Abstract
In the hydroxyurea era, insights into mechanisms downstream of erythrocyte sickling have led to new therapeutic approaches for patients with sickle cell disease (SCD). Therapies have been developed that target vascular adhesion, inflammation and hemolysis, including innovative biologics directed against P-selectin and invariant natural killer T cells. Advances in hematopoietic stem cell transplant and gene therapy may also provide more opportunities for cures in the near future. Several clinical studies are underway to determine the safety and efficacy of these new treatments. Novel approaches to treat SCD are desperately needed, since current therapies are limited and rates of morbidity and mortality remain high.
Similar content being viewed by others
Introduction
“Peculiar, elongated and sickle-shaped” cells in the blood of a 20-year-old dental student led Dr. James Herrick to describe the first case of sickle cell disease (SCD) in 1910 (1,2). The dental student, Walter Clement Noel, had repeated hospital admissions for episodes of pain accompanied by red cell hemolysis. Noel died when he was only 32 years old from a respiratory illness, likely acute chest syndrome (ACS) related to SCD. Unfortunately, frequent pain and early deaths still characterize the clinical course for many patients with SCD today.
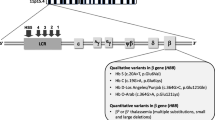
Since publication of this index case, there have been great strides toward understanding the mechanistic underpinnings of SCD. In 1949, SCD was heralded as the first molecular disease after the discovery of sickle hemoglobin by Pauling et al. (3). The genetic basis for this abnormal hemoglobin was later found to be a missense mutation in the β-globin gene, resulting in the substitution of a valine for glutamic acid at position 6. This deoxy-sickle hemoglobin undergoes structural changes that promote its polymerization into long fibrils, distorting the red cell into a crescent or sickle shape. The sickle erythrocytes are dehydrated, rigid and prone to hemolysis. They occlude the microvasculature causing acute, and critically important, chronic tissue ischemia and injury. The two most common acute morbidities in patients with SCD, vaso-occlusive pain crises (VOC) and ACS, are due to sudden occlusion of small vessels in the bone marrow and lungs (4,5) On a chronic basis, vaso-occlusion may damage the lungs, kidneys or brain and ultimately may lead to end-organ dysfunction (6). These acute and chronic complications of vaso-occlusion account for most deaths in patients with SCD in the modern era (7).
Clinical care improvements for patients with SCD have lagged behind the science. During the two decades that followed the identification of the hemoglobinopathy, only 50% of afflicted children survived into adulthood (8). By the 1990s, however, widespread mandatory newborn screening and the routine administration of penicillin to prevent pneumococcal sepsis increased childhood survival to over 90% (9,10). Then in 1998, hydroxyurea became the only U.S. Food and Drug Administration-approved therapy for SCD (11). First described as a potential therapy in 1984, hydroxyurea enhances the production of fetal hemoglobin production in sickle erythrocytes (12). Clinical studies of hydroxyurea use have demonstrated a decreased rate of VOC, ACS and improved survival (11,13,14).
Although hydroxyurea has been a major therapeutic breakthrough for patients with SCD, additional treatments are surely needed. Up to 50% of patients will not benefit from hydroxyurea long term because of poor response, toxicity, nonaggressive therapists or noncompliance (15). Potentially related to suboptimal hydroxyurea use, life expectancy for patients with SCD remains around 50 years (7,16). The focus of this article will be on advances in SCD therapies in the hydroxyurea era, excluding further progress in antisickling approaches. Recent insights into the regulation of fetal hemoglobin will likely lead to new therapies in the near future, but a careful description of these developments is beyond the scope of this article (17). Since the approval of hydroxyurea, a deeper understanding of how sickle cells interact with and affect other blood cells, the vasculature and vital organs has uncovered new ways to treat SCD. There has also been much effort directed toward a cure. From these discoveries, therapies have emerged that target cell adhesion, inflammation and hemolysis, as well as innovations in curative approaches.
Cell Adhesion and Inflammation
The process of vaso-occlusion begins with the adhesion of sickle erythrocytes and neutrophils to activated endothelium (18,19). Aggregates of blood cells, including platelets, form on these adhered cells and occlude the microvasculature, ultimately causing acute and chronic tissue ischemia and injury. Integral to these cellular interactions are inflammatory mediators, which activate endothelial cells, leukocytes and platelets and attract additional leukocytes to the site of occlusion (20). After the resolution of the microvascular occlusion, ischemia-reperfusion injury may further amplify inflammation, creating a vicious cycle that sustains and propagates vasoocclusion (21,22). New therapeutic targets have emerged as understanding of vaso-occlusion has progressed beyond the simple concept of a “log jam” of sick-led erythrocytes in small vessels. Interruption of the process of vaso-occlusion downstream from erythrocyte sickling may either dampen the severity of an ongoing VOC or prevent one altogether, depending on the approach and efficacy of therapy.
Adhesion: Selectin-Based Therapies
Several lines of evidence suggest that the selectin family of adhesion receptors may be a target for VOC therapies. Selectins are expressed on endothelial cells, platelets and leukocytes, as well as other cell types (23). P-selectin and E-selectin mediate rolling and tethering of blood cells to the endothelium, which may initiate vaso-occlusion in the postcapillary venules (24). Cellular and animal models of SCD demonstrate that interruption of selectin-mediated cellular adhesion decreases erythrocyte and leukocyte adhesion to the endothelium and improves blood flow (25–29). Several therapies that target selectins, including rivipansel (GMI-1070) and the anti-P-selectin monoclonal antibody SelG1, have been or are currently being studied in patients with SCD.
Rivipansel
Rivipansel is a synthetic pan-selectin inhibitor with effects predominantly mediated through E-selectin (25). After a loading dose, rivipansel is administered intravenously every 12 h, with the intent to diminish the severity of an ongoing VOC. A randomized, double-blind, placebo-controlled phase IIb trial of rivipansel (n = 76) was completed, although results have not yet been published. Communications from the company report that rivipansel decreased the duration of hospital stay and the amount of parenteral opioids used during VOC compared with placebo (30).
SelGl
SelG1 is a humanized monoclonal antibody directed against P-selectin. Investigators examined the pharmacokinetics and safety of monthly doses in a phase I study of SelG1 designed to prevent VOC. No results have yet been published. A phase 2 study is now planned in which patients will be randomized to high-dose SelG1, low-dose SelG1 or placebo and the effect on VOC will be measured (NCT01895361).
Inflammation: Invariant NKT (iNKT) Cell-Based Therapies
Reduction of inflammation constitutes another strategy to treat VOC. Studies of corticosteroids provided some of the first evidence that antiinflammatories may improve VOC in patients with SCD. When administered during VOC or ACS episodes, corticosteroids decreased length of hospital stay compared with placebo (31,32). Unfortunately, there was a high rate of readmission to the hospital because of rebound vaso-occlusion after the abrupt cessation of corticosteroids (31,32). A follow-up trial to evaluate the benefits of a tapering regimen of corticosteroids during ACS episodes was, unfortunately, stopped because of poor accrual (33).
iNKT cells
Therapies designed to target iNKT cells have recently been evaluated. iNKT cells comprise <1% of circulating lymphocytes in humans. Albeit small in number, iNKT cells possess potent characteristics of adaptive and innate immunity and “jumpstart” larger inflammatory responses (34). Similar to T cells that produce adaptive immune responses, iNKT cells express a T-cell receptor (TCR) that requires binding of an antigen presented on an antigen-presenting cell. Unlike T cells, which express a diverse TCR repertoire that recognizes different peptides, the iNKT cell receptor is invariant and recognizes only the pattern of a lipid antigen, akin to innate immune responses. Cytokines secreted from the antigen-presenting cell, in response to toll-like receptor activation, further enhance iNKT cell activation. Within hours, activated iNKT cells rapidly produce large quantities of cytokines (interferon-γ, tumor necrosis factor-α, interleukin [IL]-2 and IL-4), which may activate B cells, T cells, NK cells and dendritic cells (35). In addition, interferon-γ stimulates the production of the chemokines C-X-C motif chemokine 9 precursor (CXCL9), CXCL10 and CXCL11, potent chemoattractants for CXC receptor 3 (CXCR3)-expressing lymphocytes (36). In a mouse model of SCD, interruption of iNKT cell activation or depletion of iNKT cells reduces tissue injury (22).
Regadenoson
Administration of an adenosine A2A receptor agonist is one strategy used to block iNKT cell activation in a SCD mouse model (22,37). On the basis of that preclinical data, a phase I study of the A2A receptor agonist, regadenoson, was performed in patients (38). Twenty-seven SCD patients were administered infusional regadenoson. The target dose of 1.44 µg/kg/h was achieved without toxicity. During VOC, a 24-h infusion of regadenoson decreased the percentage of activated iNKT cells by a median of 50%. A multicenter, randomized, double-blind, placebo-controlled phase IIb trial is currently underway to examine the potential clinical benefits of regadenoson during VOC (clinicaltrials.gov, NCT01788631).
NKTT 120
NKTT 120 is a humanized monoclonal antibody directed against a unique epitope on the invariant T-cell receptor of iNKT cells (39). Preliminary results of an ongoing phase I study (clinicaltrials.gov, NCT01783691) demonstrate that NKTT 120 is safe in doses up to 0.01 mg/kg and rapidly depletes iNKT cells in a dose-dependent fashion (40). The return of iNKT cells to circulation is also dose-dependent and inversely related to the concentration of iNKT cells. Further studies will be needed to determine if long-term depletion of iNKT cells with NKTT 120 decreases VOC rate and chronic morbidity.
Limitations of selectin- and iNKT cell-based therapies
Even though rivipansel and regadenoson may improve outcomes during VOC, they are unlikely to completely abrogate the associated risks of VOC, including the risk of death. Monoclonal antibodies, such as SelG1 or NKTT 120, offer the potential to prevent VOC, a much superior approach. Long-term inhibition of P-selectin or iNKT cells in patients with SCD may, however, carry concomitant risks. Both play important roles in immunity, and their chronic inhibition may increase susceptibility to infection in an already infection-prone patient population.
Hemolysis
Recent studies have suggested an adverse impact of red cell hemolysis on SCD beyond that of anemia. Sickled red cells have a shortened lifespan of 10–20 d, and irreversibly sickled cells may be removed in hours (41). This rapid clearance of sickle erythrocytes may be due to engulfment by monocytes or macrophages, complement deposition or entrapment in the microvasculature (42). Rapid hemolysis releases red cell contents, including hemoglobin, free heme and arginase, into the circulation with a myriad of downstream effects that ultimately affect the pathogenesis of SCD (43).
Nitric Oxide and Free Heme
Deleterious effects of hemolysis are mediated in part by a reduction in NO bioavailability and the actions of free heme (44–47). When hemoglobin is disgorged into the circulation, heme iron reacts with and depletes nitric oxide (NO) to form nitrate (NO3−). Intraery-throcyte arginase is also released and metabolizes l-arginine, a key substrate for NO synthesis. The actions of NO, including vasodilation, platelet inhibition and decreased inflammation, oppose many of the pathogenic mechanisms that contribute to vaso-occlusion. Therefore, a disease model emerges whereby release of cell-free hemoglobin during hemolysis and depletion of NO may lead to vasoconstriction and a prothrombotic, proinflammatory environment that promotes vaso-occlusion (48). Whether cell-free hemoglobin and arginase are released in amounts sufficient to deplete NO and induce VOC in most patients is a matter of dispute (49).
Heme groups liberated from cell-free hemoglobin during hemolysis may also contribute to the toxicity associated with hemolysis (45–47). In mouse models of SCD, free heme has been shown to activate endothelial cells through toll-like receptor 4 signaling, inducing a proadhesive endothelial cell phenotype that promotes the red cell, white cell and platelet interactions that underlie vaso-occlusion (46,47). These negative effects of heme can be blocked by inhibition of toll-like receptor 4 or the administration of the heme-binding protein hemopexin (46,47).
NO and Pulmonary Hypertension
Pulmonary hypertension is the most notable complication of SCD thought to be, in part, secondary to hemolysis and reduced NO bioavailability. In a prospective cohort study of 195 patients with SCD, 30% had evidence of pulmonary hypertension, defined as a peak tricuspid regurgitant jet velocity (TRJV) ≥2.5 m/s on echocardiogram (50). Individuals with pulmonary hypertension had a 10-fold higher risk of death than those without. The pulmonary hypertension described in this study was mild compared with the definitions used for the general population, raising questions as to whether the pulmonary hypertension was actually a contributing cause of death or just a marker of severe SCD.
More controversy surrounding the role of pulmonary hypertension arose when a prospective cohort study of 398 adult patients with SCD, 96 of who underwent right heart catheterization, was published (51). Although 27% of the cohort had TRJV ≥2.5 m/s, only 6% had evidence of pulmonary hypertension that was confirmed on right heart catheterization, defined as a mean pulmonary artery systolic pressure >25 mmHg. Of those patients with confirmed pulmonary hypertension, there was mix of pulmonary arterial hypertension, consistent with an NO depletion model, and pulmonary venous hypertension, more consistent with left-sided heart disease. A TRJV ≥2.5 m/s had a positive predictive value of only 25% for pulmonary hypertension, confirmed on right heart catheterization; however, the positive predictive value increased to 64% when a TRJV cutoff of 3.0 m/s was used. The group with TRJV ≥3.0 m/s was older and had worse exercise capacity and a higher brain naturetic peptide level (consistent with symptomatic, physiologically relevant disease).
Sildenafil for Pulmonary Hypertension
Extending these findings to a therapeutic trial, investigators sought to determine whether treating pulmonary hypertension with sildenafil in adults with SCD would improve exercise capacity (52). Sildenafil is a phosphodiesterase-5 inhibitor that increases levels of cyclic guanosine monophosphate (cGMP), which mediates the vasodilation effects of NO. Unfortunately, the trial was stopped because a significantly high percentage of patients in the group receiving sildenafil required hospitalization for pain. The most likely explanation for the increased rate of pain in the sildenafil group is cGMP-related effects on pain signaling as opposed to worsened vaso-occlusion (53).
Limitations of Hemolysis- and Pulmonary Hypertension-Based Therapies
The contribution of pulmonary hypertension to morbidity and mortality of patients with SCD remains an important concern whether the hypertension is produced by NO depletion or other mechanisms such as pulmonary arterial thrombosis (54,55). There is a growing concern in the field that even mild pulmonary hypertension, defined by a TRJV ≥2.5 m/s, might contribute to SCD mortality, perhaps because of exacerbations of pulmonary pressures during acute VOC. Whether treatment of patients with SCD and pulmonary hypertension reduces morbidity or mortality remains an open question. In the absence of definitive data, an American Thoracic Society consensus group recently recommended treating patients with TRJV ≥2.5 m/s with aggressive SCD-based therapy, either hydroxyurea or chronic transfusions, while reserving pulmonary hypertension therapies (for example, endothelin-1 receptor antagonists) for those with right heart catheterization-proven pulmonary arterial hypertension (56). The use of chronic transfusion with its attendant risks for patients with TRJV ≥2.5 m/s will be disputed in many quarters.
Hematopoietic Stem Cell Transplant/Gene Therapy
Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) offers the potential for a cure from SCD. Largely as an extension of the thalassemia experience, HSCT has been investigated in patients with SCD for the past 30 years. In 1984, there was an initial report of a successful HSCT in a child with SCD who developed acute myeloid leukemia (57). Thereafter, a larger study (n = 22) using matched-sibling donors and high-intensity preparatory chemotherapy demonstrated 90% survival and 70% disease-free survival, along with stabilization of end-organ dysfunction (58). Despite these promising results, high mortality rates in patients older than 16 years and a paucity of suitable HLA-identical donors have limited the widespread implementation of HSCT in this patient population (59). Overcoming these obstacles to allow more patients with SCD to undergo HSCT is the focus of current investigations.
Reduced-intensity HSCT. Recent data suggest that reduced-intensity regimens before HSCT decrease toxicity in adults with SCD. Preparatory therapy eliminates the recipient hematopoietic cells and creates space in the bone marrow, allowing for donor engraftment and hematopoiesis. Reduced-intensity regimens do not completely ablate the bone marrow, but do make enough space for donor hematopoiesis, often creating a state of chimerism between the recipient and donor. Thus, the patient still produces sickle erythrocytes, albeit at a lower percentage of the circulating cells that may reverse the SCD phenotype. Using a reduced-intensity regimen, 10 adults with SCD, ages 16–45 years, underwent HSCT from matched-sibling donors (60). No deaths occurred, and there was no graft versus host disease, which has complicated other lower-intensity regimens (61). Stable engraftment was achieved in 9 of 10 subjects. In these nine engrafted subjects, the percentage of sickle hemoglobin was similar to donors, five of whom had sickle cell trait. Similar to studies in children, endorgan disease stabilized and pain symptoms improved. Recently, this group published findings from a larger cohort of 30 adults with SCD who underwent an HSCT after reduced-intensity conditioning. Consistent with the prior study, the regimen was well tolerated with a high rate of stable engraftment (87%) and low rate of graft versus host disease (62). This approach addressed the problem of toxicity in adults. Identification of suitable donors remained a problem, however, since only 20% of patients screened as part of the original study had an HLA-identical donor (60).
HSCT with alternative donors. The use of haploidentical donors has the potential to increase the number of patients with SCD able to undergo HSCT. Nearly all patients will have a haploidentical donor, who is half-matched at HLA antigens (for example, a parent). The downside of this approach is a graft failure rate of nearly 50%. Fourteen patients underwent HSCT from a haploidentical donor after a low-intensity preparatory regimen (63). Although six patients rejected the graft (43%), there were also five patients who achieved full donor chimerism. Those who rejected their grafts reverted back to SCD phenotype. There were no deaths.
Based on these data, HSCT is recommended for those children with an HLA-identical sibling donor. HSCT is not yet standard for adults with SCD, although a 50% chance for a cure with a haploidentical transplant may be a viable treatment option in the future, since the risk of mortality appears to be low.
Gene Therapy
Correction of the β-globin gene may be the ideal approach to cure SCD in the future. In mouse models, strategies to transplant genetically modified hematopoietic stem cells (HSCs), expressing normal β-globin gene or an antisickling globin, have been investigated (64–67). One approach relies on a lentivirus vector to integrate β-globin or antisickling globin genes into the genome of mouse HSCs, which are then transplanted into irradiated mice (68). Insertional mutagenesis is a theoretical and factual concern. If the lentivirus disrupts a tumor suppressor gene or activates an oncogene, leukemia may result. Despite these concerns, there are trials ongoing to evaluate this approach in patients with β-thalassemia major (clinicaltrials.gov, NCT01639690) and SCD (clinicaltrials.gov, NCT02186418). Homologous recombination, a strategy to edit DNA that may avoid insertional mutagenesis, is also being studied. This technique has the potential to change a βS-globin (sickle) gene to a normal β-globin gene in a site-specific manner. In mice with SCD, investigators have used homologous recombination to correct a β-globin gene in fibroblast-derived induced pluripotent stem (iPS) cells (69). Corrected iPS clones are then differentiated to HSCs and transplanted into irradiated mice, reversing the SCD phenotype. Genetic modification of human iPS cells could cure patients with SCD; however, there are many challenges to overcome before such clinical trials become a reality (70).
Conclusion
With several drugs in the investigational pipeline and new approaches to gene therapy under development, the prospect of new therapies for patients with SCD has never been better. The goal of any of these therapies is to help patients with SCD and to ease suffering. To this end, some of the approaches discussed are theoretically better than others. A guiding principle when considering the potential benefit of SCD therapies is: the further upstream the target, the better. In SCD, a single gene defect causes red cell sickling, which has widespread downstream effects on nearly every organ. If the gene is fixed, red cell sickling does not occur, and all of the downstream pathologies are prevented. Approaches that address mechanisms downstream of the mutation and erythrocyte sickling (for example, adhesion, inflammation and hemolysis) attempt to minimize the damage, conceding that some damage from erythrocyte sickling is probably inevitable. Potentially, the greatest value from these downstream therapies is in combination with other therapies, such as an antisickling therapy. Short of a cure, multiple drugs to target multiple mechanisms, similar to chemotherapy regimens in cancer, may be the optimal approach for SCD.
Finally, health care delivery markedly influences the potential benefits of new therapies for patients with SCD. Much of the care provided for patients with SCD occurs in the primary care setting, outside of specialized centers. Because primary care physicians have not been trained to treat patients with SCD, new treatments may not be prescribed, even if they are highly effective. Bear in mind that nearly two decades after the approval of hydroxyurea, most patients with SCD are suboptimally treated with it, or not treated at all. Any of the new therapies discussed here may be similarly underused, which may be the most difficult problem of all.
Disclosure
DG Nathan and JJ Field receive research support from NKT Therapeutics and Astellas. DG Nathan and JJ Field are the PIs of the phase 2 regadenoson study supported by NIH.
References
Savitt TL, Goldberg MF. (1989) Herrick’s 1910 case report of sickle cell anemia: the rest of the story. JAMA. 261:266–71.
Herrick JB. (1910) Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. Arch. Intern. Med. 6:517–21.
Pauling L, et al. (1949) Sickle cell anemia: a molecular disease. Science. 110:543–8.
Platt OS, et al. (1991) Pain in sickle cell disease: rates and risk factors. N. Engl. J. Med. 325:11–6.
Castro O, et al. (1994) The acute chest syndrome in sickle cell disease: incidence and risk factors: the Cooperative Study of Sickle Cell Disease. Blood. 84:643–9.
Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. (2005) Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 84:363–76.
Platt OS, et al. (1994) Mortality in sickle cell disease: life expectancy and risk factors for early death. N. Engl. J. Med. 330:1639–44.
Scott RB. (1970) Health care priority and sickle cell anemia. JAMA. 214:731–4.
Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. (2010) Improved survival of children and adolescents with sickle cell disease. Blood. 115:3447–52.
Gaston MH, et al. (1986) Prophylaxis with oral penicillin in children with sickle cell anemia: a randomized trial. N. Engl. J. Med. 314:1593–9.
Charache S, et al. (1995) Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 332:1317–22.
Platt OS, et al. (1984) Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J. Clin. Invest. 74:652–6.
Steinberg MH, et al. (2003) Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 289:1645–51.
Steinberg MH, et al. (2010) The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5 year follow-up. Am. J. Hematol. 85:403–8.
Steinberg MH. (1997) Determinants of fetal hemoglobin response to hydroxyurea. Semin. Hematol. 34:8–14.
Lanzkron S, Carroll CP, Haywood C Jr. (2013) Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep. 128:110–6.
Xu J, et al. (2011) Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 334:993–6.
Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. (2002) Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. U. S. A. 99: 3047–51.
Hebbel RP. (1997) Adhesive interactions of sickle erythrocytes with endothelium. J. Clin. Invest. 100:S83–6.
Hebbel RP, Osarogiagbon R, Kaul D. (2004) The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 11:129–51.
Kaul DK, Hebbel RP. (2000) Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J. Clin. Invest. 106:411–20.
Wallace KL, et al. (2009) NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 114:667–76.
Tedder TF, Steeber DA, Chen A, Engel P. (1995) The selectins: vascular adhesion molecules. FASEB J. 9:866–73.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–89.
Chang J, et al. (2010) GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 116:1779–86.
Matsui NM, et al. (2001) P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood. 98:1955–62.
Matsui NM, Varki A, Embury SH. (2002) Heparin inhibits the flow adhesion of sickle red blood cells to P-selectin. Blood. 100:3790–6.
Embury SH, et al. (2004) The contribution of endothelial cell P-selectin to the microvascular flow of mouse sickle erythrocytes in vivo. Blood. 104:3378–85.
Kutlar A, et al. (2012) A potent oral P-selectin blocking agent improves microcirculatory blood flow and a marker of endothelial cell injury in patients with sickle cell disease. Am. J. Hematol. 87:536–9.
Telen MJ. (2014) Cellular adhesion and the endothelium: E-selectin, L-selectin, and pan-selectin inhibitors. Hematol. Oncol. Clin. North Am. 28:341–54.
Bernini JC, et al. (1998) Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 92:3082–9.
Griffin TC, McIntire D, Buchanan GR. (1994) High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N. Engl. J. Med. 330:733–7.
Quinn CT, et al. (2011) Tapered oral dexamethasone for the acute chest syndrome of sickle cell disease. Br. J. Haematol. 155:263–7.
Brennan PJ, Brigl M, Brenner MB. (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13:101–17.
Tupin E, Kinjo Y, Kronenberg M. (2007) The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5:405–17.
Singh UP, Venkataraman C, Singh R, Lillard JW Jr. (2007) CXCR3 axis: role in inflammatory bowel disease and its therapeutic implication. Endocr. Metab. Immune Disord. Drug Targets. 7:111–23.
Wallace KL, Linden J. (2010) Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 116:5010–20.
Field JJ, et al. (2013) Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood. 121:3329–34.
Field JJ, Nathan DG, Linden J. (2011) Targeting iNKT cells for the treatment of sickle cell disease. Clin. Immunol. 140:177–83.
Field JJ, et al. (2013) A phase 1 single ascending dose study of NKTT120 in stable adult sickle cell patients [abstract]. Blood. 122:977.
Bertles JF, Milner PF. (1968) Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J. Clin. Invest. 47:1731–41.
Mohandas N, Hebbel R. (1994) Pathogenesis of Hemolytic Anemia. In: Sickle Cell Disease: Basic Principles and Clinical Practice. Embury SH, Hebbel RP, Mohandas N, Steinberg MH (eds.) New York, Raven Press, pp. 327–334.
Reiter CD, et al. (2002) Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8:1383–9.
Gladwin MT, et al. (2003) Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 107:271–8.
Belcher JD, et al. (2006) Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J. Clin. Invest. 116:808–16.
Belcher JD, et al. (2014) Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 123:377–90.
Ghosh S, et al. (2013) Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J. Clin. Invest. 123:4809–20.
Kato GJ, Gladwin MT, Steinberg MH. (2007) Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 21:37–47.
Bunn HF, et al. (2010) Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 116:687–92.
Gladwin MT, et al. (2004) Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N. Engl. J. Med. 350:886–95.
Parent F, et al. (2011) A hemodynamic study of pulmonary hypertension in sickle cell disease. N. Engl. J. Med. 365:44–53.
Machado RF, et al. (2011) Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 118:855–64.
Schmidtko A, Tegeder I, Geisslinger G. (2009) No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 32:339–46.
Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. (2001) Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch. Pathol. Lab. Med. 125:1436–41.
Weil JV, et al. (1993) NHLBI Workshop Summary: Pathogenesis of lung disease in sickle hemoglobinopathies. Am. Rev. Respir. Dis. 148:249–56.
Klings ES, et al. (2014) An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am. J. Respir. Crit. Care Med. 189:727–40.
Johnson FL, et al. (1984) Bone-marrow transplantation in a patient with sickle-cell anemia. N. Engl. J. Med. 311:780–3.
Walters MC, et al. (1996) Bone marrow transplantation for sickle cell disease. N. Engl. J. Med. 335:369–76.
Bhatia M, Walters MC. (2008) Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 41:109–17.
Hsieh MM, et al. (2009) Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N. Engl. J. Med. 361:2309–17.
Srinivasan R, et al. (2006) Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br. J. Haematol. 133:305–14.
Hsieh MM, et al. (2014) Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 312:48–56.
Bolanos-Meade J, et al. (2012) HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 120:4285–91.
Levasseur DN, et al. (2004) A recombinant human hemoglobin with anti-sickling properties greater than fetal hemoglobin. J. Biol. Chem. 279:27518–24.
Pawliuk R, et al. (2001) Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 294:2368–71.
Pestina TI, et al. (2009) Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol. Ther. 17:245–52.
Perumbeti A, et al. (2009) A novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: critical determinants for successful correction. Blood. 114:1174–85.
Chandrakasan S, Malik P. (2014) Gene therapy for hemoglobinopathies: the state of the field and the future. Hematol. Oncol. Clin. North Am. 28:199–216.
Hanna J, et al. (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 318:1920–3.
Townes TM. (2008) Gene replacement therapy for sickle cell disease and other blood disorders. Hematology Am. Soc. Hematol. Educ. Program. 2008:193–6.
Acknowledgment
This paper is dedicated to Dr. Anthony Cerami, founder of the journal Molecular Medicine and longtime friend and colleague.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Field, J.J., Nathan, D.G. Advances in Sickle Cell Therapies in the Hydroxyurea Era. Mol Med 20 (Suppl 1), S37–S42 (2014). https://doi.org/10.2119/molmed.2014.00187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2014.00187