Abstract
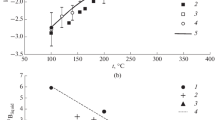
The hydrogen isotopic fractionation factor between brucite and water has been determined in the temperature range of 100°–510° C. Brucite is always depleted in deuterium relative to the coexisting water, and the degree of depletion becomes larger with decreasing temperature. The fractionation factor changes smoothly in the temperature range of 144°–510° C and its temperature dependence was obtained by the method of least square fit in the following form: 103Inα=8.72×106 T −2−3.86×104 T −1+14.5
However, a marked decrease of about 5‰ was observed at 100°–144° C. The D/H fractionation factor for the brucite-water system is not similar to that for serpentine-water system presented by Sakai and Tsutsumi (1978), though all the hydroxyl ions coordinate to magnesium ion in both minerals. This discrepancy cannot be attributed to hydrogen bonding but to distortion of Mg-octahedron of serpentine, in which the Mg-OH bonding length is shorter than the sum of ionic radius of Mg2+ and O2− and there is no distortion in brucite. It is indicated that aside from hydrogen bonding, the structure effect also controls the D/H fractionation between hydrous mineral and water.
Similar content being viewed by others
References
Bigeleisen J, Perlman ML, Prosser HC (1952) Conversion of hydrogenic materials to hydrogen for isotopic analysis. Anal Chem 24:1356–1357
Brindley GW, Ogilvie GJ (1952) The texture of single crystals of brucite. Acta Cryst 5:412–413
Deer WA, Howie RA, Zussman J (1962) Rock-forming minerals, vol 3. Longmans, London
Elleman DD, Williams D (1956) Proton positions in brucite crystals. J Chem Phys 25:742–744
Evans BW (1977) Metamorphism of alpine peridotite and serpentinite. Ann Rev Earth Planet Sci 5:397–447
Graham CM (1981) Experimental hydrogen isotope studies III: diffusion of hydrogen in hydrous minerals and stable isotope exchange in metamorphic rocks. Contrib Mineral Petrol 76:216–228
Graham CM, Sheppard SMF (1980) Experimental hydrogen isotope studies II: fractionations in the systems epidote-NaCl H2O, epidote-CaCl2-H2O and epidote-seawater, and the hydrogen isotope composition of natural epidotes. Earth Planet Sci Lett 49:237–251
Graham CM, Sheppard SMF, Heaton THE (1980) Experimental hydrogen isotope studies I. systematics of hydrogen isotope fractionation in the systems epidote-H2O, zoisite-H2O and AlO(OH)-H2O. Geochim Cosmochim Acta 44:353–364
Graham CM, Harmon RS, Borthwick J, Sheppard SMF (1982) Experimental studies of hydrogen isotope exchange between amphiboles and water. Abstracts of Fifth International Conf. Geochronology, Cosmochronology, Isotope Geology. Nikko, Japan
Marumo K, Nagasawa K, Kuroda Y (1980) Mineralogy and hydrogen isotope geochemistry of clay minerals in the Ohnuma geothermal area, northeastern Japan. Earth Planet Sci Lett 47:255–262
McKay HAC (1938) Kinetics of exchange reactions. Nature 142:997–998
Mellini M (1982) The crystal structure of lizardite 1T: hydrogen bonds and polytypism. Am Mineral 67:587–598
Meyer JW, Yang JC (1962) Some observations in the system MgO-H2O. Am J Sci 260:707–717
Morimoto N, Sunagawa I, Miyashiro A (1975) Mineralogy. Iwanami, Tokyo, pp 640 (in Japanese)
Northrop DA, Clayton RN (1966) Oxygen isotope fractionations in systems containing dolomite. J Geol 74:174–196
O'Neil JR, Kharaka YK (1976) Hydrogen and oxygen isotope exchange reactions between clay minerals and water. Geochim Cosmochim Acta 40:241–246
Sakai H, Tsutsumi M (1978) D/H fractionation factors between serpentine and water at 100° to 500° C and 2000 bar water pressure and the D/H ratios of natural sepentines. Earth Planet Sci Lett 40:231–242
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Cryst B 25:935–946
Stewart MK, Friedman I (1975) Deuterium fractionation between aqueous salt solutions and water vapor. J Geophys Res 80:3812–3818
Suzuoki T, Epstein S (1976) Hydrogen isotope fractionation between OH-bearing silicate minerals and water. Geochim Cosmochim Acta 40:1229–1240
Taylor HP (1974) The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ Geol 9:843–883
Wenner DB, Taylor HP (1973) Oxygen and hydrogen isotope studies of the serpentinization of ultramafic rocks in oceanic environments and continental ophiolite complexes. Am J Sci 273:207–239
Wenner DB, Taylor HP (1974) D/H and O18/O16 studies of serpentinization of ultramafic rocks. Geochim Cosmochim Acta 38:1255–1286
Whittaker EJW (1953) The structure of chrysotile. Acta Cryst 6:747–748
Whittaker EJW (1956) The structure of chrysotile II. clinochrysotile. Acta Cryst 9:855–864
Wicks FJ, Whittaker EJW (1975) A reappraisal of the structures of the serpentine minerals. Can Mineral 13:227–243
Yamaoka S, Fukunaga O, Saito S (1970) Phase equilibrium in the system MgO-H2O at high temperatures and very high pressures. J Am Ceramic Soc 53:179–181
Zigan F, Rothbauer R (1976) Neutronenbeugungsmessungen am Brucit. Neues Jahrb Mineral Monatsh 4:137–142
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satake, H., Matsuo, S. Hydrogen isotopic fractionation factor between brucite and water in the temperature range from 100° to 510° C. Contr. Mineral. and Petrol. 86, 19–24 (1984). https://doi.org/10.1007/BF00373707
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373707