Abstract
Animal models are needed to study and understand a human complex disease. Because of their similarities in anatomy, structure, physiology, and pathophysiology, the pig has proven its usefulness in studying human gastrointestinal diseases, such as inflammatory bowel disease, ischemia/reperfusion injury, diarrhea, and cancer. To understand the pathogenesis of these diseases, a number of experimental models generated in pigs are available, for example, through surgical manipulation, chemical induction, microbial infection, and genetic engineering. Our interests have been using amino acids as therapeutics in pig and human disease models. Amino acids not only play an important role in protein biosynthesis, but also exert significant physiological effects in regulating immunity, anti-oxidation, redox regulation, energy metabolism, signal transduction, and animal behavior. Recent studies in pigs have shown that specific dietary amino acids can improve intestinal integrity and function under normal and pathological conditions that protect the host from different diseases. In this review, we summarize several pig models in intestinal diseases and how amino acids can be used as therapeutics in treating pig and human diseases.
Similar content being viewed by others
Introduction
Rodents have been widely used as models of human nutrition, physiology and pathophysiology in health and in disease. However, in numerous cases rodents cannot accurately replicate the human conditions. Compared to rodents, the pig has a closer match of human biochemistry, cell biology, anatomy, physiology, and pathophysiology. The pig shows a high homology in DNA sequence and chromosomal structure with those of humans (Verma et al. 2011), and there are considerable anatomical and physiological similarities of the organ systems, for example, the intestine, between pigs and humans (Clouard et al. 2012; Heinritz et al. 2013). Furthermore, pigs are monogastric omnivores, and their dietary requirements and physiology in digestion and nutrient absorption are closely resembled to those of humans (Clouard et al. 2012). Moreover, pigs have an ability to ferment nutrients in the colon, and possess similar intestinal microbial ecosystem and microbiota to those of humans (Heinritz et al. 2013; Gonzalez et al. 2015). Taken together, these characteristics have made the pig an ideal model for investigating human intestinal diseases, such as inflammatory bowel disease (IBD) (Pouillart et al. 2010), ischemia/reperfusion (I/R) injury (Spanos et al. 2007), diarrhea (Kocher et al. 2014), necrotizing enterocolitis (Jiang and Sangild 2014), short bowel syndrome (Jiang and Sangild 2014; Gonzalez et al. 2015), stress-induced intestinal dysfunction (Gonzalez et al. 2015; Wu et al. 1996b), and cancer (Flisikowska et al. 2012).
In general, amino acids are absorbed and used by the host to synthesize proteins and other important substances, and are oxidized as a source of energy (Wu 2013b). Recent studies indicated that amino acids possess additional, novel functions in growth, health, and disease. For example, some amino acids can attenuate intestinal damage, maintain barrier function and intestinal integrity, restore mucosal immune homeostasis, reduce oxidative stress and inflammatory cytokine production, and increase the level of immune regulatory cytokines (Li et al. 2016; Ruth and Field 2013; Wu et al. 2015; Yi et al. 2016). Based on our and other’s findings in pig models, amino acids, such as arginine (Liu et al. 2008), glutamine (Ewaschuk et al. 2011), glycine (Wu 2015), cysteine (Song et al. 2016), N-acetylcysteine (NAC) (Hou et al. 2012; Yi et al. 2016), and proline (Kang et al. 2014), are beneficial to gut health and hold great promise in treating a wide array of gut-related disorders in both pigs and humans. In this article, we highlight several intestinal diseases, pig models, and the potential therapeutic roles of amino acids in diseases of the gut.
Intestinal diseases and models
Due to the multifactorial etiology of disease, many different experimental strategies (such as surgical manipulation, and feeding or injection with chemicals or microorganisms) have been used to induce intestinal lesions in pig models for the study of molecular changes, histopathology, mechanisms, and treatment strategies (Table 1).
IBD
IBD, a chronic, remitting and relapsing intestinal inflammatory response, harbors two diseases, ulcerative colitis and Crohn’s disease (Randhawa et al. 2014). IBD can affect the entire gastrointestinal tract and mucosal layer, which may increase the risk of colorectal cancer (CRC) (Clevers 2004; Kaser et al. 2010). Clinically, severe diarrhea, bleeding, abdominal pain, loss of fluid and electrolytes are the characteristics of IBD (Randhawa et al. 2014). Though the etiology of IBD remains undetermined, several factors, including immunologic abnormalities, loss of tolerance to commensal bacteria, disruption of mucosal barrier, and increase in inflammatory mediators and oxidative stress, are related to the pathogenesis of IBD (Goyal et al. 2014).
To date, multiple models, such as spontaneous, chemical-induced, bacteria-induced, genetically engineered, transgenic, mutation knock-in and gene knock-out have been established to study IBD and their related complications (Goyal et al. 2014). Among the various models, chemical [for example, trinitrobenzene sulfonic acid (TNBS), dextran sodium sulphate (DSS) and acetic acid]-induced and bacteria (for example, Salmonella)-induced colitis models in the pig are extensively used: (1) Crohn’s disease can be induced by rectal instillation of TNBS (15 mg/kg in 5 mL 50% ethanol solution) (Pouillart et al. 2010). The pathologic changes include extensive ulceration, inflammation, and bloody stools (Pouillart et al. 2010). (2) Ulcerative colitis can be induced by injection of DSS at 1.25 g/kg BW/day for 5 days (Kim et al. 2010) or 0.75 g/kg BW/day for 7 days (O’Shea et al. 2016). The signs of DSS-induced ulcerative colitis include severe and bloody diarrhea, elevated gut permeability and concentrations of cytokines [for example, interleukin (IL)-6 and tumor necrosis factor (TNF)-α] (Kim et al. 2010), increased proximal colon pathology score and colonic Enterobacteriaceae (O’Shea et al. 2016). The distorted crypt architecture, infiltration of inflammatory cells into the mucosa and submucosa, crypt abscesses and cryptitis are observed in the hematoxylin and eosin-stained colon sections in DSS-treated pigs (Kim et al. 2010). (3) Colitis can be induced by intrarectal administration of 10 mL of 10% acetic acid to the pig (Wang et al. 2013a). It was found that acetic acid administration caused increase in the histopathology score, intraepithelial lymphocyte number and density of colon, myeloperoxidase activity, concentrations of malondialdehyde and pro-inflammatory mediators in the plasma and colon, and reduction in goblet cell number in colonic mucosa (Wang et al. 2013a). (4) Ulcerative colitis can be induced by Salmonella typhimurium (Cho and Chae 2004). Salmonella typhimurium DT104 infection (1011 cells per animal) decreases transepithelial ion conductance, histamine flux and histamine N-methyltransferase activity, and increases diamine oxidase (DAO) activity (Aschenbach et al. 2007). The pathophysiologic processes of this disease may be association with the expression of cyclooxygenase-2 and nitric oxide synthase 2 (Cho and Chae 2004).
I/R injury
I/R injury, a crucial research field for studying small bowel transplantation, is known to cause allograft rejection, tissue injury, and organ dysfunction (Yandza et al. 2012; Lenaerts et al. 2013). I/R injury is characterized by altered permeability of vascellum and epithelium, and dramatical damage of villus (Spanos et al. 2007). The pig model of I/R injury is commonly used to capture the disease process in humans. A surgical model in pigs showed that ischemia can be established by clamping the superior mesenteric artery at its origin and is sustained for 2 h, and duration of reperfusion is 2 h after release of the clamp (Spanos et al. 2007). The related studies found that I/R induces inflammation and tissue injury by producing reactive oxygen species (ROS) and pro-inflammatory cytokines (Spanos et al. 2007). Similarly, Kostopanagiotou et al. (2011) reported that I/R affected the function and structure of small bowel transplantation and induced inflammatory cascades by the production of cytokines (e.g., TNF-α, IL-8), hyaluronic acid, and reactive nitrogen species (e.g., nitric oxide).
Diarrhea
Diarrhea is one of the most concerning and important public health problems that cause considerable morbidity and mortality among children. In particular, viral pathogens, such as norovirus and rotavirus, can cause outbreaks of gastroenteritis and diarrhea (Heinritz et al. 2013; Zhang et al. 2016). Thus, norovirus (or rotavirus)-infected pig models have been used to study the mechanisms of diarrhea, dehydration, and intestinal lesions in humans (Souza et al. 2007; Meurens et al. 2012; Kocher et al. 2014; Mao et al. 2015). For example, the gnotobiotic pig is suitable for the investigation of pathogenesis, host immunity, and vaccine development of viral diarrhea (Meurens et al. 2012; Kocher et al. 2014), as the gnotobiotic pig model is deficient in maternal antibodies and is pathogen free. Souza et al. (2007) found that the diarrhea caused by human norovirus genogroup II.4 (HS66 strain) in gnotobiotic pigs was related to systemic and intestinal antibody, antibody-secreting cells, cytokine and cytokine-secreting cells. Mao et al. (2015) reported that in the rotavirus-infected pigs, frequency of diarrhea, serum rotavirus antibody concentration, and intestinal crypt depth were increased. On the other hand, ratios of villus height and crypt depth, concentrations of mucin 1 and 2, numbers of goblet cells, and levels of phosphorylated mammalian target of rapamycin (mTOR) of intestinal mucosa were decreased (Mao et al. 2015). In another trial, the rotavirus infection also altered both gut microbial diversity and composition of the microbial community (Li et al. 2014). These may reflect the pathobiological mechanism of viral diarrhea in infected children. In addition, studies using pigs have found that not only viruses but also Escherichia coli is associated with diarrhea and the release of toxic materials to impair intestinal barrier function (Heinritz et al. 2013; Yang et al. 2014). In addition, it has been reported that pigs challenged with E. coli K88 showed intestinal barrier damage, increased urinary lactulose:mannitol ratio, plasma endotoxin concentration, and intestinal mucosal injury, such as shorter villi, deeper crypts and the decreased expression of tight junction protein zonula occludens (ZO)-1 and occludin (Yang et al. 2014). Furthermore, the early weaning of piglets provides a natural model for the occurrence of diarrhea and its prevention by dietary supplementation with glutamine (Wang et al. 2015b; Wu et al. 1996b).
Colorectal cancer (CRC)
This year over 600,000 people worldwide will die of CRC. It is the third most frequently diagnosed and third most deadly cancer in the United States. Standard treatment for CRC showed some efficacy but fall short in terms of increasing long-term survival. CRC is a malignant disease, and its etiology includes genetic background and environmental risk factors (Gao et al. 2017). Recent studies indicate that gut microbiota may be an important contributor factor in the initiation and development of CRC (Gao et al. 2017). In addition, the familial adenomatous polyposis (FAP), a genetic disorder resulting from mutations in the adenomatous polyposis coli (APC) gene, is one of the major sources of hereditary CRC (Bong et al. 2016). The vast majority of FAP patients will develop CRC if they cannot be treated at an early stage (Bong et al. 2016). FAP is classically characterized by hundreds of adenomatous polyps in the rectum and colon, which, if not removed, ultimately progress to tumor (Croner et al. 2005). The pig has its advantages in human cancer research because of its size, easy handling, and drug delivery in the same way as in human patients, and for follow-up blood and imaging work over time, as well as its genetic, biochemical, and physiological similarities to humans. In addition, high-throughput genome sequencing and a collection of precision-genetic tools combined with bioinformatics analysis, and profiling of transcriptomics/proteomics/metabolomics/secretomics/interactomics can be applied in the pig. The ability to modify pig genomes through targeted nucleases combined with the development of novel reproductive technologies including cloning allows researchers to create complex and unique models of cancer in pigs that are more applicable to human disease. Previously, Flisikowska et al. (2012) produced gene-targeted cloned pigs carrying mutations in the APC gene (APC1061 and APC1311), which are orthologous to human FAP mutations (APC1061 and APC1309), to model the symptoms of FAP patients and to develop diagnostics and therapeutics for CRC. These pigs showed classic features of aberrant crypt foci and low- and high-grade dysplastic adenomas in the large bowel (Flisikowska et al. 2012). Without a doubt, pig models in cancer help us understand the molecular bases of tumorigenesis and to develop immunotherapeutic, pharmaceutical, endoscopic, and surgical interventions.
The impact of amino acids on intestinal diseases
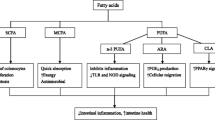
Amino acids are necessary not only for the biosynthesis of various proteins, but also for the regulation of key metabolic pathways (Hou et al. 2015a, b; Li et al. 2007). Despite these beneficial effects, studies in animals and humans with intestinal diseases have demonstrated that dietary supplementation with amino acids sustains intestinal integrity and normal immunocompetence, reduces oxidative stress, and protects the host from different diseases, thereby decreasing morbidity and mortality (Ruth and Field 2013; Li et al. 2007). In this section, we consider their roles in pathological states and display their treatment outcomes in intestinal diseases in pig models (Table 2). The possible mechanisms responsible for the beneficial effects of amino acids in intestine are shown in Fig. 1.
Possible mechanisms responsible for the beneficial effects of amino acids in intestine. Akt protein kinase B, AMPK AMP-activated protein kinase, CRH corticotropin-releasing hormone, CRHR CRH receptor, ERK extracellular signal-regulated kinase, Foxo forkhead box o, GSK glycogen-synthase kinase, MAPK mitogen-activated protein kinase, mTOR mammalian target of rapamycin, NF-κB nuclear factor-κB, NOD nucleotide-binding oligomerization domain protein, Nrf2 NF erythroid 2-related factor 2, PI3K phosphatidylinositol 3-kinase, PPAR peroxisome proliferator-activated receptor, P5C ∆1-pyrroline-5-carboxylate, ROS reactive oxygen species, TLR toll-like receptor
Arginine
Arginine is the nitrogenous precursor for synthesizing nitric oxide. Arginine activates mTOR signaling in enterocytes to promote protein synthesis and prevent lipopolysaccharide (LPS)-induced cell death (Tan et al. 2010). This amino acid also enhances angiogenesis in the small intestine to augment nutrient absorption (Yao et al. 2011), as well as immune status in early-weaned piglets (Tan et al. 2009). Interestingly, a recent finding indicated that arginine played important roles in pig nutrition partially via modulating amino acids utilization and metabolism in the small-intestinal microbiota (Dai et al. 2012). Numerous experiments have shown that arginine and nitric oxide play a modulatory role in physiology of gastrointestinal tract. We previously demonstrated that 0.5 or 1.0% arginine ameliorated the adverse effects of E. coli LPS on the pig intestine, including improving intestinal morphology (villus height and crypt depth), regulating cell proliferation and apoptosis, and also decreasing the expression of pro-inflammatory cytokines (IL-6 and TNF-α) via activating peroxisome proliferator-activated receptor γ (Liu et al. 2008). Zhu et al. (2013) reported that arginine increased the numbers of IgA-secreting cells, CD8+ and CD4+ T cells, and decreased mast cell number and lymphocyte apoptosis of Peyer’s patches in piglets challenged by LPS. In addition to reducing intestinal injury induced by LPS, supplementation with arginine has also been reported to augment intestinal protein synthesis in part by p70S6k stimulation in piglet rotavirus enteritis (Corl et al. 2008). Besides, Spanos et al. (2007) observed amelioration of intestinal I/R injury with administration of arginine. Further, studies in humans with CRC have found that oral 30 g of arginine once a day can inhibit the formation and development of colorectal tumors (Ma et al. 2007). Thus, arginine supplementation can ameliorate intestinal diseases.
Glutamate and glutamine
Glutamine and glutamate, along with aspartate, are the major energy substrates for enterocytes (Wu 1998). A number of animal studies indicated that glutamate and glutamine play versatile roles in the metabolism and function of gut. As a specific precursor for the synthesis of glutathione and other amino acids (alanine, aspartate, ornithine, and proline) (Ruth and Field 2013), glutamate shows positive effect in improving intestinal mucosa morphology (Wu et al. 2012), reducing intestinal hyperpermeability (Vermeulen et al. 2011) and enhancing mucosal barrier and anti-oxidative functions (Jiao et al. 2015). Moreover, in our previous work, dietary supplementation of 1.0 or 2.0% glutamate appears to be therapeutic in intestine during inflammatory states via (1) improving intestinal barrier function by suppression of corticotropin-releasing hormone (CRH)/CRH receptor 1 signalling pathway; (2) decreasing pro-inflammatory cytokine production by regulation of toll-like receptor (TLR4) and nucleotide-binding oligomerization domain protein (NOD) signaling pathways; (3) inhibiting protein degradation by maintenance of mTOR signaling (Ren 2015; Wang 2015). Dietary supplementation of glutamate has been reported to enhance anti-oxidative capacity in the small intestine of weanling piglets and reduce the incidence of diarrhea in these neonates (Rezaei et al. 2013a).
Glutamine exerts key role in the maintenance of intestinal structure and function, regulates amino acid utilization by intestinal bacteria, and beneficially alters endogenous gut microbiota (Dai et al. 2013; Zhang et al. 2017). Compared to glutamate, much more research on the therapeutic effect of glutamine in gastrointestinal diseases has been documented (e.g., Haynes et al. 2009; Wu et al. 1996b; Yi et al. 2015). Yi et al. (2005) reported that 2% glutamine mitigated villous atrophy, intestinal morphology impairment, and diarrhea in weaned pigs challenged with E. coli K88+. Similarly, Ewaschuk et al. (2011) found that supplementing the weaning diet of piglets with 4.4% glutamine regulated the mucosal cytokine response, and decreased damage to tight junction proteins and intestinal electrolyte movement after E. coli (K88AC or K88 wild-type) challenge. In addition, glutamine plus transforming growth factor-alpha treatment can synergistically restore mucosal architecture (e.g., recovery of villous surface area) via increasing the activity of extracellular signal-regulated kinase (ERK) in porcine ischemic-injured intestine (Blikslager et al. 1999). Glutamine also stimulates jejunal sodium and chloride absorption in pig rotavirus enteritis (Rhoads et al. 1991). Furthermore, based on the previous results in mouse model, glutamine establishes a protective role in colitis-associated CRC (Tian et al. 2016). As noted above, glutamine is recognized as an important dietary component in maintaining intestinal health.
Glycine
Glycine, the simplest amino acid, is the most abundant amino acid in the plasma of postnatal pigs (Wang et al. 2013b). This amino acid is remarkably deficient in sow’s milk (Wu and Knabe 1994) and in plant-based diets for postweaning pigs (Wu et al. 2014). There is evidence that endogenous synthesis of glycine is inadequate to support optimal intestinal health or maximum growth of the whole-body (including the small intestine) in young pigs (Wang et al. 2014a). Of note, glycine has been proved to be an anti-inflammatory, immunomodulatory, and cytoprotective agent (Zhong et al. 2003). In vitro studies using intestinal porcine epithelial cells showed that glycine inhibits oxidative stress (Wang et al. 2014b) and improves intestinal mucosal barrier by regulating the expression and distribution of claudin-7 and ZO-3 (Li et al. 2016). In recent years, mounting evidence has documented the protective effect of glycine in intestinal diseases in animals and humans. We reported that supplementing the weaning diet of piglets with 1.0 or 2.0% glycine was beneficial in attenuating LPS-induced protein degradation [by regulation of AMP-activated protein kinase (AMPK) and mTOR signaling] and inflammatory response (by regulation of TLR4 and NOD signaling) (Wu 2015). During LPS-induced sepsis, less intestinal hemorrhage is observed in the rats supplemented with glycine (Effenberger-Neidnicht et al. 2014). Glycine appears to exert great protective effects in preventing I/R injury to intestine, which is clearly suggested by a large body of researches with experimental animals (Zhong et al. 2003; Petrat et al. 2012). Moreover, dietary glycine prevents hypoxia–reoxygenation-induced necrotizing enterocolitis (Meyer et al. 2006) and chemical-induced colitis (Tsune et al. 2003) in rats. However, the research is mainly focused on the rat models, evidence from the pig models is limited. Nevertheless, based on the reported findings, dietary glycine supplementation may provide an effective strategy in keeping intestinal health.
Proline
Proline is an indispensable amino acid in young mammals, due to a limited ability to synthesize proline from glutamine, glutamate or arginine in the small intestine of young pigs (Wu et al. 1994, 1996a). Proline is a major precursor for the synthesis of polyamines (Wu et al. 2000a, b) and arginine (Wu 1997) in enterocytes of pigs to support intestinal cell growth and migration. It has been well demonstrated that dietary proline supplementation plays an important role in the gut of the weaned piglets regulating cell differentiation and de novo synthesis of arginine and polyamines (Wu et al. 2011). Recently, we showed that proline supplementation can increase immunostimulatory effects on inactivated Pasteurella multocida vaccine-immunized mice (Ren et al. 2013) and improve growth performance, increase superoxide dismutase activities, and has a positive effect on the gastrointestinal tract digestibility in early-weaned pigs (Kang et al. 2014). In addition, the villus height, percentage of proliferating cell nuclear antigen-positive cells, alkaline phosphatase activity, the protein expressions of tight junction proteins (ZO-1, occludin and claudin-3) and voltage-gated K+ channel (Kv) 1.1 protein are increased in the intestine of proline-treated piglets (Wang et al. 2015c). Metabolism of proline in mammals involves four other amino acids, glutamate, glutamine, ornithine, and arginine, and seven proximal enzymatic activities, ∆1-pyrroline-5-carboxylate (P5C), reductase, proline oxidase/proline dehydrogenase, P5C dehydrogenase, P5C synthase, glutamine synthetase, glutaminase, and ornithine-δ-aminotransferase (OAT) (Hu et al. 2008; Hu and Hou 2014). With the exception of OAT, which catalyzes a reversible reaction, the other four enzymes are unidirectional (Hu et al. 2008; Hu and Hou 2014). In addition, proline metabolism also links with three other pivotal metabolic systems, namely the TCA cycle, the urea cycle, and the pentose phosphate pathway (Hu et al. 2008; Hu and Hou 2014). Thus, proline metabolism involves in NADP+, NAD+, ROS, and ATP production, redox balance, and ammonia detoxification in intestinal epithelial cells (Hu et al. 2008; Hu and Hou 2014; Phang et al. 2015; Wu 1998).
Sulfur-containing amino acids
Methionine and cysteine are involved in the biosynthesis of proteins of the immune system (Li et al. 2007). A sufficient intake of dietary methionine is important for mucosal integrity (Chen et al. 2014), morphological development (Shen et al. 2014; Zhong et al. 2016), and intestinal antioxidant capacity (Shen et al. 2014; Zhong et al. 2016). S-Adenosylmethionine, the activated form of methionine, participates in plenty of essential metabolic processes, such as the methylation of DNA and proteins and the synthesis of spermidine and spermine (Li et al. 2007). In addition, dietary methionine intake may be related to reduce the risk of CRC (de Vogel et al. 2008; Zhou et al. 2013). Moreover, Tang et al. (2015) reported that methionine deficiency inhibited autophagic response and accelerated death in IPEC-1 cells infected with enterotoxigenic E. coli. However, in the methionine restriction experiments in rats, reduction in dietary intake of methionine results in improved colon tight junction barrier function (Ramalingam et al. 2010) and inhibited colon carcinogenesis (Komninou et al. 2006).
Cysteine is important for normal intestinal function, including the immune surveillance of the intestinal epithelial layer and regulation of the mucosal response to foreign antigens (Fang et al. 2010). Cysteine also involves in the biosynthesis of glutathione and taurine, both of which possess potent anti-oxidative activity and can suppress oxidative stress during IBD (Kim et al. 2009). Kim et al. (2009) reported that cysteine supplementation (0.144 g/kg BW/day) improved intestinal permeability, local chemokine expression, neutrophil influx and colon histology, and regulated the expression of pro-inflammatory cytokines, apoptosis initiator, and pro-survival genes in a porcine model of colitis, supporting the importance of cysteine in attenuating local inflammation and restoring gut homeostasis. Moreover, cysteine in diet (0.25 or 0.5%) protected intestinal integrity, as demonstrated by increased proliferating cell nuclear antigen, occludin and claudin-1 expression, and down-regulated caspase-3 activity in weaned piglets after LPS challenge (Song et al. 2016). This beneficial effect of cysteine is resulted from its anti-inflammation, anti-oxidation, and regulatory effect on suppressing nuclear factor-κB (p65) nuclear translocation and enhancing NF erythroid 2-related factor 2 translocation (Song et al. 2016). However, excessive cysteine (5–10 mmol/L) may induce vacuole-like cell death by activating endoplasmic reticulum stress and mitogen-activated protein kinase signaling in intestinal porcine epithelial cells (Ji et al. 2016).
Because cysteine is detrimental at a high concentration, NAC (the precursor of cysteine) is commonly used to deliver cysteine. NAC can be rapidly metabolized by the small intestine and restore intestinal function (Wang et al. 2013a). Xu et al. (2014) reported that NAC can improve intestinal bacteria in piglets by enhancing Lactobacillus and Bifidobacterium counts and reducing E. coli counts. In the acetic acid-induced colitis model, dietary supplementation with 500 mg/kg NAC regulates anti-oxidative responses, apoptosis, and epidermal growth factor expression in colonic mucosa, and thus partially ameliorates the adverse effects of acetic acid in pigs (Wang et al. 2013a). In addition, NAC preconditioning also attenuates ischemia–reperfusion injury in piglet small bowel transplantation (Kostopanagiotou et al. 2011). Moreover, results from recent studies indicated that NAC alleviates LPS-induced intestinal alterations, such as DAO activity (a marker of intestinal injury), d-xylose concentration (a marker of intestinal absorption) in the circulation, levels of tight junction proteins, and ratios of villus height to crypt depth, RNA/DNA and protein/DNA (Hou et al. 2012; Yi et al. 2017). Further examination revealed that NAC attenuates LPS-induced intestinal inflammation through multiple signaling pathways, such as redox, epidermal growth factor, TLR4, PI3K/Akt/mTOR, and AMPK signaling (Hou et al. 2013; Yi et al. 2017).
Threonine
Threonine is of great importance in intestinal health needed especially for synthesis of mucin (Mao et al. 2011). Increasing dietary threonine intake can increase serum IgG concentration and promote a healthy microbiota (Trevisi et al. 2015; Wang et al. 2006). Trevisi et al. (2015) reported that the diet with 9.0 g threonine/kg reduced E. coli counts in feces of pigs and led to favorable impact on average daily feed intake in the first week after weaning, as compared to 8.5 g threonine/kg diet. However, either an excess or a deficiency of dietary threonine is deleterious to the intestinal mucosal integrity and barrier function (Wang et al. 2010). Some of the negative consequences include villus atrophy, increased apoptosis, and decreased mucin concentration (Wang et al. 2010). Consistent with these, Hamard et al. (2010) reported that paracellular permeability and the expression of genes associated with immune and inflammatory responses (e.g., the complement C1s subcomponent, the MHC class I antigen, the T cell differentiation antigen CD6, the C–C motif chemokine 16, and chemokine receptors) were increased in piglets given to a 30% reduced threonine diet for 2 weeks. Further, threonine requirement may be increased under pathological conditions, such as ileitis and sepsis (Mao et al. 2011). Baird et al. (2013) showed that threonine increased heat-shock protein expression and decreased apoptosis in heat-stressed intestinal epithelial cells. Wang (2006) also reported that dietary threonine increased concentrations of IgG and IgA in jejunal mucosa and improved intestinal morphological features in piglets after E. coli K88+ challenge. Interestingly, however, very little research has been done to investigate the therapeutic potential of threonine on intestinal diseases.
Tryptophan
As the precursor of multiple bioactive compounds (e.g., kynurenine, serotonin, melatonin, and picolinic acid) (Wu 2013b), tryptophan is important to regulate physiological function in the intestine, such as intestinal permeability, motility, and secretion (Wang et al. 2015a; Tossou et al. 2016). Dietary tryptophan also has a role in microbiota diversity in the gut of pigs (Messori et al. 2013). Kim et al. (2010) showed that tryptophan (0.115 g/kg BW day) given to pigs after DSS-induced colitis improved colitis symptoms and histological parameters. Furthermore, the expression of the pro-inflammatory cytokines (for example, TNF-α, IL-6, interferon-γ, IL-12p40, IL-1β, and IL-17) and intracellular adhesion molecule-1 was reduced, and the expression of apoptosis initiators (caspase-8 and Bax) was increased in tryptophan-supplemented pigs (Kim et al. 2010). Moreover, Trevisi et al. (2009) found that a tryptophan-enriched diet (1 g of tryptophan/kg to the basal diet) was beneficial in attenuating the changes of feed intake and growth performance in susceptible weaned piglets orally challenged with E. coli K88. However, Koopmans et al. (2012) compared the basal diet group (apparent ileal digestible tryptophan = 1.9 g/kg) with a tryptophan-enriched basal diet group (+5 g of free tryptophan/kg), and found that there was limited effect of surplus dietary tryptophan on stress and immunology in a pig model of systemic endotoxemia. Nevertheless, a recent study conducted by Tossou et al. (2016) concluded that dietary tryptophan at a high level (0.75%) could negatively influence intestinal epithelial morphology and tight junction proteins. Taken together, the efficacy and functions of tryptophan on intestinal diseases are controversial, and thus further research is warranted to determine the dosage and molecular bases of tryptophan functioning in human and animal health and disease.
Aspartate and asparagine
Like glutamine and glutamate, aspartate and asparagine are abundant in sow’s milk (Rezaei et al. 2016). Aspartate is also one of the major metabolic fuels in mammalian enterocytes and metabolize through mitochondrial oxidation (Wu 2013a). Asparagine, with a similar chemical structure to glutamine, can stimulate cell proliferation in intestinal epithelial cells via increasing ornithine decarboxylase activity and cellular polyamine levels. Both aspartate and asparagine contribute to mounting a successful immune response and attenuating intestinal injury (Li et al. 2007; Pi et al. 2014; Wang et al. 2015d, 2016; Chen et al. 2016). However, the evident from pig is limiting. We recently demonstrated that a supplementation of aspartate or asparagine (0.5 or 1.0%) improved intestinal morphological features, development, digestion, and barrier function under pathological conditions (Pi et al. 2014; Wang et al. 2015d, 2016; Chen et al. 2016). Our previous research showed that aspartate or asparagine supplementation improved intestinal mucosal energy status and enhanced activities of tricarboxylic acid cycle key enzymes through inhibiting the AMPK signaling pathway in weaned piglets challenged with LPS (Pi et al. 2014; Wang et al. 2015d). We also found that these beneficial effects are associated with the decrease of intestinal pro-inflammatory cytokine (via TLR4, NODs, and p38 pathways) and of enterocyte apoptosis (via p38 and ERK 1/2 pathways) (Chen et al. 2016; Wang et al. 2016).
Branched-chain amino acids (BCAA)
BCAA, including valine, leucine, and isoleucine, are essential amino acids and important regulators of protein metabolism and autophagy (Rezaei et al. 2013b). The growth performance, intestinal development, and expression of amino acid transporters in weaned piglets are elevated by BCAA supplementation to a low-protein diet (17.1% crude protein) (Zhang et al. 2013). In addition, feeding a diet with BCAA is found to enhance intestinal immune defense system via the improvement of morphological integrity and of immunoglobulin production in the intestine (Ren et al. 2015). Furthermore, dietary leucine supplementation promotes intestinal development in young pigs (Sun et al. 2015). Some studies indicate that isoleucine induces the expressions of β-defensins in human (Konno et al. 2012) and porcine (Mao et al. 2013) intestinal epithelial cells, which are essential for the mammalian innate immunity. Moreover, BCAA possess therapeutic effects on diseases. For example, Alam et al. (2011) reported that adding isoleucine in oral rehydration salts solution showed some beneficial effects on decreasing stool output of non-cholera acute watery diarrhea in children. Other studies in pigs have also shown that 1% leucine supplementation attenuated the effects of porcine rotavirus infusion on feed efficiency, diarrhea, mucin production, and goblet cell numbers in the jejunal mucosa (Mao et al. 2015). Of note, these amino acids can activate some signaling pathways in intestinal cells. Taking leucine as an example, researches in pigs and humans have demonstrated that leucine can reduce mucosal proteasome activity (Coëffier et al. 2011), enhance cell proliferation via phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/glycogen-synthase kinase-3α/β-catenin pathway (Coëffier et al. 2011), and up-regulate amino acid transporter expression by PI3K/Akt/mTOR and ERK signaling pathways (Zhang et al. 2014) in the intestine. Thus, BACC play an important role in intestinal growth, integrity, and function.
Other amino acids
Lysine, histidine, phenylalanine, tyrosine, serine, and alanine are required for protein synthesis and important for immune function (Li et al. 2007). These amino acids have also been demonstrated to exert beneficial effects in intestine. Studies in pigs have shown that lysine in the diet influences apparent nutrient digestibility and expression of cationic amino acid transporter in the small intestine (Wang et al. 2012). Peterson et al. (1998) reported that histidine protected the mouse intestinal tissue from Salmonella-induced injury. In addition, Dietary supply with specific amino acids (containing threonine, serine, proline, and cysteine) can promote mucin synthesis and improve the gut microbiota in DSS-treated rats (Faure et al. 2006). However, the effects of these amino acids on intestinal disease are still rarely studied compared to other amino acids. Thus, future studies including the information about the molecular mechanisms that regulate the actions of amino acids on intestine are needed.
Conclusions
Amino acids serve as the building blocks of protein and also regulate metabolic pathways to improve the survival, growth, and development of amino acids (Hou et al. 2015b). They are called functional amino acids (Wu 2010). Emerging evidence shows that pigs fed conventional diets cannot synthesize many amino acids that are required for optimal intestinal health and growth (Hou et al. 2016). Aside from the importance of generating pig models for the understanding and treating pig diseases, considering the similarities in anatomy, nutritional needs, immunology and physiology of the gut, the pig offers an attractive model to exploit the mechanisms of human intestinal diseases. In addition, the size and ease in handling piglets allows for drugs to be administered in the same way as in human patients and for follow-up blood work over time. The pig is also ideal for screening and developing new therapeutics. Furthermore, with the new development and insights into nutritional research, amino acids show great promises in maintaining or improving intestinal integrity and functions under pathological events. Recent studies in pigs indicate that specific dietary amino acids, in particular, arginine, glutamine, glycine, cysteine, NAC, and proline can regulate the intestinal microbial milieu and host immune system by fine-tuning inflammatory cytokine secretion and the redox status of intestinal cells, and thus exert protective effects on cells. Of note, arginine, glutamine, glycine, and proline can be synthesized de novo in pigs via interorgan metabolism of amino acids (Wu 2013b). Although they were traditionally considered as “nutritionally nonessential amino acids”, this term has now been recognized as a misnomer in nutritional sciences (Hou and Wu 2017). Supplementation with peptides (Hou et al. 2017) or crystalline amino acids (Wu 2013b) is effective in improving intestinal health and alleviating intestinal dysfunction under diseased conditions. More research endeavors are warranted in terms of using functional amino acids to their full potentials in improving health and treating diseases in humans and animals.
Abbreviations
- Akt:
-
Protein kinase B
- AMPK:
-
AMP-activated protein kinase
- APC:
-
Adenomatous polyposis coli
- BCAA:
-
Branched-chain amino acids
- CRC:
-
Colorectal cancer
- CRH:
-
Corticotropin-releasing hormone
- DAO:
-
Diamine oxidase
- DSS:
-
Dextran sodium sulphate
- ERK:
-
Extracellular signal-regulated kinase
- FAP:
-
Familial adenomatous polyposis
- I/R:
-
Ischemia/reperfusion
- IBD:
-
Inflammatory bowel disease
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- mTOR:
-
Mammalian target of rapamycin
- NAC:
-
N-Acetylcysteine
- NOD:
-
Nucleotide-binding oligomerization domain protein
- OAT:
-
Ornithine-δ-aminotransferase
- PI3K:
-
Phosphatidylinositol 3-kinase
- P5C:
-
∆1-Pyrroline-5-carboxylate
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptor
- TNBS:
-
Trinitrobenzene sulfonic acid
- TNF:
-
Tumor necrosis factor
- ZO:
-
Zonula occludens
References
Alam NH, Raqib R, Ashraf H, Qadri F, Ahmed S, Zasloff M, Agerberth B, Salam MA, Gyr N, Meier R (2011) l-Isoleucine-supplemented oral rehydration solution in the treatment of acute diarrhoea in children: a randomized controlled trial. J Health Popul Nutr 29:183–190
Aschenbach JR, Ahrens F, Schwelberger HG, Fürll B, Roesler U, Hensel A, Gäbel G (2007) Functional characteristics of the porcine colonic epithelium following transportation stress and Salmonella infection. Scand J Gastroenterol 42:708–716
Baird CH, Niederlechner S, Beck R, Kallweit AR, Wischmeyer PE (2013) l-Threonine induces heat shock protein expression and decreases apoptosis in heat-stressed intestinal epithelial cells. Nutrition 29:1404–1411
Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA (1999) Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery 125:186–194
Bong YS, Assefnia S, Tuohy T, Neklason DW, Burt RW, Ahn J, Jiang HJ, Byers SW (2016) A role for the vitamin D pathway in non-intestinal lesions in genetic and carcinogen models of colorectal cancer and in familial adenomatous polyposis. Oncotarget 7:80508–80520
Chen Y, Li D, Dai Z, Piao X, Wu Z, Wang B, Zhu Y, Zeng Z (2014) l-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46:1131–1142
Chen S, Liu Y, Wang X, Wang H, Li S, Shi H, Zhu H, Zhang J, Pi D, Hu CA, Lin X, Odle J (2016) Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun 22:577–587
Cho WS, Chae C (2004) Expression of cyclooxygenase-2 and nitric oxide synthase 2 in swine ulcerative colitis caused by Salmonella typhimurium. Vet Pathol 41:419–423
Clevers H (2004) At the crossroads of inflammation and cancer. Cell 118:671–674
Clouard C, Meunier-Salaün MC, Val-Laillet D (2012) Food preferences and aversions in human health and nutrition: how can pigs help the biomedical research? Animal 6:118–136
Coëffier M, Claeyssens S, Bensifi M, Lecleire S, Boukhettala N, Maurer B, Donnadieu N, Lavoinne A, Cailleux AF, Déchelotte P (2011) Influence of leucine on protein metabolism, phosphokinase expression, and cell proliferation in human duodenum. Am J Clin Nutr 93:1255–1262
Corl BA, Odle J, Niu X, Moeser AJ, Gatlin LA, Phillips OT, Blikslager AT, Rhoads JM (2008) Arginine activates intestinal p70(S6k) and protein synthesis in piglet rotavirus enteritis. J Nutr 138:24–29
Croner RS, Brueckl WM, Reingruber B, Hohenberger W, Guenther K (2005) Age and manifestation related symptoms in familial adenomatous polyposis. BMC Cancer 5:24
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY (2012) Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY (2013) l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 45:501–512
de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP (2008) Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr 138:2372–2378
Effenberger-Neidnicht K, Jägers J, Verhaegh R, de Groot H (2014) Glycine selectively reduces intestinal injury during endotoxemia. J Surg Res 192:592–598
Ewaschuk JB, Murdoch GK, Johnson IR, Madsen KL, Field CJ (2011) Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection. Br J Nutr 106:870–877
Fang Z, Yao K, Zhang X, Zhao S, Sun Z, Tian G, Yu B, Lin Y, Zhu B, Jia G, Zhang K, Chen D, Wu D (2010) Nutrition and health relevant regulation of intestinal sulfur amino acid metabolism. Amino Acids 39:633–640
Faure M, Mettraux C, Moennoz D, Godin JP, Vuichoud J, Rochat F, Breuillé D, Obled C, Corthésy-Theulaz I (2006) Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr 136:1558–1564
Flisikowska T, Merkl C, Landmann M, Eser S, Rezaei N, Cui X, Kurome M, Zakhartchenko V, Kessler B, Wieland H, Rottmann O, Schmid RM, Schneider G, Kind A, Wolf E, Saur D, Schnieke A (2012) A porcine model of familial adenomatous polyposis. Gastroenterology 143:1173–1175
Gao R, Gao Z, Huang L, Qin H (2017) Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. doi:10.1007/s10096-016-2881-8
Gonzalez LM, Moeser AJ, Blikslager AT (2015) Porcine models of digestive disease: the future of large animal translational research. Transl Res 166:12–27
Goyal N, Rana A, Ahlawat A, Bijjem KR, Kumar P (2014) Animal models of inflammatory bowel disease: a review. Inflammopharmacology 22:219–233
Hamard A, Mazurais D, Boudry G, Le Huërou-Luron I, Sève B, Le Floc’h N (2010) A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J Nutr Biochem 21:914–921
Haynes TE, Li P, Li X, Shimotori K, Sato H, Flynn NE, Wang J, Knabe DA, Wu G (2009) l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Heinritz SN, Mosenthin R, Weiss E (2013) Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr Res Rev 26:191–209
Hou Y, Wu G (2017) Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 8:137–139
Hou Y, Wang L, Zhang W, Yang Z, Ding B, Zhu H, Liu Y, Qiu Y, Yin Y, Wu G (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Hou Y, Wang L, Yi D, Ding B, Yang Z, Li J, Chen X, Qiu Y, Wu G (2013) N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Hou Y, Wang L, Yi D, Wu G (2015a) N-acetylcysteine and intestinal health: a focus on its mechanism of action. Front Biosci (Landmark Ed) 20:872–891
Hou Y, Yin Y, Wu G (2015b) Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp Biol Med (Maywood) 240:997–1007
Hou Y, Yao K, Yin Y, Wu G (2016) Endogenous synthesis of amino acids limits growth, lactation, and reproduction in animals. Adv Nutr 7:331–342
Hou Y, Wu Z, Dai Z, Wang G, Wu G (2017) Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol 8:24
Hu CA, Hou Y (2014) Mammalian P5CR and P5CDH: protein structure and disease association. SOJ Biochem 1:4
Hu CA, Bart Williams D, Zhaorigetu S, Khalil S, Wan G, Valle D (2008) Functional genomics and SNP analysis of human genes encoding proline metabolic enzymes. Amino Acids 35:655–664
Ji Y, Wu Z, Dai Z, Sun K, Zhang Q, Wu G (2016) Excessive l-cysteine induces vacuole-like cell death by activating endoplasmic reticulum stress and mitogen-activated protein kinase signaling in intestinal porcine epithelial cells. Amino Acids 48:149–156
Jiang P, Sangild PT (2014) Intestinal proteomics in pig models of necrotising enterocolitis, short bowel syndrome and intrauterine growth restriction. Proteom Clin Appl 8:700–714
Jiao N, Wu Z, Ji Y, Wang B, Dai Z, Wu G (2015) l-Glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J Nutr 145:2258–2264
Kang P, Zhang L, Hou Y, Ding B, Yi D, Wang L, Zhu H, Liu Y, Yin Y, Wu G (2014) Effects of l-proline on the growth performance, and blood parameters in weaned lipopolysaccharide (LPS)-challenged pigs. Asian Australas J Anim Sci 27:1150–1156
Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621
Kim CJ, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y (2009) l-Cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim Biophys Acta 1790:1161–1169
Kim CJ, Kovacs-Nolan JA, Yang C, Archbold T, Fan MZ, Mine Y (2010) l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem 21:468–475
Kocher J, Bui T, Giri-Rachman E, Wen K, Li G, Yang X, Liu F, Tan M, Xia M, Zhong W, Jiang X, Yuan L (2014) Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs. J Virol 88:9728–9743
Komninou D, Leutzinger Y, Reddy BS, Richie JP Jr (2006) Methionine restriction inhibits colon carcinogenesis. Nutr Cancer 54:202–208
Konno Y, Ashida T, Inaba Y, Ito T, Tanabe H, Maemoto A, Ayabe T, Mizukami Y, Fujiya M, Kohgo Y (2012) Isoleucine, an essential amino acid, induces the expression of human β defensin 2 through the activation of the G-protein coupled receptor-ERK pathway in the intestinal epithelia. Food Nutr Sci 3:548–555
Koopmans SJ, van der Staay FJ, Le Floc’h N, Dekker R, van Diepen JT, Jansman AJ (2012) Effects of surplus dietary l-tryptophan on stress, immunology, behavior, and nitrogen retention in endotoxemic pigs. J Anim Sci 90:241–251
Kostopanagiotou G, Avgerinos ED, Markidou E, Voiniadis P, Chondros C, Theodoraki K, Smyrniotis V, Arkadopoulos N (2011) Protective effect of NAC preconditioning against ischemia–reperfusion injury in piglet small bowel transplantation: effects on plasma TNF, IL-8, hyaluronic acid, and NO. J Surg Res 168:301–305
Lenaerts K, Ceulemans LJ, Hundscheid IH, Grootjans J, Dejong CH, Olde Damink SW (2013) New insights in intestinal ischemia–reperfusion injury: implications for intestinal transplantation. Curr Opin Organ Transplant 18:298–303
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li M, Monaco MH, Wang M, Comstock SS, Kuhlenschmidt TB, Fahey GC Jr, Miller MJ, Kuhlenschmidt MS, Donovan SM (2014) Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J 8:1609–1620
Li W, Sun K, Ji Y, Wu Z, Wang W, Dai Z, Wu G (2016) Glycine regulates expression and distribution of claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. J Nutr 146:964–969
Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, Yin Y, Yi G, Shi J, Fan W (2008) Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr 100:552–560
Ma Q, Wang Y, Gao X, Ma Z, Song Z (2007) l-Arginine reduces cell proliferation and ornithine decarboxylase activity in patients with colorectal adenoma and adenocarcinoma. Clin Cancer Res 13:7407–7412
Mao X, Zeng X, Qiao S, Wu G, Li D (2011) Specific roles of threonine in intestinal mucosal integrity and barrier function. Front Biosci (Elite Ed) 3:1192–1200
Mao X, Qi S, Yu B, He J, Yu J, Chen D (2013) Zn(2+) and l-isoleucine induce the expressions of porcine β-defensins in IPEC-J2 cells. Mol Biol Rep 40:1547–1552
Mao X, Liu M, Tang J, Chen H, Chen D, Yu B, He J, Yu J, Zheng P (2015) Dietary leucine supplementation improves the mucin production in the jejunal mucosa of the weaned pigs challenged by porcine rotavirus. PLoS One 10:e0137380
Messori S, Trevisi P, Simongiovanni A, Priori D, Bosi P (2013) Effect of susceptibility to enterotoxigenic Escherichia coli F4 and of dietary tryptophan on gut microbiota diversity observed in healthy young pigs. Vet Microbiol 162:173–179
Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V (2012) The pig: a model for human infectious diseases. Trends Microbiol 20:50–57
Meyer KF, Martins JL, de Freitas Filho LG, Oliva ML, Patrício FR, Macedo M, Wang L (2006) Glycine reduces tissue lipid peroxidation in hypoxia-reoxygenation-induced necrotizing enterocolitis in rats. Acta Cir Bras 21:161–167
O’Shea CJ, O’Doherty JV, Callanan JJ, Doyle D, Thornton K, Sweeney T (2016) The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J Nutr Sci 5:e15
Peterson JW, Boldogh I, Popov VL, Saini SS, Chopra AK (1998) Anti-inflammatory and antisecretory potential of histidine in Salmonella-challenged mouse small intestine. Lab Invest 78:523–534
Petrat F, Boengler K, Schulz R, de Groot H (2012) Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischemia–reperfusion injury: current knowledge. Br J Pharmacol 165:2059–2072
Phang JM, Liu W, Hancock CN, Fischer JW (2015) Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care 18:71–77
Pi D, Liu Y, Shi H, Li S, Odle J, Lin X, Zhu H, Chen F, Hou Y, Leng W (2014) Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem 25:456–462
Pouillart PR, Dépeint F, Abdelnour A, Deremaux L, Vincent O, Mazière JC, Madec JY, Chatelain D, Younes H, Wils D, Saniez MH, Dupas JL (2010) Nutriose, a prebiotic low-digestible carbohydrate, stimulates gut mucosal immunity and prevents TNBS-induced colitis in piglets. Inflamm Bowel Dis 16:783–794
Ramalingam A, Wang X, Gabello M, Valenzano MC, Soler AP, Ko A, Morin PJ, Mullin JM (2010) Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern. Am J Physiol Cell Physiol 299:C1028–C1035
Randhawa PK, Singh K, Singh N, Jaggi AS (2014) A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18:279–288
Ren XR (2015) Regulatory effect of glutamic acid or glycine on intestinal mucosal immune barrier injury in piglets after lipopolysaccharide challenge. Dissertation, Wuhan Polytechnic University
Ren W, Zou L, Ruan Z, Li N, Wang Y, Peng Y, Liu G, Yin Y, Li T, Hou Y, Wu G (2013) Dietary l-proline supplementation confers immunostimulatory effects on inactivated Pasteurella multocida vaccine immunized mice. Amino Acids 45:555–561
Ren M, Zhang SH, Zeng XF, Liu H, Qiao SY (2015) Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian Australas J Anim Sci 28:1742–1750
Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G (2013a) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rezaei R, Wang W, Wu Z, Dai Z, Wang J, Wu G (2013b) Biochemical and physiological bases for utilization of dietary amino acids by young Pigs. J Anim Sci Biotechnol 4:7
Rezaei R, Wu Z, Hou Y, Bazer FW, Wu G (2016) Amino acids and mammary gland development: nutritional implications for milk production and neonatal growth. J Anim Sci Biotechnol 7:20
Rhoads JM, Keku EO, Quinn J, Woosely J, Lecce JG (1991) l-Glutamine stimulates jejunal sodium and chloride absorption in pig rotavirus enteritis. Gastroenterology 100:683–691
Ruth MR, Field CJ (2013) The immune modifying effects of amino acids on gut-associated lymphoid tissue. J Anim Sci Biotechnol 4:27
Shen YB, Weaver AC, Kim SW (2014) Effect of feed grade l-methionine on growth performance and gut health in nursery pigs compared with conventional dl-methionine. J Anim Sci 92:5530–5539
Song Zh, Tong G, Xiao K, le Jiao F, Ke Yl HuCh (2016) l-Cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun 22:152–161
Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ (2007) Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain). J Virol 81:9183–9192
Spanos CP, Papaconstantinou P, Spanos P, Karamouzis M, Lekkas G, Papaconstantinou C (2007) The effect of l-arginine and aprotinin on intestinal ischemia–reperfusion injury. J Gastrointest Surg 11:247–255
Sun Y, Wu Z, Li W, Zhang C, Sun K, Ji Y, Wang B, Jiao N, He B, Wang W, Dai Z, Wu G (2015) Dietary l-leucine supplementation enhances intestinal development in suckling piglets. Amino Acids 47:1517–1525
Tan B, Li XG, Kong X, Huang R, Ruan Z, Yao K, Deng Z, Xie M, Shinzato I, Yin Y, Wu G (2009) Dietary l-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 37:323–331
Tan B, Yin Y, Kong X, Li P, Li X, Gao H, Li X, Huang R, Wu G (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Tang Y, Tan B, Xiong X, Li F, Ren W, Kong X, Qiu W, Hardwidge PR, Yin Y (2015) Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids 47:2199–2204
Tian Y, Wang K, Fan Y, Wang Y, Sun L, Wang L, Wang J, Wang Z, Li J, Ye Y, Ji G (2016) Chemopreventive effect of dietary glutamine on colitis-associated colorectal cancer is associated with modulation of the DEPTOR/mTOR signaling pathway. Nutrients 8:261
Tossou MC, Liu H, Bai M, Chen S, Cai Y, Duraipandiyan V, Liu H, Adebowale TO, Al-Dhabi NA, Long L, Tarique H, Oso AO, Liu G, Yin Y (2016) Effect of high dietary tryptophan on intestinal morphology and tight junction protein of weaned pig. Biomed Res Int 2016:2912418
Trevisi P, Melchior D, Mazzoni M, Casini L, De Filippi S, Minieri L, Lalatta-Costerbosa G, Bosi P (2009) A tryptophan-enriched diet improves feed intake and growth performance of susceptible weanling pigs orally challenged with Escherichia coli K88. J Anim Sci 87:148–156
Trevisi P, Corrent E, Mazzoni M, Messori S, Priori D, Gherpelli Y, Simongiovanni A, Bosi P (2015) Effect of added dietary threonine on growth performance, health, immunity and gastrointestinal function of weaning pigs with differing genetic susceptibility to Escherichia coli infection and challenged with E. coli K88ac. J Anim Physiol Anim Nutr (Berl) 99:511–520
Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N (2003) Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology 125:775–785
Verma N, Rettenmeier AW, Schmitz-Spanke S (2011) Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 11:776–793
Vermeulen MA, de Jong J, Vaessen MJ, van Leeuwen PA, Houdijk AP (2011) Glutamate reduces experimental intestinal hyperpermeability and facilitates glutamine support of gut integrity. World J Gastroenterol 17:1569–1573
Wang X (2006) The effects of threonine on protein synthesis and immune function in intestinal mucosal of weaned piglets. Dissertation, China Agricultural University
Wang XY (2015) Regulative effect of glutamate on intestinal injury and muscle protein synthesis and degradation of piglets after lipopolysaccharide challenge. Dissertation, Wuhan Polytechnic University
Wang X, Qiao SY, Liu M, Ma YX (2006) Effects of graded levels of true ileal digestible threonine on performance, serum parameters and immune function of 10–25 kg pigs. Anim Feed Sci Technol 129:264–278
Wang W, Zeng X, Mao X, Wu G, Qiao S (2010) Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J Nutr 140:981–986
Wang XQ, Zeng PL, Feng Y, Zhang CM, Yang JP, Shu G, Jiang QY (2012) Effects of dietary lysine levels on apparent nutrient digestibility and cationic amino acid transporter mRNA abundance in the small intestine of finishing pigs, Sus scrofa. Anim Sci J 83:148–155
Wang Q, Hou Y, Yi D, Wang L, Ding B, Chen X, Long M, Liu Y, Wu G (2013a) Protective effects of N-acetylcysteine on acetic acid-induced colitis in a porcine model. BMC Gastroenterol 13:133
Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G (2013b) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wang W, Dai Z, Wu Z, Lin G, Jia S, Hu S, Dahanayaka S, Wu G (2014a) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wang W, Wu Z, Lin G, Hu S, Wang B, Dai Z, Wu G (2014b) Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr 144:1540–1548
Wang H, Ji Y, Wu G, Sun K, Sun Y, Li W, Wang B, He B, Zhang Q, Dai Z, Wu Z (2015a) l-Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J Nutr 145:1156–1162
Wang H, Zhang C, Wu G, Sun Y, Wang B, He B, Dai Z, Wu Z (2015b) Glutamine enhances tight-junction protein expression and modulates CRF signaling in the jejunum of weanling piglets. J Nutr 145:25–31
Wang J, Li GR, Tan BE, Xiong X, Kong XF, Xiao DF, Xu LW, Wu MM, Huang B, Kim SW, Yin YL (2015c) Oral administration of putrescine and proline during the suckling period improves epithelial restitution after early weaning in piglets. J Anim Sci 93:1679–1688
Wang X, Liu Y, Li S, Pi D, Zhu H, Hou Y, Shi H, Leng W (2015d) Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br J Nutr 114:553–565
Wang H, Liu Y, Shi H, Wang X, Zhu H, Pi D, Leng W, Li S (2016) Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-κB and p38 signaling in weaned pigs after LPS challenge. Eur J Nutr. doi:10.1007/s00394-016-1189-x
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 272:G1382–G1390
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2010) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2013a) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2013b) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
Wu HT (2015) Regulative effect of glycine on intestinal injury and muscle protein synthesis and degradation of piglets after lipopolysaccharide challenge. Dissertation, Wuhan Polytechnic University
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Wu G, Knabe DA, Flynn NE (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Knabe DA, Flynn NE, Yan W, Flynn SP (1996a) Arginine degradation in developing porcine enterocytes. Am J Physiol Gastrointest Liver Physiol 271:G913–G919
Wu G, Meier SA, Knabe DA (1996b) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Flynn NE, Knabe DA (2000a) Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets. Am J Physiol Endocrinol Metab 279:E395–E402
Wu G, Flynn NE, Knabe DA, Jaeger LA (2000b) A cortisol surge mediates the enhanced polyamine synthesis in porcine enterocytes during weaning. Am J Physiol Regul Integr Comp Physiol 279:R554–R559
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li X, McKnight JR, Satterfield MC, Spencer TE (2011) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu X, Zhang Y, Liu Z, Li TJ, Yin YL (2012) Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets. J Anim Sci 90:337–339
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Wu Z, Hu CA, Wu G, Zhaorigetu S, Chand H, Sun K, Ji Y, Wang B, Dai Z, Walton B, Miao Y, Hou Y (2015) Intimacy and deadly feud: the interplay of autophagy and apoptosis mediated by amino acids. Amino Acids 47:2089–2099
Xu CC, Yang SF, Zhu LH, Cai X, Sheng YS, Zhu SW, Xu JX (2014) Regulation of N-acetylcysteine on gut redox status and major microbiota in weaned piglets. J Anim Sci 92:1504–1511
Yandza T, Tauc M, Saint-Paul MC, Ouaissi M, Gugenheim J, Hébuterne X (2012) The pig as a preclinical model for intestinal ischemia–reperfusion and transplantation studies. J Surg Res 178:807–819
Yang KM, Jiang ZY, Zheng CT, Wang L, Yang XF (2014) Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Sci 92:1496–1503
Yao K, Guan S, Li T, Huang R, Wu G, Ruan Z, Yin Y (2011) Dietary l-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr 105:703–709
Yi GF, Carroll JA, Allee GL, Gaines AM, Kendall DC, Usry JL, Toride Y, Izuru S (2005) Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J Anim Sci 83:634–643
Yi D, Hou Y, Wang L, Ouyang W, Long M, Zhao D, Ding B, Liu Y, Wu G (2015) l-Glutamine enhances enterocyte growth via activation of the mTOR signaling pathway independently of AMPK. Amino Acids 47:65–78
Yi D, Hou Y, Wang L, Long M, Hu S, Mei H, Yan L, Hu CA, Wu G (2016) N-Acetylcysteine stimulates protein synthesis in enterocytes independently of glutathione synthesis. Amino Acids 48:523–533
Yi D, Hou YQ, Xiao H, Wang L, Zhang Y, Chen HB, Wu T, Ding BY, Hu CA, Wu GY (2017) N-Acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways. Amino Acids. doi:10.1007/s00726-017-2389-2
Zhang S, Qiao S, Ren M, Zeng X, Ma X, Wu Z, Thacker P, Wu G (2013) Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45:1191–1205
Zhang S, Ren M, Zeng X, He P, Ma X, Qiao S (2014) Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/Akt/mTOR and ERK signaling pathways. Amino Acids 46:2633–2642
Zhang SX, Li L, Yin JW, Jin M, Kong XY, Pang LL, Zhou YK, Tian LG, Chen JX, Zhou XN (2016) Emergence of human caliciviruses among diarrhea cases in southwest China. BMC Infect Dis 16:511
Zhang Y, Lu T, Han L, Zhao L, Niu Y, Chen H (2017) l-Glutamine supplementation alleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. Biomed Res Int 2017:4862861
Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ (2003) l-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care 6:229–240
Zhong H, Li H, Liu G, Wan H, Mercier Y, Zhang X, Lin Y, Che L, Xu S, Tang L, Tian G, Chen D, Wu D, Fang Z (2016) Increased maternal consumption of methionine as its hydroxyl analog promoted neonatal intestinal growth without compromising maternal energy homeostasis. J Anim Sci Biotechnol 7:46
Zhou ZY, Wan XY, Cao JW (2013) Dietary methionine intake and risk of incident colorectal cancer: a meta-analysis of 8 prospective studies involving 431,029 participants. PLoS One 8:e83588
Zhu HL, Liu YL, Xie XL, Huang JJ, Hou YQ (2013) Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun 19:242–252
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31372318 and 31422053) and State’s Key Project of Research and Development Plan (2016YFD0501210).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Hence, no informed consent was required for any part of this review.
Additional information
Handling Editor: J. D. Wade.
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, X., Hou, Y. et al. Roles of amino acids in preventing and treating intestinal diseases: recent studies with pig models. Amino Acids 49, 1277–1291 (2017). https://doi.org/10.1007/s00726-017-2450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2450-1