Abstract
Objective: PET has the ability to evaluate functional information as well as visualization of radiotracer uptake. Compartmental model is a basic idea to analyze dynamic PET data. C-HED has been the most frequently used PET tracer for the evaluation of cardiac sympathetic nervous system (SNS) function. The washout of norepinephrine from myocardium is associated with increasing SNS activity in heart failure (HF). However, the existence of washout of 11C-HED from the myocardium is controversial. Although “retention index” (RI) is commonly calculated to quantify the uptake of HED, RI is not purely able to distinguish washout parameter and uptake parameter. Therefore, in this study, we aimed to evaluate whether HED was washed out from the myocardium using compartment model analysis.
Material and Methods: We compared HED parameters in ten normal volunteers (32.4 ± 9.6 years) and nine HF patients (age: 57.3 ± 17.3 years, LVEF: 36.1 ± 16.7 %). Each subject underwent rest 11C-HED PET. We estimated RI, inflow rate K1, and washout rate k2 using single-compartment model analysis using 11C-HED PET.
Result: HF patients showed lower RI and inflow rate K1 compared to normal volunteers (RI: 0.06 ± 0.02 vs. 0.15 ± 0.03 min−1, p < 0.001, K1: 0.14 ± 0.05 vs 0.20 ± 0.03 ml/min/g, p < 0.001). Washout rate k2 also significantly increased in HF patients (k2: 0.036 ± 0.026 vs. 0.016 ± 0.011 min−1, p = 0.041).
Conclusion: HF patients showed reduced RI, reduced K1, and higher washout rate k2 compared to normal. This result may imply that HED PET is able to evaluate washout parameter using compartment model.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Positron emission tomography (PET) is a powerful tool to evaluate functional information imaging as well as anatomical information [1, 2]. PET is the most reliable modality for assessing functional information, especially in cardiovascular imaging [3, 4]. When biomedical functions are analyzed using PET images, compartment model analysis is generally applied [5, 6]. Compartment model analysis enables to observe the pharmacokinetics of radiotracer in human body. Thus, we apply compartment model analysis to evaluating pharmacokinetics of 11C-hydroxyephedrine (HED).
11C- HED has been the most frequently used PET tracer for the estimation of cardiac sympathetic nervous system (SNS) function [7–9]. In general, 11C- HED data has been evaluated using the retention index (RI) [8]. RI is a parameter that can be calculated easily compared to other quantitative parameters. RI includes uptake and washout parameters. However, RI does not differentiate washout parameters from cardiac HED data. Cardiac washout parameter is widely used for evaluation of SNS function and increased cardiac washout is associated with cardiac events in heart failure (HF) [10]. Therefore, it would be important to evaluate the washout parameters using HED PET. Compartment model analysis might have a potential to evaluate precise pharmacokinetics of 11C-HED and also has a potential to evaluate purely washout parameter [11].
In this study, we aimed to analyze HED uptake parameter and washout parameter using single-compartment model analysis in patients with HF.
2 Methods
2.1 Study Subjects
Ten healthy volunteers and nine HF patients participated in the current study. The healthy volunteers (ten men, 32.4 ± 9.6 years) had a low pretest likelihood of coronary artery disease (<5 %) based on risk factors [12]. HF patients were recruited from a group of patients who underwent HED PET for the assessment of sympathetic neuronal function. They were six men and three women (57.3 ± 17.3 years). The study was approved by the Hokkaido University Graduate School of Medicine Human Research Ethics Board. Written informed consent was obtained from all participants.
2.2 Positron Emission Tomography/Computed Tomography 11C-HED PET/CT Imaging
11C-HED was produced from 11C-methyl iodide and metaraminol (free base) using standard methods with high purity and high specific activity [13].
All participants were instructed to fast overnight. PET/CT imaging was performed with a 64-slice PET/CT scanner (Biograph Siemens/CTI, Knoxville, TN, USA). A low-dose CT was performed for attenuation correction. The CT co-registered to standard orthogonal PET images was then re-sliced into series of short-axis, horizontal long-axis, and vertical long-axis images.
Immediately after the administration of 5 mCi(185 MBq) of intravenous 11C-HED, participants underwent 40-min 3D list-mode PET acquisition. The images were reconstructed using filtered back correction with a12-mm Hann filter and were reconstructed into 23 frames (10 × 10 s; 1 × 60 s; 5 × 100 s; 3 × 180 s; 4 × 300 s) [14].
2.3 RI Estimation
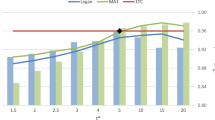
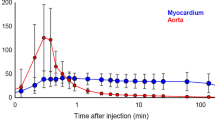
RI is obtained by normalizing late phase of tracer activity concentrations (30–40 min) of left ventricular (LV) myocardium divided by the integral of the arterial input function (AIF). The time-activity curve was derived from a small circular region of interest in the left ventricular cavity (Fig. 5.1, [ 10]).
Analysis methods of 11C-hydroxyephedrine. (a) Calculation method of retention index: retention index was obtained by normalizing late activity concentrations of left ventricular myocardium divided by the integral of the arterial input function.(b) Single-tissue compartment model: single-tissue compartment model enables to monitor inflow rate K1 and washout rate k2 of radiotracer between arterial input function (AIF) and tissue activity curve (TAC). AIF and TAC were obtained from LV cavity and myocardial tissue, respectively
2.4 Compartment Model Analysis
Harms HJ et al. reported the single-tissue model was more robust than two-tissue compartment model and results obtained were similar to more precise models [11]. Thus, we used single-compartment model to evaluate 11C-HED washout parameter.
In single-compartment model analysis, tracer kinetics are consisted by only two parameters, which are inflow rate K1 and washout rate k2 (Fig. 5.1, [ 6]). In this study, K1 and k2 were estimated using the nonlinear least squares method. This approach used AIF arterial input function and tissue activity carve (TAC) of LV myocardium [15, 16]. Distribution volume was also calculated [17]. The equation of distribution volume was inflow rate K1 divided by washout rate k2.
2.5 Statistical Analysis
Data are expressed as mean ± SD. The differences between the means of two volumetric results were examined using the unpaired two t-test. Fisher’s exact tests were used for categorical variables. P-value of less than 0.05 was considered indicative of a statistically significant difference. Statistical calculations were carried out using JMP software version 12.0 (SAS Institute, Inc., Cary, NC).
3 Results
3.1 Subjects’ Background
Table 5.1 summarizes the baseline characteristics of the volunteers and HF patients. HF patients also had laboratory data and echocardiography data. The HF patients were older than normal.
3.2 HED PET Data Normal Volunteers and HF Patients
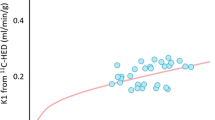
HF patients significantly decreased RI compared to normal volunteers (0.060 ± 0.020 vs. 0.150 ± 0.032 1/min, P < 0.001, Fig. 5.2). In compartment model analysis, HF patients showed decreased inflow rate K1 (0.14 ± 0.03 vs. 0.20 ± 0.05 ml/min/g, P = 0.004, Fig. 5.3a) and reduced distribution volume (5.17 ± 2.93 vs. 22.4 ± 23.1 mL/g, P = 0.04, Fig. 5.3c). In addition, HF patients significantly increased washout rate k2 compared to normal volunteers (0.036 ± 0.026 vs. 0.016 ± 0.011 1/min, P = 0.044, Figs. 5.3b and 5.4.).
4 Discussion
HF patients showed decreased RI, inflow rate K1, and distribution volume compared to normal volunteers. In contrast, the HF patients increased washout parameter k2.
Many previous studies reported patients with imparted SNS function showed lower 11C-HED uptake [8, 17]. Thus, the present data agree with previous reports.
In this study, HF patients showed significantly increased washout rate k2 compared to normal volunteers. Previous studies using 123I-MIBG reported HF patient showed increased washout rate [10, 18]. Previous study also reported that washout parameter of 11C-HED was well correlated with plasma and cardiac norepinephrine in experiments with rats [7]. Therefore, current data that HF patient showed increased washout rate may be considered to be appropriate.
4.1 Study Limitation
Our study had a small population and HF patients were significantly older than normal volunteers. Therefore, further investigations with larger and age-matched populations are required.
In addition, washout parameters were not compared to other clinical indexes. Comparison washout of 11C-HED and other clinical parameter such as ejection fraction, laboratory parameter, and washout of 123I-MIBG should be the next step.
5 Conclusion
In this study, we applied compartment model analysis to evaluating washout of 11C-hydroxyephedrine (HED).
As a result, HF patients showed reduced RI, K1, and distribution volume and higher washout rate k2 compared to normal. This result may imply that HED PET is able to evaluate washout parameter using compartment model.
References
Yoshinaga K, Burwash IG, Leech JA, Haddad H, Johnson CB, deKemp RA, Garrard L, Chen L, Williams K, DaSilva JN, Beanlands RS. The effects of continuous positive airway pressure on myocardial energetics in patients with heart failure and obstructive sleep apnea. J Am Coll Cardiol. 2007;49:450–8.
Katoh C, Morita K, Shiga T, Kubo N, Nakada K, Tamaki N. Improvement of algorithm for quantification of regional myocardial blood flow using 15O-water with PET. J Nucl Med. 2004;45:1908–16.
Tomiyama Y, Manabe O, Oyama-Manabe N, Naya M, Sugimori H, Hirata K, Mori Y, Tsutsui H, Kudo K, Tamaki N, Katoh C. Quantification of myocardial blood flow with dynamic perfusion 3.0 Tesla MRI: validation with o-water PET. J Magn Reson Imaging. 2015;42(3):754–62.
Kikuchi Y, Oyama-Manabe N, Naya M, Manabe O, Tomiyama Y, Sasaki T, Katoh C, Kudo K, Tamaki N, Shirato H. Quantification of myocardial blood flow using dynamic 320-row multi-detector CT as compared with (1)(5)O-H(2)O PET. Eur Radiol. 2014;24:1547–56.
Yoshinaga K, Tomiyama Y, Suzuki E, Tamaki N. Myocardial blood flow quantification using positron-emission tomography: analysis and practice in the clinical setting. Circ J. 2013;77:1662–71.
Klein R, Beanlands RS, deKemp RA. Quantification of myocardial blood flow and flow reserve: technical aspects. J Nucl Cardiol. 2010;17:555–70.
Thackeray JT, Renaud JM, Kordos M, Klein R, Dekemp RA, Beanlands RS, DaSilva JN. Test-retest repeatability of quantitative cardiac 11C-meta-hydroxyephedrine measurements in rats by small animal positron emission tomography. Nucl Med Biol. 2013;40:676–81.
Schwaiger M, Kalff V, Rosenspire K, Haka MS, Molina E, Hutchins GD, Deeb M, Wolfe Jr E, Wieland DM. Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation. 1990;82:457–64.
Bengel FM, Ueberfuhr P, Ziegler SI, Nekolla S, Reichart B, Schwaiger M. Serial assessment of sympathetic reinnervation after orthotopic heart transplantation. A longitudinal study using PET and C-11 hydroxyephedrine. Circulation. 1999;99:1866–71.
Matsunari I, Aoki H, Nomura Y, Takeda N, Chen WP, Taki J, Nakajima K, Nekolla SG, Kinuya S, Kajinami K. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging. 2010;3:595–603.
Harms HJ, de Haan S, Knaapen P, Allaart CP, Rijnierse MT, Schuit RC, Windhorst AD, Lammertsma AA, Huisman MC, Lubberink M. Quantification of [(11)C]-meta-hydroxyephedrine uptake in human myocardium. EJNMMI Res. 2014;4:52.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–8.
Rosenspire KC, Haka MS, Van Dort ME, Jewett DM, Gildersleeve DL, Schwaiger M, Wieland DM. Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: a false transmitter agent for heart neuronal imaging. J Nucl Med. 1990;31:1328–34.
Allman KC, Wieland DM, Muzik O, Degrado TR, Wolfe Jr ER, Schwaiger M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol. 1993;22:368–75.
Katoh C, Yoshinaga K, Klein R, Kasai K, Tomiyama Y, Manabe O, Naya M, Sakakibara M, Tsutsui H, deKemp RA, Tamaki N. Quantification of regional myocardial blood flow estimation with three-dimensional dynamic rubidium-82 PET and modified spillover correction model. J Nucl Cardiol. 2012;19:763–74.
Mori Y, Manabe O, Naya M, Tomiyama Y, Yoshinaga K, Magota K, Oyama-Manabe N, Hirata K, Tsutsui H, Tamaki N, Katoh C. Improved spillover correction model to quantify myocardial blood flow by 11C-acetate PET: comparison with 15O-H 2O PET. Ann Nucl Med. 2015;29:15–20.
Schafers M, Dutka D, Rhodes CG, Lammertsma AA, Hermansen F, Schober O, Camici PG. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ Res. 1998;82:57–62.
Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–77.
Acknowledgments
The authors thank Keiichi Magota, PhD; Ken-ichi Nishijima, PhD; Daisuke Abo, MSc; and Eriko Suzuki for their support for this study. This manuscript has been reviewed by a North American English-language professional editor Ms. Holly Beanlands. The authors also thank Ms. Holly Beanlands for critical reading of the manuscript.
This study was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Category B, No. 23390294) and grants from the Innovation Program of the Japan Science and Technology Agency. Tomiyama was supported by postgraduate students’ Travel Award Program by Hokkaido University. Dr. Yoshinaga is supported by the Imura Clinical Research Award (Adult Vascular Disease Research Foundation).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Conflicts of Interest
None
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/) which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this paper
Cite this paper
Tomiyama, Y., Yoshinaga, K. (2016). Kinetic Analysis for Cardiac PET. In: Kuge, Y., Shiga, T., Tamaki, N. (eds) Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55894-1_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55894-1_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55892-7
Online ISBN: 978-4-431-55894-1
eBook Packages: MedicineMedicine (R0)