O stretching vibration in carbonyl compounds which may be characterized by the presence of high content of terpenoids and flavonoids. A broad intense band at 3,431.36 cm−1 in both leaf extract and AgNP spectra can be linked to the N–H stretching frequency arising from the peptide linkages present in the proteins of the extract [71, 78]. Therefore, it may be inferred that these biomolecules are responsible for capping and efficient stabilization of synthesized nanoparticles. Thus, the analysis of FTIR spectrum from the green fabricated AgNP showed the presence of different functional groups from alkane, methylene, alkene, amine, and carboxylic acid, previously reported as reducing agents in the nano-biosynthesis [33]. Polyphenols have been also reported as potential reducing agent in the biosynthesis of AgNP [79, 100]. The adsorption on the surface of metal nanoparticles is a characteristic of flavanones and terpenoids, since they easily interacted through carbonyl groups in the lack of other strong ligating agents in sufficient concentration [113].
Particle size and size distribution are the most important characteristics of nanoparticle systems. In our analysis, zeta potential of AgNP was −18.5 mV (Fig. 7). Similarly, [42] noted that C. album-synthesized silver and gold nanoparticles were stable under a wide pH range due to their high zeta potential. In agreement with TEM results, size analysis showed a distribution of particle diameters ranging from 10 to 90 nm with an average particle size of 25 nm. The particle sizes determined the in vivo distribution, biological fate, toxicity and the targeting ability of nanoparticle systems [13]. [40] have reported that 100 nm nanoparticles had a 2.5 fold greater uptake than1 µm microparticles, and sixfold greater uptake than 10 µm microparticles on Caco-2 cell line.
Toxicity on Aedes albopictus
In laboratory conditions, the C. ambrosioides ethanolic leaf extract showed larvicidal and pupicidal toxicity against A. albopoictus, with LC50 values ranging from 124.55 ppm (I instar larva) to 237.06 ppm (pupa), respectively (Table 2). A number of plant extracts has been reported as effective against larvae and pupae of mosquito vectors [10, 61, 122, 123]. More recently, the green biosynthesis of mosquitocidal nanoparticles is advantageous over chemical and physical methods, since it is cheap, single-step, and does not require high pressure, energy, temperature, and the use of highly toxic chemicals [87, 102, 103]. In this study C. ambrosioides-synthesized AgNP were toxic against A. albopictus larvae and pupae, with LC50 values ranging from 13.37 ppm (I instar) to 19.77 ppm (pupa) (Table 3). In agreement with our data, [126] showed that Mimusops elengi leaf aqueous extract was moderately effective against malarial vector, Anopheles stephensi and arbovirus vector A. albopictus while the LC50 of AgNP fabricated using this plant and tested on A. stephensi ranged from 12.53 (I instar larvae) to 23.55 ppm (pupae), and LC50 against A. albopictus ranged from 11.72 ppm (I) to 21.46 ppm (pupae). Low doses of AgNP biosynthesized using Euphorbia hirta leaf extract have been reported as highly toxic against A. stephensi, withLC50 values ranging from 10.14 ppm (I instar larvae) to 34.52 ppm (pupae) [101]. Another good example is the larvicidal activity of Leucas aspera-synthesized AgNP, with LC50 ranging from 13.06 to 25.54 ppm for A. aegypti, and from 12.45 to 22.26 ppm for A. stephensi [117]. Nelumbo nucifera-synthesized AgNP were toxic to the larvae of A. subpictus (LC50 = 0.69 ppm) and C. quinquefasciatus (LC50 = 1.10 ppm; LC90 = 3.59 ppm), respectively [110]. In the field, the application of C. ambrosioides aqueous extract and C. ambrosioides-synthesized AgNP (10 × LC50) in water storage reservoirs led to the complete elimination of larval populations of A. albopictus after 72 h (Table 4). [104] reported that the stable neem fractions were as effective as mosquito larvicides in the field. Plant based insecticides have been evaluated successfully in different habitats of mosquito vectors, tested species include Clerodendron inerme, Acanthus ilicifolius [63], M. elengi and green-synthesized AgNP [125], Phyllanthus niruri and green-synthesized AgNP [128]. Further research aimed to clarify the exact mechanism(s) of action of AgNP against mosquito young instars is ongoing [13].
In adulticidal experiments, the C. ambrosioides leaf extract and green-synthesized AgNP were toxic to A. albopictus (Table 5). LC50 values were 154.99 ppm (C. ambrosioides extract) and 14.29 ppm (AgNP). At the highest concentration tested, the adults of both species remained still for a short time period (i.e. 1–3 min) following application, showed fast wagging movements and then died. The adulticidal efficacy of a number of plant borne extracts and essential oils against adult mosquitoes of public health importance has been reported by several recent studies (e.g. [2, 97, 123, 125, 126]. For example, Subramaniam al. [125] reported that the adulticidal activity of methanol extracts of seaweeds D. dichotoma, P. pavonica and V. pachynema on the costal malarial vector Anopheles sundaicus, LC50 values were 147.18 ppm, 161.94 ppm and 133.79 ppm, respectively. On Ae. aegypti, an high adulticidal effect was reported for Piper sarmentosum, followed by Piper ribesoides and Piper longum, with LD50 values of 0.14, 0.15 and 0.26 microg/female, respectively [36]. [49] have reported that the adulticidal activity was observed testing the methanol extracts of E. alba and A. paniculata on An. stephensi, LC50 and LC90 values were of 150.36, 130.19 ppm and 285.22, and 244.16 ppm respectively. Besides this interesting data, few efforts have been done to shed light on the contact toxicity of green-fabricated AgNP on mosquito adults [11]. We hypothesized that an important toxicity effect can be due to the magnified action of bioactive botanicals capping the wide surfaces of the nanocomposite.
Impact of Ag nanoparticles on Oryzias melastigma predation
Biological control of mosquito larval populations using aquatic predators, such as insects, fishes, copepods, and tadpoles recently received renewed attention (e.g. [19, 81–85, 126, 127]; see [17] for a review). In this present study, the rice fish, O. melastigma showed high predation rates on the dengue vector A. albopictus 2nd and 3rd instar larvae post-treatment with very low doses of AgNP. In standard conditions, after 24 h, the predation rates of II and III instar larvae of A. albopictus were 65.5 and 59.0%. Predation by O. melastigma post-treatment with ultra-low dosages of C. ambrosioides aqueous extract were 75.0 and 83.0%, while post-treatment with green-synthesized AgNP reached 91.0 and 85.5% against II and III instar larvae, resepctively (Table 6). No detectable toxicity effects were observed on O. melastigma individuals exposed to the AgNP-contaminated aquatic environment (post-treatment observation period: 10 days; data not shown). In agreement with our data, previous studies testing other aquatic species, including Gambusia affinis [28, 126];), Hoplobatrachus tigerinus [84], Poecilia reticulata [85], Aplocheilus lineatus [127], Carassius auratus (Linneaus) [29], Xenontodon cancila [27], Ctenopharyngodon idella, Cyprinus carpio [30], Oreochromis niloticus niloticus [25, 46], Betta splendens, Pseudotropheus tropheops tropheops, Osphronemus goramy Lacépède, and Pterophyllum scalare [45], showed good predatory ability on mosquito larvae under similar testing conditions. Recently, Chobua et al. [34] studied G. affinis and C. auratus as control agents of A. gambiae. In addition, our data, in agreement with recent researches [54, 58, 88, 126, 127], highlighted the limited toxicity of plant extracts and green nanocomposites on mosquito natural enemies, as well as the chance to use both tools in synergy to successfully manage mosquito young instar populations.
Conclusions
Overall, in the present work we reported the synthesis of AgNP at room temperature using the aqueous extract of C. ambrosioides, and their high toxicity against larvae, pupae and adults of the invasive mosquito A. albopictus. It is worthy to note that extremely low doses of the reducing extract and AgNP magnify the predation rates of O. melastigma fishes against A. albopictus 2nd and 3rd instar larvae, highlighting the concrete potential of C. ambrosioides-synthesized AgNP to develop effective and cheap tools to control young instars and adults of the invasive mosquito A. albopictus.
References
A. Aioi, T. Shimizu, and K. Kuriyama (1995). Int. J. Pharm. 113, 159.
D. Amerasan, K. Murugan, K. Kovendan, P. M. Kumar, C. P. Selvam, J. Subramaniam, S. John William, and J. S. Hwang (2012). Parasitol. Res 111, 1953.
D. Amerasan, T. Nataraj, K. Murugan, P. Madhiyazhagan, C. Panneerselvam, M. Nicoletti, and G. Benelli (2016). J. Pest Sci. 89, 249.
A. Azizullah, Z. U. Rehman, I. Ali, W. Murad, N. Muhammad, W. Ullah, and D.-P. Hader (2014). Parasitol. Res 113, 4321.
H. Bar, K. R. Dipak Bhui, P. Gobinda Sahoo, and P. Sarkar (2009). Coll. Surf. A. Physicochem. Eng. Asp. 339, 134.
M. E. Barbercheck and J. Wang (1996). J. Insect. Pathol. 68, (2), 141.
S. K. Batabyal, C. Basu, A. R. Das, and G. S. Sanyal (2007). J. Biobased Mater. Bioenerg. 1, 143.
N. A. Begum, S. Mondal, S. Basu, R. A. Laskar, and D. Mandal (2009). Coll. Surf. B 71, 113.
G. Benelli (2015). Parasitol. Res 114, 2801.
G. Benelli (2015). Parasitol. Res 114, 3201.
G. Benelli (2016). Parasitol. Res 115, 23.
G. Benelli (2016). Asia Pacif. J. Trop. Biomed. 6, 353.
G. Benelli (2016). Enzyme. Microb. Technol. doi:10.1016/j.enzmictec.2016.08.022.
G. Benelli and H. Mehlhorn (2016). Parasitol. Res 115, 1747.
G. Benelli, A. Lo Iacono, A. Canale, and H. Mehlhorn (2016). Parasitol. Res 115, 2131.
G. Benelli, R. Pavela, A. Canale, and H. Mehlhorn (2016). Parasitol. Res. doi:10.1007/s00436-016-5095-1.
G. Benelli, C. L. Jeffries, and T. Walker (2016). Insects. 7, 52.
S. Bhatt, P. W. Gething, and O. J. Brady (2013). Nature. 496, 504.
G. Bowatte, P. Perera, G. Senevirathne, S. Meegaskumbura, and M. Meegaskumbura (2013). Biol. Control 67, 469.
O. J. Brady, P. W. Gething, S. Bhatt, J. P. Messina, J. S. Brownstein, and A. G. Hoen (2012). PLoS Negl. Trop. Dis. 6, e1760. doi:10.1371/journal.pntd.0001760.
L. J. Bruce-Chwatt (1985). Drugs Exp. Clin. Res 11, 899.
S. D. Caruthers, S. A. Wickline, and G. M. Lanza (2007). Curr. Opin. Biotech. 18, 26.
A. M. Carvalho Plantasy sabidurıa popular del Parque Natural de Montesinho. Un estudio etnobotanico en Portugal. Biblioteca de Ciencias, 35 (Consejo Superior de Investigaciones Cientı´ficas, Madrid, 2010).
J. F. Cavalli, F. Tomi, A. F. Bernardini, and J. Casanova (2004). Phytochem Anal 15, 275.
S. K. Chand and R. S. Yadav Use of Oreochromis mossambicus (Peters) in controlling mosquito breeding in cow dung pits. in V. P. Sharma and A. Ghosh (eds.), Larvivorous Fishes of Inland Ecosystems (Malaria Research Centre, Delhi, 1994), p. 115.
G. Chandra, I. Bhattacharjee, S. N. Chatterjee, and A. Ghosh (2008). Indian J. Med. Res 127, 13.
S. N. Chatterjee and G. Chandra (1996). Environ. Ecol. 14, 173.
S. N. Chatterjee and G. Chandra (1997). Sci. Cult. 63, 51.
S. N. Chatterjee, S. Das, and G. Chandra (1997). Transact. Zool. Soc. India 1, 112.
S. N. Chatterjee, A. Ghosh, and G. Chandra (2001). Transact. Zool. Soc. India 5, 83.
S. S. Cheng, J. Y. Liu, K. H. Tsai, W. J. Chen, and S. T. Chang (2004). J. Agric. Food Chem. 52, 4395.
L. Cheryl, T. Nancy, B. Gerhard, L. Grant, and G. Karla (2006). J. Ethnobiol. Ethnomed. doi:10.1186/1746-4269-2-31.
K. Cho, J. Park, T. Osaka, and S. Park (2005). Electrochim. Acta 51, 956.
M. Chobua, G. Nkwengulilaa, A. M. Mahandeb, B. J. Mwang’ondeb, and J. E. Kwekab (2015). Acta. Trop. 142, 131.
K. Y. Choi, G. Liu, S. Lee, and X. Chen (2012). Nanoscale. 4, 330.
W. Choochote, U. Chaithong, K. Kamsuk, E. Rattanachanpichai, A. Jitpakdi, P. Tippawangkosol, D. Chaiyasit, D. Champakaew, B. Tuetun, and B. Pitasawat (2006). Rev. Inst. Med. Trop. S. Paulo. 48, 33.
A. Costello, T. Dua, P. Duran, M. Gülmezoglu, O. T. Oladapo, W. Perea, J. Pires, P. R. Pardo, N. Rollins, and S. Saxena (2016). Bull. World Health Organ 94, 406. doi:10.2471/BLT.16.176990.
G. V. B. Cruz, P. V. S. Pereira, F. J. Patrıcio, G. C. Costa, S. M. Sousa, J. B. Frazao, W. C. Aragao-Filho, M. C. G. Maciel, Amaral F. M. M. SilvaLA, E. S. B. Barroqueiro, R. N. M. Guerra, and F. R. F. Nascimento (2007). J. Ethnopharmacol. 111, 148.
M. R. Das, R. K. Sarma, R. Saikia, V. S. Kale, M. V. Shelke, and P. Sengupta (2011). Coll. Surf. B 83, 16.
M. P. Desai, V. Labhasetwar, E. Walter, R. J. Levy, and G. L. Amidon (1997). Pharm. Res 14, 1568.
D. Dinesh, K. Murugan, P. Madhiyazhagan, C. Panneerselvam, M. Nicoletti, W. Jiang, G. Benelli, B. Chandramohan, and U. Suresh (2015). Parasitol. Res 114, 1519.
A. D. Dwivedi and K. Gopal (2010). Coll. Surf. A. 369, (2010), 27.
A. M. Fayaz, K. Balaji, Y. R. GirilalM, P. T. Kalaichelvan, and R. Venketesan (2010). Nanomed. Nanotechnol. Biol. Med. 6, 103.
D. J. Finney Probit Analysis (Cambridge University Press, London, 1971), p. 68.
A. Ghosh, I. Bhattacharjee, M. Ganguly, S. Mandal, and G. Chandra (2004). Bull. Penelit. Kesehat. 32, 144.
A. Ghosh, I. Bhattacharjee, and G. Chandra (2006). J Appl Zool Res 17, 114.
A. P. Goncalvez, R. E. Engle, M. St Claire, R. H. Purcell, and C. J. Lai (2007). Proc. Natl. Acad. Sci. USA. 104, 9422.
P. Gong, H. Li, X. He, K. Wang, J. Hu, W. Tan, S. Zhang, and X. Yang (2007). Nanotechnology. 18, 285604.
M. Govindarajan and R. Sivakumar (2011). Asia. Pacif. J. Trop. Med. 4, (1), 941.
M. Govindarajan and G. Benelli (2016). Parasitol. Res 115, 925.
M. Govindarajan and G. Benelli (2016). RSC. Adv. 6, 59021.
M. Govindarajan and G. Benelli (2016). J. Clust. Sci. doi:10.1007/s10876-016-1035-6.
M. M. Green and J. M. Singer (1981). J. Am. Mosq. Control Assoc. 7, 282.
K. M. Haldar, B. Haldar, and G. Chandra (2013). Parasitol. Res 112, 1451.
J. Hemingway and H. Ranson (2000). Annu. Rev. Entomol. 45, 371.
A. Henglein (1993). J. Phys. Chem. 97, 5457.
J. Huang, G. Zhan, B. Zheng, D. Sun, F. Lu, and Y. Lin (2011). Ind. Eng. Chem. Res 50, 9095.
A. Jaganathan, K. Murugan, C. Panneerselvam, P. Madhiyazhagan, D. Dinesh, C. Vadivalagan, A. T. Aziz, B. Chandramohan, U. Suresh, R. Rajaganesh, J. Subramaniam, M. Nicoletti, A. Higuchi, A. A. Alarfaj, M. A. Munusamy, S. Kumar, and G. Benelli (2016). Parasitol. Int. 65, 276.
Y. S. Jang, M. K. Kim, Y. S. Ahn, and H. S. Lee (2002). Agric. Chem. Biotechnol. 4, 131.
K. C. Jayaram The Freshwater Fishes of India, Pakistan, Bangladesh, Burma and Sri Lanka (ZSI, Calcutta, 1981).
M. Kalyanasundaram and P. K. Das (1985). Indian. J. Med. Res 82, 19.
E. G. Kamel, M. A. El-Emam, S. S. M. Mahmoud, F. M. Fouda, and F. E. Bayaumy (2011). Parasitol. Int. 60, 388.
K. Kovendan and K. Murugan (2011). Adv. Environ. Biol. 5, 335.
A. T. Le, P. T. Huy, P. D. Tam, T. Q. Huy, P. D. Cam, and A. A. Kudrinskiy (2010). Curr. Appl. Phys. 10, 910.
V. Louca, M. C. Lucas, C. Green, S. Majambere, U. Fillinger, and S. W. Lindsay (2009). J. Med. Entomol. 46, 546.
P. Madhiyazhagan, K. Murugan, A. Naresh Kumar, T. Nataraj, D. Dinesh, C. Panneerselvam, J. Subramaniam, P. Mahesh Kumar, U. Suresh, M. Roni, M. Nicoletti, A. A. Alarfaj, A. Higuchi, M. A. Munusamy, and G. Benelli (2015). Parasitol. Res. doi:10.1007/s00436-015-4671-0.
P. Magudapathy, P. Gangopadhyay, B. K. Panigrahi, K. G. M. Nair, and S. Dhara (2001). Physica 299, 142.
A. K. Manna (1989). Environ. Ecol. 7, 502.
A. K. Manna and S. Bannerjee (1984). Sci. Cult. 50, 329.
A. K. Manna and S. Bannerjee (1985). Environ. Ecol. 3, 456.
S. Marimuthu, A. A. Rahuman, G. Rajakumar, T. Santhoshkumar, A. Vishnu Kirthi, C. Jayaseelan, A. Bagavan, A. Abduz Zahir, G. Elango, and C. Kamaraj (2011). Parasitol. Res 108, 1541.
McClelland (1839) http://www.marinespecies.org/aphia.php?p=taxdetails&id=315132.
A. G. K. Menon Indigenous Larvivorous Fishes of India (NIMR, New Delhi, 1991).
H. Mehlhorn (ed.) Encyclopedia of Parasitology, 4th ed (Springer, New York, 2015).
H. Mehlhorn, K. A. Al-Rasheid, S. Al-Quraishy, and F. Abdel-Ghaffar (2012). Parasitol. Res 110, 259.
T. A. Miettinen and H. Vanhanen (1994). Am. J. Clin. Nutr. 59, 356.
Mishra A (2002) Evaluation of some higher plant products for their pesticidal activity against some storage fungi and insects. Ph D thesis. Banaras Hindu University, Varanasi, India.
P. Mukherjee, M. Roy, B. P. Mandal, G. K. Dey, P. K. Mukherjee, J. Ghatak, A. K. Tyagi, and S. P. Kale (2008). Nanotechnology 19, 075103.
K. S. Mukunthan, E. K. Elumalai, T. N. Patel, and V. R. Murty (2011). Asian Pac. J. Trop. Biomed. 1, 270.
K. Murugan, V. Vahitha, I. Baruah, and S. C. Das (2003). Ann. Med. Entomol. 12, 11.
K. Murugan, J. S. Hwang, K. Kovendan, K. Prasanna Kumar, C. Vasugi, and A. Naresh Kumar (2011). Hydrobiologia 666, 331.
K. Murugan, N. Aarthi, K. Kovendan, C. Panneerselvam, B. Chandramohan, P. M. kumar, D. Amerasan, M. Paulpandi, R. Chandirasekar, D. Dinesh, U. Suresh, J. Subramaniam, A. Higuchi, A. A. Alarfaj, M. Nicoletti, H. Mehlhorn, and G. Benelli (2015). Parasitol. Res 114, 3657.
K. Murugan, C. M. Samidoss, C. Panneerselvam, A. Higuchi, M. Roni, U. Suresh, B. Chandramohan, J. Subramaniam, P. Madhiyazhagan, D. Dinesh, R. Rajaganesh, A. A. Alarfaj, M. Nicoletti, S. Kumar, H. Wei, A. Canale, H. Mehlhorn, and G. Benelli (2015). Parasitol. Res 114, 4087.
K. Murugan, V. Priyanka, D. Dinesh, P. Madhiyazhagan, C. Panneerselvam, J. Subramaniam, U. Suresh, B. Chandramohan, M. Roni, M. Nicoletti, A. A. Alarfaj, A. Higuchi, M. A. Munusamy, H. F. Khater, R. H. Messing, and G. Benelli (2015). Parasitol. Res. doi:10.1007/s00436-015-4582-0.
K. Murugan, J. S. E. Venus, C. Panneerselvam, S. Bedini, B. Conti, M. Nicoletti, S. K. Sarkar, J. S. Hwang, J. Subramaniam, P. Madhiyazhagan, P. M. Kumar, D. Dinesh, U. Suresh, and G. Benelli (2015). Environ. Sci. Poll. Res. doi:10.1007/s11356-015-4920-x.
K. Murugan, M. Aamina Labeeba, C. Panneerselvam, D. Dinesh, U. Suresh, J. Subramaniam, P. Madhiyazhagan, J. S. Hwang, L. Wang, M. Nicoletti, and G. Benelli (2015). Res. Vet. Sci. 102, 127.
K. Murugan, P. Aruna, C. Panneerselvam, P. Madhiyazhagan, M. Paulpandi, J. Subramaniam, R. Rajaganesh, H. Wei, M. Saleh Alsalhi, Nicoletti M. DevanesanS, B. Syuhei, A. Canale, and G. Benelli (2016). Parasitol. Res 115, 651.
K. Murugan, J. Anitha, D. Dinesh, U. Suresh, R. Rajaganesh, B. Chandramohan, J. Subramaniam, M. Paulpandi, C. Vadivalagan, P. Amuthavalli, L. Wang, J. S. Hwang, H. Wei, M. S. Alsalhi, S. Devanesan, S. Kumar, K. Pugazhendy, A. Higuchi, M. Nicoletti, and G. Benelli (2016). Ecotoxicol. Environ. Saf 132, 318.
K. Murugan, C. Panneerselvam, A. T. Aziz, J. Subramaniam, P. Madhiyazhagan, J. S. Hwang, Lan Wang, D. Dinesh, U. Suresh, M. Roni, A. Higuchi, M. Nicoletti, M. Saleh Alsalhi, and G. Benelli (2016). Environ. Sci. Poll. Res. doi:10.1007/s11356-016-6832-9.
K. Murugan, D. Nataraj, P. Madhiyazhagan, V. Sujitha, B. Chandramohan, C. Panneerselvam, D. Dinesh, R. Chandirasekar, K. Kovendan, U. Suresh, J. Subramaniam, M. Paulpandi, C. Vadivalagan, R. Rajaganesh, H. Wei, B. Syuhei, A. T. Aziz, M. Saleh Alsalhi, S. Devanesan, M. Nicoletti, A. Canale, and G. Benelli (2016). Parasitol. Res 115, 1071.
M. N. Naqqash, A. Gökçe, A. Bakhsh, and M. Salim (2016). Parasitol. Res 115, 1363.
F. R. F. Nascimento, G. V. B. Cruz, P. V. S. Pereira, M. C. G. Maciel, L. A. Silva, A. P. S. Azevedo, E. S. B. Barroqueiro, and R. N. M. Guerra (2006). Life Sci. 78, 2650.
S. Navaladian, B. Viswanathan, R. P. Viswanath, and T. K. Varadarajan (2007). Nanoscale Res. Lett. 2, 44.
H. L. Newmark (1997). Cancer Epidemiol Biomark Prev. 6, 1101.
M. Nicoletti, S. Mariani, O. Maccioni, T. Coccioletti, and K. Murugan (2012). Parasitol. Res 111, 205.
S. Y. Ohba, Dida G. O. KawadaH, D. Juma, G. Sonye, N. Minakawa, and M. Takagi (2010). J. Med. Entomol. 47, 783.
C. Panneerselvam and K. Murugan (2013). Parasitol Res 112, (2), 679.
C. Panneerselvam, K. Murugan, M. Roni, A. T. Aziz, U. Suresh, R. Rajaganesh, P. Madhiyazhagan, J. Subramaniam, D. Dinesh, M. Nicoletti, A. Higuchi, A. A. Alarfaj, M. A. Munusamy, S. Kumar, N. Desneux, and G. Benelli (2016). Parasitol. Res 115, 997.
R. Pavela and G. Benelli (2016). Tr Plant Sci. doi:10.1016/j.tplants.2016.10.005.
T. N. V. K. V. Prasad and E. K. Elumalai (2011). Asian Pac. J. Trop. Biomed. 1, 439.
A. Priyadarshini, K. Murugan, C. Panneerselvam, S. Ponarulselvam, H. Jiang Shiou, and M. Nicoletti (2012). Parasitol. Res 111, 997.
G. Rajakumar and A. A. Rahuman (2011). Acta Trop. 118, 196.
R. Rajan, K. Chandran, S. L. Harper, S. I. Yun, and P. T. Kalaichelvan (2015). Ind. Crops Prod. 70, 356.
C. V. Rao, H. L. Newmark, and B. S. Reddy (1998). Carcinogenesis. 19, 287.
J. V. Rao and P. Kavitha (2010). Z Naturforsch. C. 65, 303.
B. K. Ravindra and A. H. Rajasab (2014). Int. J. Pharm. Pharm. Sci. 6, 372.
M. Roni, K. Murugan, C. Panneerselvam, J. Subramaniam, M. Nicoletti, P. Madhiyazhagan, D. Dinesh, U. Suresh, H. F. Khater, H. Wei, Alarfaj A. A. CanaleA, M. A. Munusamy, A. Higuchi, and G. Benelli (2015). Ecotoxicol. Environ. Saf. doi:10.1016/jecoenv201507005.
D. E. Rosen and L. R. Parenti (1981). Am. Mus. Novit. 27, 1.
M. A. Rude and A. Schirmer (2009). Curr. Opin. Microbiol. 12, 274.
T. Santhoshkumar, A. A. Rahuman, G. Rajakumar, S. Marimuthu, A. Bagavan, C. Jayaseelan, A. A. Zahir, G. Elango, and C. Kamaraj (2010). Parasitol. Res 108, 693.
R. Sathyavathi, M. Balamurali Krishna, S. Venugopal Rao, R. Saritha, and D. Narayana Rao (2010). Adv. Sci. Lett. 3, 1.
Service MW (1977). J. Med. Entomol. 13, 535.
S. S. Shankar, A. Rai, A. Ahmad, and M. Sastry (2004). J. Coll. Interf. Sci. 275, 496.
V. P. Sharma and A. Ghosh Larvivorous Fishes of Inland Ecosystem (NIMR, New Delhi, 1994).
B. P. Singh, B. J. Hatton, B. Singh, A. L. Cowie, and A. Kathuria (2010). J. Environ. Qual. 39, 1.
R. Sivakumar, A. Jebanesan, M. Govindarajan, and P. Rajasekar (2011). Asia. Pacif. J. Trop. Med. 2011, 706.
S. Sivapriyajothi, P. Mahesh Kumar, K. Kovendan, J. Subramaniam, and K. Murugan (2014). J. Entomol. Acarol. Res 46, 1787.
T. J. Smith (2000). Exp. Opin. Invest. Drugs. 9, 1841.
T. J. Smith, G. Y. Yang, D. N. Seril, J. Liao, and S. Kim (1998). Carcinogenesis. 19, (4), 703.
K. S. Sreeram, M. Nidin, and B. U. Nair (2008). Bull. Mater. Sci. 31, 937.
M. Starowicz, B. Stypuła, and J. Banas (2006). Electrochem. Commun. 8, 227.
J. Subramaniam, K. Murugan, and K. Kovendan (2012). J. Biopestic. 5, 163.
J. Subramaniam and M. Murugan (2013). Evaluation of larvicidal, pupicidal, repellent, and adulticidal activity of Myristica fragrans (Family: Myristicaceae) against malarial vector Anopheles stephensi. Proceedings of the national conference on insect diversity and systematics: special emphasis on molecular approaches. pp:1–6.
J. Subramaniam and K. Murugan (2014). J. Int. J. Biol. Sci. 2014, 33.
J. Subramaniam, K. Murugan, K. Kovendan, P. M. Kumar, D. Amerasan, C. P. Selvam, T. Nataraj, D. Dinesh, B. Chandramohan, R. Chandrasekar, and J. S. Hwang (2015). Util. Manag. Med. Plants. 3, 261.
J. Subramaniam, K. Murugan, C. Panneerselvam, K. Kovendan, P. Madhiyazhagan, P. M. Kumar, D. Dinesh, B. Chandramohan, U. Suresh, M. Nicoletti, A. Higuchi, J. S. Hwang, S. Kumar, A. A. Alarfaj, M. A. Munusamy, R. H. Messing, and G. Benelli (2015). Environ. Sci. Poll. Res 22, (24), 20067.
J. Subramaniam, K. Murugan, C. Panneerselvam, K. Kovendan, P. Madhiyazhagan, D. Dinesh, P. Mahesh Kumar, B. Chandramohan, U. Suresh, R. Rajaganesh, Mohamad Saleh Alsalhi, S. Devanesan, M. Nicoletti, A. Canale, and G. Benelli (2016). Environ. Sci. Pollut. Res. doi:10.1007/s11356-015-6007-0.
U. Suresh, K. Murugan, G. Benelli, M. Nicoletti, D. R. Barnard, C. Panneerselvam, P. Mahesh Kumar, J. Subramaniam, D. Dinesh, and B. Chandramohan (2015). Parasitol. Res 114, 1551.
G. Taubes (2000). Science. 290, (5491), 434.
S. Thiripura and S. Shankar (2014). Int. J. Pharm. Tech. Res 6, 1731.
A. Voyadjoglou, V. Roussis, and P. V. Petrakis Biological control of mosquito populations: an applied aspect of pest control by means of natural enemies. in A. M. T. Elewa (ed.), Predation in Organisms: Adistinct Phenomenon (Springer, Berlin, 2007), p. 123.
W. W. Walton (2007). J. Am. Mosq. Control Assoc. 23, (S2), 184.
WHO (1981) Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides: diagnostic test. WHO/VBC/81–807, Geneva.
WHO (2015) Dengue and severe dengue. Fact sheet N°117. World Health Organization, Geneva.
WHO (2016) Weekly epidemiological record, No 7, 19 Feb 2016, Vol. 91, 73–88.
H. Yap (1985). Southeast Asian J. Trop. Med. Public Health. 16, 163.
M. Zargar, A. A. Hamid, F. A. Bakar, M. N. Shamsudin, K. Shameli, F. Jahanshiri, and F. Farahani (2011). Molecules. 6, 6667.
J. Zhu, X. Zeng, M. O. Neal, G. Schultz, B. Tucker, and J. Coats (2008). J. Am. Mosq. Control. Assoc. 24, 161.
Acknowledgements
Two anonymous reviewers kindly improved an earlier version of our manuscript. J. Subramaniam is grateful to the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), New Delhi, India for the financial support (Principal Investigator/NPDF-DST-SERB/Project File n. PDF/2015/000650).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The Authors declare no conflicts of interest.
Research Involving Human and Animal Rights
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Subramaniam, J., Murugan, K., Jebanesan, A. et al. Do Chenopodium ambrosioides-Synthesized Silver Nanoparticles Impact Oryzias melastigma Predation Against Aedes albopictus Larvae?. J Clust Sci 28, 413–436 (2017). https://doi.org/10.1007/s10876-016-1113-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1113-9