Abstract
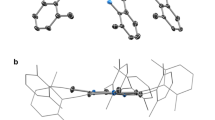
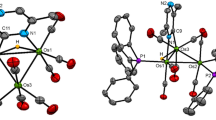
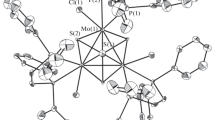
This research was an outgrowth of previous reactions with [Pd13Ni13(CO)34]4− which produced a tetragonal crystal form of Pd23(CO)20(PEt3)10 (1) that has the same cuboctahedral-based Pd23 framework with an identical number of PEt3 ligands but two fewer CO ligands than the monoclinic crystal form of Pd23(CO)22(PEt3)10 (3) originally reported from reactions with Pd10(CO)12(PEt3)6. A subsequent investigation presented herein to establish whether the carbonyl capacity is influenced by the nature of the phosphine ligands has led to syntheses of Pd23(CO) x (PR3)10 [R3=Et3 (1), Bun 3 (4), and Me2Ph (5)] with 20 CO ligands (x=20) from corresponding Pd10(CO)12(PR3)6 precursors either by deligation with Pd(OAc)2, CF3CO2H, Ni(1,5-COD)2, [NMe4]2[Ni6(CO)12], or HCO2H or by spontaneous enlargement; yields varied from 15 to 79%. Although attempts to obtain the original Pd23(CO)22(PEt3)10 (3) were unsuccessful, a highly significant outcome was the isolation (one time) of another monoclinic crystal form possessing the triethylphosphine Pd23(CO) x (PEt3)10 cluster with 21 COs (2). Both the compositions and atomic arrangements for each of five Pd23 clusters [1a (solvated); 1b (unsolvated); 2, 4, and 5] were unambiguously established from low-temperature single-crystal CCD X-ray crystallographic determinations in accordance with their nearly identical IR carbonyl frequencies. Solution 31P{1H} NMR spectra of 1 and 4 at room temperature displayed three distinct signals with expected integral ratios of 2/4/4 that are consistent with the solid-state structures of Pd23(CO)20(PR3)10 [R3=Et3 (1), Bun 3 (4)] remaining intact in solution. The metal-core geometries of all of these Pd23(CO) x (PR3)10 clusters, including the thermodynamically stable ones with 20 CO ligands and the kinetic products with additional CO ligands (x=21, 22), are essentially the same. The common Pd23 core may be best described as possessing a centered hexacapped cuboctahedral Pd19 kernel (alternatively denoted as a centered ν2 Pd19 octahedron) with four edge-connected exopolyhedral wingtip Pd(exo) atoms that reduce the pseudo metal-core symmetry from Oh to D2h. The 10 PR3 ligands are linked to the six tetracapped Pd(cap) and four edge-capped wingtip Pd(exo) atoms; the latter four Pd(exo) atoms are each composed of four trigonal-planar Pd(μ2-CO)2(PR3) units. These crystallographic results provide compelling geometrical evidence for a heretofore unknown stereochemical example involving variable carbonyl ligation (x=20, 21, 22) of a close-packed nanosized Pd n (CO) x (PR3) y cluster (in this case with identical PEt3 ligands) without significant changes being induced in either the overall metal-core architecture or steric dispositions of the same number of PR3 ligands. These experimental findings have particular relevance to the long-standing Muetterties cluster/surface science analogy in showing that the different number (as well as different modes) of carbonyl ligations observed in these large metal carbonyl clusters are directly related to pressure-induced dissociative/nondissociative migratory coverages in CO chemisorptions on metal surfaces. The observed expanded capacity of CO coordination on the same Pd23 polyhedron without notable changes in geometry is no doubt a consequence of its virtually nanosized metal-core architecture; distances between outermost centrosymmetrically related pairs of Pd(cap) and Pd(exo) atoms in the Pd23 framework are 0.8 and 0.9 nm, respectively. An electrochemical (CV) study revealed that 1 undergoes one quasi-reversible two-electron reduction to 1 2− (E1/2=−0.91 V) and two consecutive quasi-reversible one-electron oxidations to 1/1 + at E1/2=0.08 V and 1 +/1 2+ at E1/2=0.32 V (THF; Ag/AgCl as reference electrode). A stereochemical/electronic analysis with the isostructural Au2Pd21(CO)20(PEt3)10 analogue (9) and resulting implications are given.



Similar content being viewed by others
References
(a) O. A. Belyakova and Yu. L. Slovokhotov (2003). Russ. Chem. Bull. 52, 2299 (Engl. Transl.). (b) T. A. Stromnova and I. I. Moiseev (1998). Usp. Khim. 67, 542. [Russ. Chem. Rev. 1998, 67, 485 (Engl. Transl.)]. (c) A. D. Burrows and D. M. P. Mingos (1996). Coord. Chem. Rev. 154, 19. (d) K. R. Dixon and A. C. Dixon, in R. J. Puddephatt (ed.) Comprehensive Organometallic Chemistry II, Vol. 9 (Pergamon, London, 1995), p. 193. (e) A. D. Burrows and D. M. P. Mingos (1993). Transition Met. Chem. 18, 129. (f) R. B. King (1992). Gazz. Chim. Ital. 122, 383. (g) N. K. Eremenko and S. P. Gubin (1990). Pure Appl. Chem. 62, 1179. (h) N. K. Eremenko, E. G. Mednikov, and S. S. Kurasov (1985). Usp. Khim. 54, 671. [Russ. Chem. Rev. 1985, 54, 394 (Engl. Transl.)].
(a) E. G. Mednikov, N. K. Eremenko, Yu. L. Slovokhotov, and Yu. T. Struchkov (1987). Zhurnal Vsesoyuzn. Khim. Obschestva im. D.I. Mendeleeva (Rus. J. Mendeleev’s Chem. Soc.), 32, 101. (b) E. G. Mednikov (1993). Izv. Acad. Nauk. Ser. Khim. 1299. [Russ. Chem. Bull. 1993, 42, 1242 (Engl. Transl.)]. (c) E. G. Mednikov, S. A. Ivanov, J. Wittayakun, and L. F. Dahl (2003). J. Chem. Soc., Dalton Trans. 1686. (d) E. G. Mednikov, N. K. Eremenko, Yu. L. Slovokhotov, and Yu. T. Struchkov (1986). J. Organomet. Chem. 301, C35.
(a) J. Wittayakun, Ph.D. Thesis, University of Wisconsin–Madison, 2000. (b) J. Wittayakun and L. F. Dahl (2000). Unpublished research.
(a) E. G. Mednikov and N. K. Eremenko (1982). Izv. Acad. Nauk SSSR. Ser. Khim. 2540. [Bull. Acad. Sc. USSR, Div. Chem. Sc. 1982, 31, 2240 (Engl. Transl.)], and references therein. (b) D. M. P. Mingos and C. M. Hill (1995). Croat. Chim. Acta 68, 745. (c) E. G. Mednikov, Yu. L. Slovokhotov, and Yu. T. Struchkov (1991). Metalloorgan. Khim. 4, 123. [Organomet. Chem. in the USSR, 1991, 4, 65 (Engl. Transl.)]. (d) E. G. Mednikov and N. I. Kanteeva (1995). Izv. Acad. Nauk. Ser. Khim. 167. [Russ. Chem. Bull. 1995, 44, 163 (Engl. Transl.)].
N. T. Tran, D. R. Powell, and L. F. Dahl (2004). J. Chem. Soc., Dalton Trans. 209.
(a) E. G. Mednikov (1991). Metalloorgan. Khim. 4, 885. [Organomet. Chem. in the USSR, 1991, 4, 433 (Engl. Transl.)]. (b) E. G. Mednikov (1991), unpublished research.
I. I. Moiseev, T. A. Stromnova, M. N. Vargaftik, G. Ya. Mazo, L. G. Kuz’mina, and Yu. T. Struchkov (1978). J. Chem. Soc., Chem. Commun. 27.
The large independently refined least-squares site-occupancy factors α(CO)n for the positions in the front two octahedral Pd6 faces of the five carbonyl ligands corresponding to the major component of the (21 CO)-ligated 2 are 0.64 for C(21)O(21), 0.78 for C(1)O(1), 1.0 for C(2)O(2), 1.0 for C(18)O(18) and 0.64 for C(20)O(20). The small site-occupancy factors for the positions of the two observed crystal-disordered COs of the minor component [related to the corresponding CO in the major component by 1-α(CO) n ] are 0.22 for C(1A)O(1A) and 0.36 for C(20A)O(20A). The latter is in exact agreement with the independently found occupancy factor of 0.64 for C(21)O(21) ligand, which cannot sterically coexist with the triply-bridging C(20A)O(20A). The refined major occupancy factor of 1.0 for the bridging C(18)O(18) ligand in 2 is consistent with that of the corresponding C(18)O(18) ligand in 1 not being affected by the formal addition of the fifth C(21)O(21) ligand to the front octahedral Pd6 face in 2. The nonbonding separations between the C(1)O(1) and C(2)O(2) ligands are 2.91 Å for C(1)...C(2) and 2.74 Å for O(1)...O(2) in 2 and 2.89 Å for C(1)...C(2) and 2.71 Å for O(1)...O(2) in 3.
Mlynek P.D., Kawano M., Kozee M.A. and Dahl L.F. (2001). J. Cluster Sci. 12: 313
(a) E. L. Muetterties, T. N. Rhodin, E. Band, C. F. Brucker, and W. R. Pretzer (1979). Chem. Rev. 79, 91, and references therein. (b) C. M. Friend, R. M. Gavin, E. L. Muetterties, and M.-C. Tsai (1980). J. Am. Chem. Soc. 102, 1717. (c) E. Shustorovich, R. G. Baetzold, and E. L. Muetterties (1983). J. Phys. Chem. 87, 1100. (d) G. Pacchioni and J. Koutecky (1987). J. Phys. Chem. 91, 2658. (e) R. Hoffmann (1988). Rev. Mod. Phys. 60, 601. (f) J. E. Adams (1990). J. Chem. Phys. 92, 1849. (g). B. C. Gates (1993). Angew. Chem. Int. Ed. Engl. 32, 228.
(a) D. M. P. Mingos (1983). J. Chem. Soc., Chem. Commun. 706. (b) D. M. P. Mingos (1983). J. Chem. Soc., Chem. Commun. 1352. (c) D. M. P. Mingos (1984). Acc. Chem. Res. 17, 311. (d) D. M. P. Mingos (1984), Polyhedron, 3, 1289. (e) K. P. Hall and D. M. P. Mingos (1984). Progr. Inorg. Chem. 32, 237. (f) D. M. P. Mingos and R. L. Johnson (1985). J. Organomet. Chem. 281, 419. (g) D. M. P. Mingos and L. Zhenyang (1988). J. Chem. Soc., Dalton Trans. 1657 and references therein; (h) D. M. P. Mingos and A. P. May, in D. F. Shriver, H. D. Kaesz, and R. D. Adams, (eds.) The Chemistry of Metal Cluster Complexes, (VCH Publishers, New York, 1990). Chap. 2, pp. 11–119; (i) D. M. P. Mingos and D. J. Wales, Introduction to Cluster Chemistry, (Prentice Hall, Old Tappan, NJ, 1990).
(a) B. K. Teo and H. Zhang (1988). Inorg. Chim. Acta. 144, 173. (b) B. K. Teo and H. Zhang (1990). Polyhedron. 9, 1985. (c) B. K. Teo, H. Zhang, Y. Kean, H. Dang, and X. Shi (1993). J. Chem. Phys. 99, 2929. (d) B. K. Teo and N. J. Sloane (1986). Inorg. Chem. 25, 2315.
Ciani G. and Sironi A. (1980). J. Organomet. Chem. 197: 233
Kotz J.C., Nivert C.L., Lieber J.M. and Reed R.C. (1975). J. Organomet. Chem. 91: 87
de Biani F.F., Ienco A., Laschi F., Leoni P., Marchetti F., Marchetti L., Mealli C. and Zanello P. (2005). J. Am. Chem. Soc. 127: 3076
(a) This reversibility is remarkable and was observed during more than 20 full cycles without any signs of decomposition of 10 and its electrochemically generated derivatives; (b) it should be noted that majority of carbonyl/phosphine clusters of palladium and their derivatives are labile compounds, for which dilute solutions are particularly unstable. The most striking example of such kind of behavior is presented by the thallium(I) sandwich [TlPd6(CO)6L6]+ monocation (L=PEt3) [16c]. (c) E. G. Mednikov and L. F. Dahl (2003). J. Chem. Soc., Dalton Trans. 3117.
Mednikov E.G., Struchkov Yu.T. and Slovokhotov Yu. L. (1998). J. Organomet. Chem. 566: 15
T. A. Stephenson, S. M. Morehouse, A. R. Powell, J. P. Heffer, and G. Wilkinson (1965). J. Chem. Soc. 3632.
(a) G. Longoni, P. Chini, and A. Cavalieri (1976). Inorg. Chem. 15, 3025. (b) A. Ceriotti, G. Longoni, G. Piva (1989). Inorg. Synth. 26, 312.
G. Sheldrick (2000). All crystallographic software and sources of the scattering factors are contained in the SHELXTL (version 6.10, 2000) program library, Bruker Analytical X-Ray Systems, Madison, WI.
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation. The CCD area detector system was purchased, in part, from NSF Grant, CHE-9310428. The Bruker AC-300 NMR spectrometer was purchased, in part, by funds from NSF CHE-9208963 and NIH SIO RR 08389-01. We thank Dr. Sergei Ivanov (Los Alamos National Laboratory) for crystallographic assistance at the initial stage of this work and Dr. Ilia Guzei (UW–Madison) for helpful crystallographic advice. The authors are also grateful to Dr. Carlo Mealli (Istituto di Chimica dei Composti Organometallici, Firenze, Italy) for providing a preprint of the manuscript “Formation and Characterization of the Hexanuclear Platinum Cluster [Pt6(μ-PBut 2)4(CO)6](CF3SO3)2 through Structural, Electrochemical, and Computational Analyses” which in particular contains some electrochemical information relevant to this work [15]. Drawings were prepared with Crystal Maker, Interactive Crystallography. David C. Palmer (P.O. Box 183 Bicester, Oxfordshire, UK OXG TBS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to Professor Ilya I. Moiseev on the occasion of his 75th birthday in recognition of his many outstanding contributions to Cluster Chemistry and Homogeneous Catalysis. His exceptional and prolific research successfully combines the synthesis of new cluster compounds, which includes a major breakthrough into the field of giant palladium clusters, with their remarkable catalytic applications.
Rights and permissions
About this article
Cite this article
Mednikov, E.G., Wittayakun, J. & Dahl, L.F. Synthesis and Stereochemical/Electrochemical Analyses of Cuboctahedral-Based Pd23(CO) x (PR3)10 Clusters (x=20 with R3=Bun 3, Me2Ph; x=20, 21, 22 with R3=Et3): Geometrically Analogous Pd23(PEt3)10 Fragments with Variable Carbonyl Ligations and Resulting Implications. J Clust Sci 16, 429–454 (2005). https://doi.org/10.1007/s10876-005-0003-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-005-0003-3