Abstract
The XCIND syndrome is named after distinct hypersensitivity to ionizing (X-ray) irradiation, cancer susceptibility, immunodeficiency, neurological abnormality, and double-strand DNA breakage. The disorders comprising XCIND syndrome are usually inherited in an autosomal recessive manner. Ataxia telangiectasia (A-T) is one such disease, and is caused by biallelic germline mutation of the Ataxia telangiectasia mutated (ATM) gene. Heterozygous carriers of the ATM mutation, who do not show A-T-like clinical symptoms, are estimated to comprise 1 % of the population. Thus, understanding the biological basis of XCIND, including A-T, should help shed light on the pathogenesis of genetic diseases with cancer susceptibility.
Similar content being viewed by others
Introduction
Human beings are exposed to various insults to their cellular DNA in their everyday lives. However, the human body is equipped with DNA repair systems, which enable us to survive without developing devastating diseases due to the accumulation of DNA damage. There are two types of DNA damage: endogenous and exogenous. Endogenous DNA damage is caused by stresses associated with cellular oxygenation, DNA replication in cell proliferation, and DNA recombination of antigen receptor genes, while exogenous insults are caused by such agents as UV irradiation, natural and/or medical ionizing radiation, and DNA-damaging agents, such as anti-cancer drugs or environmental toxic factors. Ionizing radiation causes double-strand breaks of DNA associated with the generation of oxygen radicals. DNA damage responses and repair systems are prerequisites for normal development and differentiation of embryonic stem cells. However, in some individuals these repair systems are genetically defective, causing genetic hypersensitivity to ionizing radiation and hence genomic instability.
One of the most reliable and traditional means for testing hypersensitivity to X-irradiation (X-IR) is colony-survival assay (CSA). But evaluating sensitivity to X-IR-induced DNA damage requires substantial experience. Instead of employing CSA, we can also use a foci formation assay for γH2AX, 53BP1, or BRCA1 as surrogate marker, which is called the irradiation-induced foci (IRIF) kinetics method [1].
In this review we describe the X-IR hypersensitive syndrome XCIND, focusing on ataxia telangiectasia as a main representative disorder. Although XCIND disorders are rather rare, they may provide essential insights for understanding mechanisms of human diseases caused by concomitant effects of genetic and environmental factors, such as X-IR.
XCIND as a disease characterized by hypersensitivity to X-IR
XCIND is usually inherited in an autosomal recessive manner. Diseases belonging to the XCIND family are listed in Table 1 [2, 3]. Ruling out the possibility of XCIND is essential when patients are to be treated by chemotherapy and/or transplantation of hematopoietic stem cells for immunodeficiency or hematological malignancies, as hypersensitivity to X-IR leads to concomitant hypersensitivity to conditioning regimens, including chemotherapeutic agents which cause DNA damage.
The repair pathways for DNA double-strand breaks (DSB) include homologous recombination (HR) and non-homologous end joining (NHEJ) [4]. NHEJ repair requires KU70/80, DNA-PKcs (PRKDC), Artemis (DCLRE1C), DNA Ligase IV (LIG4), XRCC4, and Cernunnos/XLF (NHEJ1) proteins regardless of whether the source of the insult is endogenous or exogenous. One endogenous form of DNA DSB is that of antigen receptor genes [5], which is the reason defects in these proteins lead to lymphocyte deficiency as well as hypersensitivity to X-IR associated with frequent chromosomal abnormalities. A-T, Nijmegen breakage syndrome (NBS), and A-T-like disorder (ATLD) are often associated with chromosomal translocation of chromosome 7 and 14, mainly involving the T cell receptor (TCR) or immunoglobulin (Ig) locus, while LIG4 deficiency is associated with random chromosomal translocations. To maintain chromosomal integrity, cell cycle checkpoints also play essential roles. This is because DNA replication or cell division should not be allowed to initiate until DNA repair is completed, as revealed by extensive studies on A-T [6].
More recently, RNF 168 deficiency, RAD50 deficiency, and DNA-PKcs deficiency have been found to be involved in human radiation sensitivity disorders. RNF168 is involved in a ubiquitin ligase cascade of histone chromatin H2A, a deficiency reported to cause RIDDLE syndrome, which is characterized by radiosensitivity, immunodeficiency, dysmorphic features and learning difficulties [7, 8]. RAD50 is one of the components of the MRE11/RAD50/NBS (MRN) complex, which functions in DSB repair by recognizing DSB sites. The loss of RAD50 function was found to lead to increased radiosensitivity, as well as microcephaly [9]. These clinical features are also shared by NBS, DNA LIG4 deficiency, and Cernunnos/XLF deficiency. In these cases, patients usually carry hypomorphic mutations of the genes; complete deficiency of the protein leads to embryonic lethality. DNA-PKcs deficiency has previously been shown to lead to severe combined immunodeficiency (SCID) in mice, horses, and dogs. It has long been a question whether germline mutations of DNA-PKcs are involved in human SCID. Hypomorphic mutation has recently been discovered in human SCID [10]. Details of the individual diseases are described below and shown in Table 2.
Ataxia telangiectasia, a representative XCIND disease
A-T is a progressive neurodegenerative disorder with onset of truncal ataxia usually before 3 years of age. Ocular apraxia is also present in most patients. By the age of 10, patients are wheelchair-bound due to ataxia. One-third of patients have severe immunodeficiencies, accompanied by severe sino-pulmonary infections with non-opportunistic organisms. Conjunctival telangiectasia is a well-known characteristic feature that appears several years after the onset of neurologic symptoms [11].
Increase of serum alpha-fetoprotein (AFP), chromosomal translocations involving chromosomes 7 and 14, and a decrease in the lymphocytes of B cell and T cell compartments are the most useful test for the diagnosis of A-T. AFP is elevated in almost all the cases of A-T, and is the most useful clinical laboratory test for bedside diagnosis. T cell numbers are usually low, and many patients show only marginal deficiencies. γ/δ T cell numbers are usually elevated. B cell numbers are normal or slightly elevated. Deficiencies of IgE, IgG2, and IgA are often marked. Glucose intolerance is also frequently observed, suggesting that ATM may somehow be involved in the pathogenesis of DM in the general population.
Radiosensitivity is the most traditional and reliable test to confirm the clinical diagnosis of A-T. Colony-survival assay (CSA) and radioresistant DNA synthesis have been validated for clinical diagnostic testing and CSA was reported to be one of the most reliable assays to identify A-T patients [1], when tested in experienced laboratories.
The responsible gene in A-T is localized at chromosome at 11q22.3 and called the ATM gene, which contains 66 exons spanning approximately 150 kb of genomic DNA. Its cDNA consists of 10140 bp. ATM mutation in A-T is inherited in an autosomal recessive manner. The frequency of A-T patients is estimated as 1 in 40000–300000, and heterozygous carriers of the ATM gene mutation reportedly comprise 0.5–1 % of the general population.
ATM protein and its function
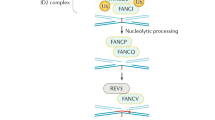
The ATM protein is composed of 3056 amino acids encoded by a 9168-bp open reading frame [6].The ATM gene mutation is not limited to A-T patients, and the ATM gene is mutated secondarily in various hematological malignancies, which lead to the speculation that ATM plays a role as a tumor suppressor gene [12]. The ATM protein encodes phosphoinositide 3-kinase-related kinase. When cells are exposed to genotoxic stresses, such as anticancer drugs or X-IR-induced DNA double-strand breaks, the ATM protein plays a central role in the DNA damage response (Fig. 1). ATM is present as a dimer or higher-order multimer in unstressed cells. During DNA damage response (DDR), the canonical pathway is activated in which ATM undergoes autophosphorylation on at least three sites (Ser367, Ser1893, and Ser1981). Autophosphorylation of these sites breaks apart the inactive, non-covalently bound ATM dimer, leading to the activation of the ATM-induced repair pathway [6, 13].
The activation of ATM protein kinase activity has been shown to be dependent on the presence of the MRN complex (Mre11/Rad50/Nbs1), which is a kind of sensor of DNA breakage. Once activated, ATM then phosphorylates a series of substrates that participate in signaling to the cell cycle checkpoints, DNA repair, or cell death. One of the most important substrates is the tumor suppressor protein p53 [14]. p53 is activated by phosphorylation in an ATM-dependent manner and prevents the passage of cells from G1 to S phase, or induces apoptosis when DNA damage is too profound to be repaired. Other well-characterized ATM substrates are the checkpoint kinase Chk2, the breast cancer susceptibility protein BRCA1, and Nbs1(NBN), which is mutated in the human genetic disorder Nijmegen breakage syndrome (NBS) [15], though there are many substrates yet not characterized.
ATM and cancer
The most frequent cancers in A-T are lymphoid tumors, which are observed in 15–30 % of A-T patients [11]. Lymphoid tumors frequently involve chromosomal translocation at chromosome 7 and 14 [16]. Recently, it was reported that TCRδ, but not the TCRα region, is involved in chromosome 14 translocation [17] and this is closely associated with recombination defects of the TCRβ region in the double-negative 3a (DN3a) stage of thymocyte development. In the DN3a stage, RAG-dependent chromosomal breaks occur at the TCRδ loci, and aberrant chromosomal translocations are induced while developing to DN3b. Thus, T cell deficiency and chromosomal translocation are closely related in A-T [18].
A-T patients reportedly suffer from other cancers of the stomach, liver, ovaries, salivary glands, oral tissue, breast, and pancreas as well. A-T heterozygous carriers are also reported to be at relatively higher risk for breast cancer. Breast cancer is one of the most frequent secondary cancers in Hodgkin disease (HD), and risk increases especially after irradiation. It should be noted that in childhood HD, dominant negative mutations of the germline ATM gene are often seen, suggesting the possible involvement of a genetic background for childhood HD concomitantly with breast cancer [19].
Recent studies have indicated that the progression of preneoplastic lesions to neoplasia is inhibited by the existence of tumorigenesis barriers. Response to DNA replication stress is one such barrier, leading to activation of the DNA damage checkpoint and thereby to apoptosis or cell cycle arrest. It has been shown that oncogene-induced senescence is associated with signs of DNA replication stress, such as prematurely terminated DNA replication forks and DNA double-strand breaks. In ATM-deficient mice, the induction of senescence is absent, which leads to increased tumor proliferation. Thus, it has been suggested that senescence in human preneoplastic lesions represents a barrier to malignant progression. This has been shown to be the case in early lesions of colon cancer. Precancerous colon adenoma expresses activated ATM protein, which corresponds to the autophosphorylation responding to replication stress of initial events in oncogene-induced cancer development [20–22]. It has also been shown that ATM is activated in myelodysplastic syndrome (MDS), which is a preleukemic condition caused by oncogenic stresses in hemopoietic stem cells, but is disrupted eventually when MDS evolves into overt leukemia [21]. Thus, ATM has a role in preventing the progression of cancer.
Other diseases in the XCIND syndrome
Nijmegen breakage syndrome
The Nijmegen breakage syndrome is an autosomal recessive chromosomal instability syndrome characterized by microcephaly, microgenia, “bird-like” face, growth retardation, mild mental retardation, immunodeficiency, and a predisposition to cancer with normal serum levels of alpha-fetoprotein. Cancer susceptibility is much higher than in AT. However, patients with NBS have neither ataxia nor telangiectasia. Almost all patients reportedly originate from Eastern Europe, and are homozygous for one founder hypomorphic NBN (NBS1) mutation c.657_661del5 originating from Slavic lines. Cells from NBS patients exhibit similar features with those of AT, namely hyper-radiosensitivity, radio-resistant DNA synthesis, and chromosomal rearrangements involving TCR and Ig loci. [23].
Nijmegen breakage syndrome-like disorder (NBSLD)
Waltes et al. identified compound heterozygosity for mutations in the RAD50 gene from NBS-like phenotype patients. The mutations were a combination of a nonsense mutation and a mutation that produces a larger polypeptide than the wild type. As a result, it produces a low level of unstable RAD50 protein, presumably a hypomorphic mutation. Patients exhibit microcephaly, mild mental retardation, and mild ataxia. However, patients with NBSLD reportedly show neither immunodeficiency nor predisposition to cancer, although further study is needed. Cells from NBSLD patients exhibit moderately increased radiosensitivity and chromosomal rearrangements involving TCR and Ig loci. [9].
Ataxia telangiectasia-like disorder (ATLD)
Mutation in the MRE11A gene was identified from a clinically diagnosed AT-like patient. Clinical features are much milder than AT, and the symptoms appear much more slowly than in AT. The appearance of ataxia, for example, is much later than AT, and telangiectasia and increased alpha-fetoprotein are absent. Although immunoglobulin levels are almost normal, the generation of specific functional antibodies is attenuated [24]. It has been thought that ATLD patients do not exhibit predisposition to cancer, but we have identified a Japanese ATLD family who developed lung cancer [25]. An original report described ATLD as a milder phenotype of AT. However, a more severe phenotype including microcephaly or NBS-like dimorphic features has been reported [25, 26]. MRE11A mutation affects M/R/N complex stability, and decreased NBS1 and/or RAD50 is observed. Residual activity of NBS1 and/or RAD50 may affect the difference in phenotypes. The cellular biological features are the same as in AT, including increased radiosensitivity and an increased level of spontaneously occurring chromosome aberrations involving the TCR or Ig region.
RIDDLE syndrome
RIDDLE syndrome is named after its clinical features, including increased radiosensitivity, immunodeficiency, dysmorphic features, and learning difficulties. Patients exhibit ataxia, telangiectasia, growth retardation, and microcephaly [8]. The gene responsible for RIDDLE syndrome is RNF168 [7]. Patients carry compound heterozygous nonsense mutations in the RNF168 gene, resulting in aberrant RNF168 proteins. RNF168 is a ubiquitin ligase, and RNF168-dependent chromatin modifications orchestrate the accumulation of 53BP1 and BRCA1 to DNA lesions; their loss is the likely cause of the cellular and developmental phenotypes associated with RIDDLE syndrome. Cellular biological features include inability to recruit 53BP1 to sites of DNA double-strand breaks, moderately increased radiosensitivity, and dysregulation of cell cycle checkpoints after irradiation.
Severe combined immunodeficiency (SCID)
SCID is an immunodeficiency in which both B cell and T cell functions are impaired due to defects in one of several possible genes. One of the genes responsible for SCID is the gene encoding the common gamma chain (γc), shared by receptors for interleukins such as IL-2, IL-7, and IL-15. Attenuation of NHEJ during VDJ recombination also leads to SCID development. The molecules involved in NHEJ are known to be LIG4, Artemis (DCLRE1C), XLF/Cernunnos (NHEJ1), and DNA-PKcs (PKRDC) [5]. Although all of the mutated genes are directly responsible for SCID development, the phenotypes differ according to the gene responsible. Only LIG4-deficient and XLF/Cernunnos-deficient SCID exhibits microcephaly and growth retardation.
References
Martin NT, Nahas SA, Tunuguntla R, Fike F, Gatti RA. Assessing ‘radiosensitivity’ with kinetic profiles of gamma-H2AX, 53BP1 and BRCA1 foci. Radiother Oncol. 2011;101:35–8.
Nahas SA, Gatti RA. DNA double strand break repair defects, primary immunodeficiency disorders, and ‘radiosensitivity’. Curr Opin Allergy Clin Immunol. 2009;9:510–6.
Gatti RA, Boder E, Good RA. Immunodeficiency, radiosensitivity, and the XCIND syndrome. Immunol Res. 2007;38:87–101.
Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–47.
Dvorak CC, Cowan MJ. Radiosensitive severe combined immunodeficiency disease. Immunol Allergy Clin North Am. 2010;30:125–42.
Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–68.
Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–34.
Stewart GS, Stankovic T, Byrd PJ, Wechsler T, Miller ES, Huissoon A, et al. RIDDLE immunodeficiency syndrome is linked to defects in 53BP1-mediated DNA damage signaling. Proc Natl Acad Sci USA. 2007;104:16910–5.
Waltes R, Kalb R, Gatei M, Kijas AW, Stumm M, Sobeck A, et al. Human RAD50 deficiency in a Nijmegen breakage syndrome-like disorder. Am J Hum Genet. 2009;84:605–16.
van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, et al. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest. 2009;119:91–8.
Perlman S, Becker-Catania S, Gatti RA. Ataxia-telangiectasia: diagnosis and treatment. Semin Pediatr Neurol. 2003;10:173–82.
Yamaguchi M, Yamamoto K, Miki T, Mizutani S, Miura O. T-cell prolymphocytic leukemia with der(11)t(1;11)(q21;q23) and ATM deficiency. Cancer Genet Cytogenet. 2003;146:22–6.
Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17.
Morgan SE, Kastan MB. p53 and ATM: cell cycle, cell death, and cancer. Adv Cancer Res. 1997;71:1–25.
Bensimon A, Aebersold R, Shiloh Y. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett 2011.585:1625–39.
Taylor AM, Metcalfe JA, Thick J, Mak YF. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996;87:423–38.
Zha S, Bassing CH, Sanda T, Brush JW, Patel H, Goff PH, et al. ATM-deficient thymic lymphoma is associated with aberrant tcrd rearrangement and gene amplification. J Exp Med. 2010;207:1369–80.
Isoda T, Takagi M, Piao J, Nakagama S, Sato M, Masuda K, et al. Process for immune defect and chromosomal translocation during early thymocyte development lacking ATM. Blood. 2012;120:789–99.
Takagi M, Tsuchida R, Oguchi K, Shigeta T, Nakada S, Shimizu K, et al. Identification and characterization of polymorphic variations of the ataxia telangiectasia mutated (ATM) gene in childhood Hodgkin disease. Blood. 2004;103:283–90.
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70.
Horibe S, Takagi M, Unno J, Nagasawa M, Morio T, Arai A, et al. DNA damage check points prevent leukemic transformation in myelodysplastic syndrome. Leukemia. 2007;21:2195–8.
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42.
Demuth I, Digweed M. The clinical manifestation of a defective response to DNA double-strand breaks as exemplified by Nijmegen breakage syndrome. Oncogene. 2007;26:7792–8.
Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NG, et al. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–87.
Uchisaka N, Takahashi N, Sato M, Kikuchi A, Mochizuki S, Imai K, et al. Two brothers with ataxia-telangiectasia-like disorder with lung adenocarcinoma. J Pediatr. 2009;155:435–8.
Matsumoto Y, Miyamoto T, Sakamoto H, Izumi H, Nakazawa Y, Ogi T, et al. Two unrelated patients with MRE11A mutations and Nijmegen breakage syndrome-like severe microcephaly. DNA Repair (Amst). 2011;10:314–21.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mizutani, S., Takagi, M. XCIND as a genetic disease of X-irradiation hypersensitivity and cancer susceptibility. Int J Hematol 97, 37–42 (2013). https://doi.org/10.1007/s12185-012-1240-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1240-5