Abstract
PDZ domain containing 1 (PDZK1) is a scaffold protein that plays a role in the fate of several proteins. Estrogen can induce PDZK1 gene expression; however, our recent report showed that PDZK1 expression in the breast cancer cell line MCF-7 is indirect and involves insulin-like growth factor (IGF)-1 receptor function. Such a relationship was established in cell culture systems and human breast cancer tissues. Here we show that overexpression of PDZK1 promoted an increase in cyclin D1 and enhanced anchorage-independent growth of MCF-7 cells in the absence of 17β-estradiol, suggesting that PDZK1 harbors oncogenic activity. Indeed, PDKZ1 overexpression enhanced epidermal growth factor receptor (EGFR)-stimulated MEK/ERK1/2 signaling and IGF-induced Akt phosphorylation. PDZK1 appeared to play this role, in part, by stabilizing the integrity of the growth promoting factors Akt, human epidermal growth factor receptor 2 (Her2/Neu) and EGFR. Increased Akt levels occurred via a decrease in the ubiquitination of the kinase. PDZK1 overexpression was associated with resistance to paclitaxel/5-fluorouracil/etoposide only at low concentrations. Although the increased stability of Akt was sensitive to heat shock protein 90 (HSP90) inhibition, increased levels of the cochaperone cell division cycle 37 (Cdc37), as well as its ability to bind PDZK1, appear to play a larger role in kinase stability. Using human tissue microarrays, we show strong positive correlation between PDZK1, Akt and Cdc37 protein levels, and all correlated with human breast malignancy. There were no positive correlations between PDZK1 and Cdc37 at the mRNA levels, confirming our in vitro studies. These results demonstrate a relationship between PDZK1, Akt and Cdc37, and potentially Her2/Neu and EGFR, in breast cancer, representing a new axis that can be targeted therapeutically to reduce the burden of human breast cancer.
Similar content being viewed by others
Introduction
Breast cancers that develop genetic and/or epigenetic changes increase the incidence of disease recurrence and distant metastases, as well as the development of resistance to standard therapies that ultimately decrease the overall survival of affected patients (1–3). Changes leading to increased expression of Akt, human epidermal growth factor receptor 2 (HER2/Neu) or epidermal growth factor receptor (EGFR), for example, are associated with highly metastatic and therapy-resistant breast cancers (4,5). The posttranslational fate of these factors is often governed by their association with chaperones such as heat shock protein 90 (Hsp90) (6,7). Inhibition of Hsp90, for instance, was shown to compromise the integrity of several proon-congenic factors, thus making it a rather appealing therapeutic stagey against cancer (6,8). The efficiency of Hsp90 in promoting the stability of client proteins is mediated by cochaperones such as cell division cycle 37 (Cdc37) (9). Cdc37 is primarily responsible for securing the integrity of protein kinases such as Akt and EGFR (7,10). Akt, a serine/threonine kinase, is critical for the mediation of cell signaling initiated by growth factors, cytokines and other cellular stimuli (11). Perturbations in Akt activity are associated with numerous pathologies including breast cancer (11). Akt promotes a reduction of cell cycle inhibitors such as the cyclin-dependent kinase inhibitor 1 (p21/WAF1) and p27Kip1 and an enhancement of cell cycle proteins such as c-Myc and cyclin D1 (12).
PDZ domain containing 1 (PDZK1) is a 70-kDa adapter protein expressed primarily in the apical brush-border membrane of the proximal tubules of the kidney (13) and has an important role in lipid metabolism. PDZK1 helps maintain the integrity of SR-B1 in a posttranscriptional manner (13,14). It is noteworthy that in normal conditions, PDZK1 is marginally expressed in other organs, including the liver and intestine, but is not detected in the breast (13). The PDZK1 gene was initially reported to be estrogen-responsive in the breast cancer cell line MCF-7 (15). Additionally, a significant association between plasma 17β-estradiol (E2) levels and PDZK1 mRNA expression levels in estrogen receptor (ER)-a(+) human breast cancer was established (16), strengthening the relationship between ER-α and PDZK1. In a recent investigation (17), we conducted a series of studies in which we found that E2-induced PDZK1 mRNA expression was slow and was blocked upon inhibition of protein synthesis. These data suggest indirect involvement of ER-α and a requirement for an intermediate ER-regulated gene product. Gene expression array analysis identified insulin-like growth factor 1 receptor (IGF-1R) as a potential candidate. Interestingly, PDZK1 knockdown completely blocked E2-dependent growth of MCF-7 cells and reduced c-Myc expression, suggesting its role during cell proliferation. PDZK1 overexpression stimulated MCF-7 cell proliferation and enhanced E2 growth promotion, potentially through an increase in c-Myc expression. This function may be related to the physical interaction between the PDZK1 and the Src/ER/EGFR complex and enhanced EGFR-mediated signal transduction. This enhancement of the extracellular-signal-regulated kinase 1 and 2 (ERK1/2) pathway may, in part, explain the involvement of PDZK1 in promoting E2-mediated growth of MCF-7 cells. However, these results do not explain the role of PDZK1 in enhancing cell growth in the absence of E2.
The present study was designed to specifically address the mechanism(s) by which PDZK1 enhances growth of breast cancer cells in the absence of E2 by focusing on key factors involved in cell growth, particularly Akt.
Materials and Methods
Materials
Dulbecco’s modified Eagle medium (DMEM), penicillin, streptomycin and fetal bovine serum (FBS) were from Invitrogen/Life Technolgies (now Thermo Fisher Scientific, Minneapolis, MN, USA). Charcoal/dextran-treated FBS was purchased from Hyclone (Logan, UT, USA); E2, 5-fluorouracil, paclitaxel and etoposide were from Sigma-Aldrich (St. Louis, MO, USA); recombinant human epidermal growth factor (EGF) was from R&D Systems (Minneapolis, MN, USA), MG132 was from Calbiochem (San Diego, CA, USA); AG1478 was from Cell Signaling Technology (Danvers, MA, USA); and geldanamycin-derivative 17-AAG was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Unless otherwise indicated, all other drugs were purchased from Sigma-Aldrich.
Cell Culture, Cell Proliferation, Cell Survival, Transfection, Immunoblot Analysis and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
MCF-7 cells (ATCC, Manassas, VA, USA) were cultured according to ATCC specifications. Before treatment with E2 or EGF, medium was changed to DMEM supplemented with 5% charcoal/dextran-treated FBS. Cell proliferation was measured by MTT assay kit (Roche, Indianapolis, IN, USA). MCF-7 cells were transiently transfected with specific siRNAs targeting Cdc37 (sc-29255 from Santa Cruz Biotechnology or O3 from Thermo Scientific, Pittsburgh, PA, USA) or the negative control (scrambled) siRNA NEG3 (SA Bioscience, Frederick, MD, USA) by using Lipofectamine 2000 (Invitrogen/Life Technologies) according to the manufacturer’s instructions. Cells were also stably transfected with an expression vector encoding human PDZK1 or empty vector (pcDNA3.1), and positive clones were isolated after selection. Clones were combined to avoid clonal differences except where indicated. Cells were then treated as described in the figure legends before being subjected to total RNA or protein preparation. Isolated RNA was reverse-transcribed and the resulting cDNA was subjected to conventional PCR with primer sets (IDT, San Jose, CA, USA) specific to human PDZK1, Cdc37, Akt1 or β-actin. Protein extracts were subjected to immunoblot analysis with antibodies to PDZK1(EPR3751) (Novus Biological), phospho-ERK1/2, ERK1/2 (total), HSP90(C45G5), EGFR, Akt (pan)(C67E7), phospho(Ser473)-Akt (all purchased from Cell Signaling Technology), mono- and polyubiquitinylated conjugates (FK2) (Enzo Life Sciences, Farmingdale, NY, USA), poly (ADP-ribose) polymerase 1 (PARP-1; BD Biosciences, San Jose, CA, USA), the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) (Abcam, Cambridge, MA, USA), Cdc37, Her2/Neu, cyclin D1 or GADPH (all from Santa Cruz Biotechnology). Immune complexes were detected with appropriate secondary antibodies and chemiluminescence reagents (Pierce, Rockford, IL, USA). For cell viability/death, cells were seeded in 96-well plates at a density of 5,000 cells/well and then treated with different concentrations of 5-fluorouracil, paclitaxel or etoposide for 48 h. Cell death was determined by using the MTT assay.
Clonogenic Soft Agar Assay
Anchorage-independent cell growth was measured in six-well plates. A 1-mL layer of 0.5% agar (Gibco/Life Technologies [now Thermo Fisher Scientific]) in tissue culture medium was solidified in the bottom of each well. Cells to be assayed were suspended at 37°C in 1 mL of 0.35% agar in tissue culture medium, and then 2.5 × 103 pcDNA3.1 MCF-7 or PDZK1 over-expressing MCF-7 cells were added per well. Both cells were then added in 2 mL medium to the top of the agar, and the medium was changed every 3 d. After 10 d, all of the colonies were counted under a dissection microscope.
Immunoprecipitation
The lysates were initially pre-cleared by incubation with protein A/G plusagarose beads (Santa Cruz Biotechnology) before an overnight incubation at 4°C with the indicated specific antibodies or normal IgG. Protein A/G plus-agarose beads were then added to the lysates, and the mixtures were incubated on a rotating wheel at 4°C for an additional 4 h. The beads were pelleted and washed several times in wash buffer (0.1% NP-40, 50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 2 µg/mL leupeptin, 2 µg/mL aprotinin, 1 mmol/L phenylmethylsul-fonyl fluoride, 5 mmol/L NaF and 100 µmol/L Na3VO4). Antibody-antigen complexes were eluted in the sample buffer by boiling and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (18).
Immunohistochemistry
Breast cancer and normal tissue microarray (TMA) sections (US Biomax, Rockville, MD, USA), which included a total of 262 neoplastic tissue samples and 87 normal breast adjacent tissue cores (17), were subjected to immunohisto-chemistry with antibodies to PDZK1 (ProteinTech Group, Chicago, IL, USA), Akt (Cell Signaling Technology) or Cdc37 (Santa Cruz Biotechnology) essentially as described previously (8). TMA slides were processed essentially as recently described (17). Stained slides were scanned using the ×40 objective, and immunoreactivity was analyzed by using Image-Pro Plus software (version 6.0) (Media Cybernetics Inc., Rockville, MD, USA) as described previously (9,10). The measurement parameters included density mean, area sum and integrated optical density. The software system allows a computerized assessment of the density of the staining as a sum of the values for intensity of all the pixels of a counted region in an analyzed area as well as the total area in an unbiased manner. Threshold range of the colors of positive staining was selected in a way that both faint and strong signals were detected without a high background. Density of immunoreactivity was next determined for all areas with a positive signal according to the weighted histoscore method (19): Histoscore = ∑ (% negative staining × 0) + (% weak staining × 1) + (% moderate staining × 2) + (% strong staining × 3).
Statistical Analysis
Data are presented as means ± standard error of the mean from at least three separate experiments. Comparisons between multiple groups were performed with either a Student t test or one-way analysis of variance (ANOVA) with a Bonferroni test. Differences were considered statistically significant at p < 0.05. All statistical summaries and analyses were performed by using GraphPad software, version 5 (GraphPad, La Jolla, CA, USA).
All supplementary materials are available online at https://doi.org/www.molmed.org .
Results
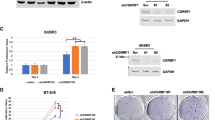
PDZK1 overexpression enhances expression of cyclin D1 and stimulates anchorage-independent growth of MCF-7 cells in the absence of E2. We previously showed that transient expression of PDZK1 was associated with increased expression of c-Myc and promotion of growth of MCF-7 cells independent of E2 (17). Consistent with the growth promoting trait of PDZK1, a stable overexpression of the protein promoted the accumulation of cyclin D1 in the complete absence of E2 (Figure 1A). These results suggest that PDZK1-overexpressing cells may have an oncogenic phenotype. Indeed, in anchorage-independent clonogenic assays, PDZK1 overexpression significantly increased the ability of MCF-7 cells to form colonies in soft agar (Figure 1B). The colonies increased in number as well as in size (Figure 1C). These results suggest that PDZK1 promotes or increases expression of growth promoting factors.
PDZK1 stimulates anchorage-independent growth of MCF-7 cells in the absence of E2. (A) MCF-7 cells were stably transfected with an expression vector encoding human PDZK1 or control empty vector (pcDNA3.1). Clones were selected and pooled. Protein extracts from these cells were subjected to immunoblot analysis with antibodies to PDZK1, cyclin D1 or GAPDH. (B) PDZKl-expressing or control vector-transfected MCF-7 cells were subjected to a clonogenic assay in soft agar in the absence of E2. Clones were counted 10 d later. *Difference from colony number generated by control vector-transfected cells, p < 0.05. (C) Depiction of representative clones generated by control vector-transfected (top panel) and PDZK1-expressing (lower panel) MCF-7 cells taken at the same magnification; the right panels depict a higher magnification of representative clones. Note the difference in size and number between the two groups.
PDZK1 overexpression stimulates growth of MCF-7 cells by increasing EGFR, Her2/Neu and Akt expression and associated signaling. In our previous report, we observed that treating MCF-7 cells with E2 induced a concomitant increase in PDZK1 and EGFR. We additionally found that PDZK1 may play a role in enhancing EGFR-mediated stimulation of the MEK/ERK pathway (where MEK is mitogen-activated protein kinase/extracellular-signal-regulated kinase [MAPK/ERK] kinase) (17). Akt and Her2/Neu are also critical factors for the proliferation and survival of breast cancer cells and are involved in signaling processes that lead to c-Myc and cyclin D1 gene expression (4,20-22). To test whether PDZK1 affects expression of the aforementioned growth factors, we examined whether the PDZK1 expression, in the absence of E2, enhances EGFR, Her2/Neu (EGFR2) and Akt expression. Figure 2A shows that stable expression of PDZK1 in MCF-7 cells was associated with a marked elevation in EGFR, Her2/Neu and Akt levels. However, expression of other proteins was not affected, including PARP-1 and DNA-PKcs. EGF-stimulated ERK1/2 phosphorylation was enhanced by PDZK1 overexpression (Figure 2B). Although EGF is not a strong inducer of Akt in MCF-7 cells (23), PDZK1 overexpression was associated with a transient but robust phosphorylation of Akt at serine-473 after 15 min in the presence of the growth factor (Figure 2B). Figure 2C shows that although the extent of Akt phosphorylation was unaffected by IGF-1, a potent inducer of Akt, the phosphorylation was persistent compared with its transient nature in MCF-7 cells expressing empty vector. Interestingly, however, the PDZK1-associated increase in Akt, Her/Neu and EGFR provided resistance to cell death induced by low concentrations of the established breast cancer chemotherapeutic drugs paclitaxel or 5-fluorouracil or the topoisomerase II inhibitor etoposide (Figure 2D). At higher concentrations, PDZK1 expression was not protective upon treatment with any of the drugs tested (data not shown). Overall, these results strongly suggest that PDZK1 influences a number of factors that are critical for breast cancer growth and are consistent with the ability of PDZK1 overexpression to enhance MCF-7 cell growth. However, increased expression of these factors only provided moderate protection against death induced by breast cancer therapeutic drugs.
PDZK1 overexpression is associated with an increase in EGFR, Her2/Neu and Akt protein levels and enhances EGF- and IGF-1-induced signal transduction. (A) Protein extracts from PDZK1-encoding or empty vector-transfected MCF-7 cells were subjected to immunoblot analysis with antibodies to PDZK1, EGFR, Her2/Neu, Akt, PARP-1, DNA-PKcs or GAPDH. (B) PDZK1-expressing or empty vector-transfected MCF-7 cells were treated with 20 ng/mL EGF for the indicated time intervals. Cells were collected and protein extracts were subjected to immunoblot analysis with antibodies to PDZK1, phospho-ERK1/2 (p-ERK), ERK1/2, phospho(S473)-Akt (p-Akt), Akt or GAPDH. (C) Cells were treated with 10 ng/mL IGF-1 for the indicated time intervals. Proteins extracts were subjected to immunoblot analysis with antibodies to p-Akt, Akt or GAPDH. (D) PDZK1-expressing or empty vector-transfected MCF-7 cells seeded in 96-well plates were treated with 5-fluorouracil, paclitaxel or etoposide at the indicated concentrations (Conc) in the absence of E2. Cell death was then determined by using the MTT assay 48 h after treatment. Data are expressed as a percentage of dead cells compared with untreated cells and are means ± standard deviation of values from six wells from a representative experiment. *Difference from respective untreated controls, p < 0.05. #Difference from treated empty vector-transfected MCF-7 cells, p < 0.05.
PDZK1 Increases Akt Levels Posttranslationally by Decreasing Its Targeting to the Ubiquitin System
Given the preceding results, it became imperative to examine how PDZK1 influences the expression of Akt, EGFR and Her2/Neu. For the purpose of the present study, we elected to concentrate our efforts on understanding the mechanism by which PDZK1 influences Akt protein levels. This stems from the observation that although Akt levels decreased after 1–2 h in the presence of IGF-1, Akt levels remained unchanged in PDZK1-expressing cells during the course of treatment. Accordingly, we hypothesized that this regulation may be at the protein level. Indeed, as shown in Figure 3A, the increase in Akt protein levels was not the result of increased mRNA levels. Because PDZK1 can regulate the stability of proteins, such as SR-B1 (14), and Akt can be regulated by proteolysis through the ubiquitin system (24), we speculated that PDZK1 increased Akt stability by preventing its ubiquitination. Therefore, we examined the effect of PDZK1 overexpression on the levels of Akt ubiquitination in the presence of the proteasome inhibitor MG132. Figure 3B shows that, whereas treating cells with MG132 dramatically increased the levels of ubiquitinated Akt, PDZK1 overexpression was associated with a blockade of this modification, as assessed by immunoprecipitation followed by immunoblot analysis. It is noteworthy that ubiquitinated proteins and Akt were not immunoprecipitated with control IgG (Supplementary Figure S1). Interestingly, these PDZK1-associated modifications were not linked to major modulations in the overall ubiquitination system (Figure 3C).
PDZK1 increases Akt levels posttranslationally by decreasing its ubiquitination. (A) Total RNA from PDZK1-expressing or empty vector-transfected MCF-7 cells were subjected to conventional RT-PCR with primers specific to human PDZK1, Akt1 or gapdh. (B) Cells were treated with the proteasome inhibitor MG132 for 12 h, after which protein extracts were prepared and subjected to immunoprecipitation (IP) with antibodies to Akt followed by immunoblot (IB) analysis with antibodies to ubiquitin (mono- and polyubiquitinylated conjugates) or Akt; input proteins (10% of total) were also subjected to immunoblot analysis with antibodies to Akt, PDZK1 or GAPDH (bottom panels). Immunoprecipitation with control IgG with immunoblot analysis is displayed in Supplementary Figure S1. (C) The same membrane as in (B) was probed with antibodies to ubiquitin (Ub); the asterisk (*) indicates that the GAPDH blot is the same as in (B).
PDZK1 Increases Akt Levels by Increasing and Interacting with the HSP90 Cochaperone Cdc37
The above results suggest that the associated increase in Akt expression observed with PDZK1 expression may be due to inhibition of Akt targeting to the proteasome system. A major mechanism by which Akt avoids being targeted to the proteasome system is via its association with the heat shock protein HSP90 (24,25). Indeed, inhibition of HSP90 by 17-AAG compromised the integrity of Akt in a dose-dependent manner in both control and PDZK1-overexpressing cells (Figure 4A). Surprisingly, the PDZK1-associated increase in Akt levels was not mirrored by a concomitant increase in HSP90 levels. Accordingly, the PDZK1-associated increase in Akt can only be associated with HSP90 function, but not its levels.
PDZK1 increases Akt levels by increasing and interacting with Cdc37. (A) PDZK1-expressing or empty vector-transfected MCF-7 cells were treated with increasing concentrations of the HSP90 inhibitor 17-AAG for 12 h, after which protein extracts were prepared and subjected to immunoblot analysis with antibodies to HSP90, Akt, PDZK1 or GAPDH. A lower exposure of the immunoblot for Akt is provided to show the increase in the kinase in PDZK1-overexpressing cells compared with the control counterpart. (B) PDZK1-expressing MCF-7 cells were transiently transfected with control siRNA (Con) or two different siRNA targeting Cdc37. Forty hours later, cells were collected and protein extracts were prepared and subjected to immunoblot analysis with antibodies to Cdc37, Akt or GAPDH. The immunoblots for Cdc37 and Akt were quantified by using ImageJ (U. S. National Institutes of Health, Bethesda, MD, USA, https://doi.org/imagej.nih.gov/ij/) and normalized to GAPDH levels (right panel); data are expressed as the fold-change from control. (C) Protein extracts from five clones of PDZK1-expressing or two clones of empty vector-transfected MCF-7 cells were subjected to immunoblot analysis with antibodies to Cdc37, PDZK1 or GAPDH. The immunoblot for Cdc37 was quantified as described above (right panel); data are expressed as the fold change from control. (D) Total RNA from PDZK1-expressing or empty vector-transfected MCF-7 cells were subjected to conventional RT-PCR with primers specific to human Cdc37 or gapdh. (E) Protein extracts from PDZK1-expressing MCF-7 cells were immunoprecipitated (IP) with antibodies to PDZK1 followed by immunoblot (IB) analysis with antibodies to Cdc37 or PDZK1. A 10% portion was loaded as input protein. (F) Protein extracts from PDZK1-expressing or empty vector-transfected MCF-7 cells were subjected to immunoprecipitation with antibodies to Cdc37. The precipitates were then analyzed by immunoblot with antibodies to Cdc37, PDZK1, Akt or GAPDH; a 10% portion was loaded as input protein. (G) The same protein extracts were subjected to immunoprecipitation with antibodies to PDZK1 followed by immunoblot analysis with antibodies to PDZK1, Cdc37 or Akt.
It is noteworthy that HSP90 functions as part of a multimeric chaperone complex that is aided by several cofactors (26). A cofactor that prominently secures the interaction of the HSP90 complex with kinases is the cell division cycle 37 homolog (Cdc37) (9). Akt is known to interact with Cdc37 (24), and Cdc37 knock-down reduces Akt levels (10). Consistent with these reports and as shown in Figure 4B, partial knockdown of Cdc37 using two different siRNAs in PDZK1-overexpressing MCF-7 cells reduced the levels of Akt, suggesting a potential connection between PDZK1 and Cdc37. Thus, we speculated that the PDZK1-associated increase in Akt levels might be associated with alterations in Cdc37 levels. We found PDZK1 overexpression was associated with a marked increase in Cdc37 protein levels (Figure 4C) without a concomitant increase in mRNA levels (Figure 4D). To determine whether PDZK1 interacts with Cdc37, we performed pull-down assays using antibodies to PDZK1. Figure 4E shows that PDZK1 interacted with Cdc37 in extracts from PDZK1-overexpressing MCF-7 cells. The interaction was confirmed by using pull-down assays with Cdc37 antibodies, followed by immunoblot analyses with antibodies to PDZK1 (Figure 4F). Interestingly, much greater levels of Akt were coimmunoprecipitated with PDZK1 and Cdc37 in extracts from PDZK1-overexpressing MCF-7 cells compared with extracts from MCF-7 cells expressing control vector. The interaction between the three proteins was also confirmed by coimmunoprecipitation by using antibodies to PDZK1 followed by immunoblot analyses with antibodies to the respective proteins. Together, these findings suggest an important interaction between PDZK1, Cdc37 and Akt that results in increased Akt stability and enhancement of associated signal transduction pathways.
Cdc35 expression is significantly increased in human breast cancer tissues at the protein, but not the mRNA, level and correlates with the expression pattern of PDZK1. Given the connection between PDZK1 and Cdc37 observed in vitro, we examined whether this relationship exists in human breast cancer tissues. In tissue microarrays of both breast cancer and normal tissues (clinical characteristics of the tissue microarray are described by Kim et al. [17]), Cdc37 protein levels were significantly higher in >75% of the carcinomas examined, as assessed by using immunohistochemistry (Figures 5A, B), suggesting a high correlation with breast malignancy. Cdc37 immunoreactivity was weak in normal tissues (Figures 5A, B) and appeared to be progressive with cancer stage, since the levels of Cdc37 were higher in invasive-ductal compared with intraductal cancer (Figure 5C). Unlike the moderate correlation between age and PDZK1 immunoreactivity reported in our previous study (17), there was no significant correlation between Cdc37 immunoreactivity and age (Supplementary Figure S2). Figure 5D depicts examples of Cdc37 immunoreactivity in normal and cancerous breast tissue. It is noteworthy that the expression of Cdc37 appeared to be mostly epithelial, since little immunoreactivity was detected in stromal cells (Figure 5D). Analyses of PDZK1 and Cdc37 immunoreactivity in serial sections revealed a significant positive correlation (r = 0.561, p < 0.001) (Figure 5E), thus supporting the association between PDZK1 upregulation in breast cancer tissues and Cdc37 levels. By using data deposited in the public domain Gene Expression Omnibus (GEO) Profiles Database (27) and conducted by Boersma et al. (28) (cohort 1: GDS3097) or Iwamoto et al. (29) (cohort 2: GDS4057), we examined correlations between PDZK1 (205380_at) and Cdc37 (209953_at) mRNA levels in fresh-frozen excised breast cancer tissues procured from a different cohort of patients. Figure 5F shows no positive correlation between the two transcripts in both cohorts; in fact, the correlation was significantly negative (r = −0.322, p = 0.028, and r = −0.252, p = 0.010, respectively). These results lend additional independent support to our observation that PDZK1 influences Cdc37 levels at the protein rather than mRNA level in breast cancer.
Correlation between PDZK1 and Cdc37 and an association with breast malignancy. (A) Depiction of scanned TMA sections (mostly normal tissues, top panel; mostly cancer tissues, bottom panel) subjected to immunohistochemical staining with antibodies to human Cdc37. (B) Histoscores of Cdc37 immunoreactivity in normal and cancer tissues. *Difference from histoscores in normal tissues, p ≤ 0.001. (C) Histoscores of Cdc37 immunoreactivity in normal tissues, tissues with intraductal carcinoma or tissues with invasive ductal carcinoma. *Difference from histoscores in normal tissues, p ≤ 0.001; difference from histoscores in tissues with intraductal carcinoma, p ≤ 0.005. (D) Tissue cores representing normal breast and cancer tissue. Scale bar: 4 µm. (E) Correlation of Cdc37 immunoreactivity with that of PDZK1 as assessed by immunohistochemistry by using TMA serial sections prepared from the same tissues where the Pearson correlation coefficient was r = 0.561 with p < 0.001. (F) Correlation between Cdc37 and PDZK1 transcripts (r = 0.322, p = 0.028) in breast cancer tissues from two different cohorts determined by using data deposited in the GEO Profiles Database (27) and conducted by Boersma et al. ((28) (cohort 1) or (29) (cohort 2)). (G) Histoscores of Akt immunoreactivity in normal and cancer tissues. *Difference from histoscores in normal tissues, p ≤ 0.001. (H) Correlation of Akt immunoreactivity with that of PDZK1 as assessed by immunohistochemistry by using TMA serial sections prepared from the same tissues (r = 0.540, p < 0.001). (I) Correlation of Akt immunoreactivity with that of Cdc37 in serial sections prepared from the same tissues (r= 0.744, p< 0.001).
Given the relationship between PDZK1 and Cdc37, it was conceivable to predict a correlation between these two factors and Akt in breast cancer tissues. In serial sections of the tissue microarray described above, Akt protein levels were found to be significantly higher in breast carcinomas compared with that in normal tissues, as assessed by using immunohistochemistry (Figure 5G). Analyses of PDZK1, Akt and Cdc37 respective immunoreactivity revealed a significant positive correlation between PZDK1 and Akt (r = 0.540, p < 0.001) (Figure 5H) and an even stronger correlation between Akt and Cdc37 (r = 0.744, p < 0.001) (Figure 5I). These results clearly support the relationship between the three different proteins suggested by our in vitro studies.
Discussion
Breast cancer heterogeneity remains a major obstacle in defining specific therapies with desirable clinical outcomes. Identifying the key players that influence the growth of breast cancer cells and resistance to available therapeutic drugs is therefore a critical step toward improving breast cancer therapy. This goal cannot be achieved without determining the mechanism(s) by which breast cancer cells gain the ability to proliferate and maintain elevated levels of growth-promoting factors, including Akt, EGFR and Her2/Neu. We previously demonstrated a strong correlation between PDZK1 protein expression and human breast cancer (17). Furthermore, we put forth a novel mechanism by which breast cancer cells may gain elevated growth traits associated with increased levels of growth-promoting factors (see model in Figure 6). More importantly, our results demonstrate a novel function for PDZK1: it contributes to E2-induced breast cancer cell growth by enhancing EGFR signaling in part through Akt stabilization. Interestingly, PDZK1 overexpression appeared to provide MCF-7 cell resistance to low concentrations of the chemotherapeutic drugs 5-fluorouracil, paclitaxel or etoposide. Increased Akt protein stability was potentially the result of elevated levels of the HSP90 cochaperone Cdc37 and interaction of PDZK with Cdc37. PDZK1 does not appear to influence Cdc37 levels at the mRNA level. This relationship was confirmed in breast cancer tissues because of a strong positive correlation between PDZK1, Akt and Cdc37. Interestingly, the correlation between PDZK1 and Cdc37 was found only at the protein but not mRNA level in two different cohorts of breast cancer tissues. To the best of our knowledge, this study is the first to report elevated levels of Cdc37, although it has long been known to influence a number of kinases and receptors known to drive breast cancer in humans.
Schematic representation of the involvement of PDZK1 in growth of cancer cells. Growth factors promote a controlled process of cell proliferation through activation of Akt via phosphorylation of the kinase followed by ubiquitination and ultimately degradation by the 26S proteasome system. In ER(+) breast cancer cells, estrogen induces expression of PDZK1 through an increase in IGF-1R. PDZK1, through an unknown mechanism, increases the protein, but not mRNA, levels of the HSP90 cochaperone Cdc37. Interaction of increased levels of Cdc37 with Akt decreases the targeting of the kinase to the ubiquitination/proteasomal degradation system. This step will then allow a promotion of a persistent and more pronounced growth signal that ultimately contributes to cancer cell proliferation and potentially resistance to chemotherapeutic drugs.
Recently, we found that although PDZK1 expression is not a direct product of ER stimulation, it may influence ER-α function through an interaction with the ER-α/EGFR/Src complex, thereby resulting in elevated levels of c-Myc (17). This observation is supported by the finding that PDZK1 knockdown reduces E2-induced c-Myc expression and EGFR-mediated ERK1/2 activation. The results of the current study show that PDZK1 expression was sufficient to enhance EGF-stimulated ERK1/2 phosphorylation, suggesting that this enhancement was not associated with any of the multiple factors that may be induced by E2 treatment in MCF-7 cells. This enhanced ERK1/2 activation may explain the association between PDZK1 expression and increased c-Myc and cyclin D1 in MCF-7 cells. An important feature of PDZK1 that may contribute to its role in breast cancer cell growth and resistance to chemotherapeutic drugs is its ability to stabilize Akt. Our results suggest that Akt stability may be related to the PDZK1-associated increase in the cochaperone Cdc37. This relationship is clearly displayed by the strong positive correlation between the three different factors. It appears that PDZK1 reduces Akt ubiquitination and thus its degradation by the proteasome system (Figure 6). Cdc37 is critical for the stability of numerous growth-promoting kinases, including Akt, and a reduction in the cochaperone drastically reduces the levels of client proteins (10).
The mechanism by which PDZK1 overexpression increases Cdc37 levels is not clear, but it appears that it occurs by a posttranslational mechanism because Cdc37 mRNA levels were not influenced by PDZK1. Given the ability of PDZK1 to maintain the integrity of the HDL receptor SR-B1 (30) in addition to its ability to interact with the cochaperone Cdc37, it is plausible that PDZK1 increases Cdc37 levels through persistent interaction. Our results do not unequivocally demonstrate a direct interaction between PDZK1 and Cdc37, and it is possible that PDZK1 interacts with any member of the HSP90 complex, including HSP90 itself, Akt or any other protein that might interact with the complex. This result is likely considering the report by Hu et al. (31), where a yeast two-hybrid screen of a random peptide library identified a number of putative proteins that may interact with PDZK1; however, Akt, Her2/Neu and EGFR were not among the candidates. Interestingly, other factors known to be involved in cell proliferation and signaling in breast cancer were identified in their screen, such as cyclin-dependent kinase 4 (CDK4) and insulin-like growth factor-binding protein 5 (IGFBP5) (32,33). PDZK1 function appears to be tissue-specific because gene deletion has been shown to completely inhibit SR-B1 expression exclusively in the liver, which explains the proatherogenic phenotype observed in PDZK1 knockout mice (14,34). It is, however, unclear whether the expression and function of PDZK1 observed in breast cancer could be applicable to other cancers. Therefore, more tissue-specific experimentation is required to clarify the exact function and specificity of PDZK1 in the context of cancer.
Conclusion
It is rather interesting that PDZK1 overexpression and the associated increase in Akt, Her2/Neu and EGFR conferred resistance of MCF-7 cells to only low concentrations of 5-fluorouracil, paclitaxel or etoposide. It is well established that overexpression of the latter factors provide substantial resistance to chemotherapeutic drugs in vitro, in animal models and in human disease. For instance, is the interaction of PDZK1 with Akt a determinant for this phenotype? This is a likely scenario. However, a study by Inoue et al. (35) examined a number of cell lines derived from multiple myelomas with high-level gene amplification at 1q12-q22 corresponding, in part, to PDZK1 with a subsequent overexpression at the protein level. The PDZK1-overexpressing cell lines exhibited resistance to melphalan-, cisplatin- and vincristin-induced death compared with cell lines with no PDZK1. Although the conclusions of the latter study were based on a correlation rather than a direct examination of PDZK1 function, detailed examinations of PDZK1 function in drug resistance are warranted.
Together, our findings provide evidence for a role of PDZK1 in driving growth of breast cancer cells by influencing the fate of critical factors in cell proliferation (Figure 6). This function may be mediated via increased Cdc37. More importantly, we report for the first time that Cdc37 protein is elevated in human breast cancer tissues and such elevation may be associated with increased PDZK1. Much work remains to decipher the exact mechanism by which PDZK1 influences Cdc37 expression in breast cancer cells. Overall, the results of the current study, along with data from our previous recent report (17), support the notion that PDZK1 and Cdc37 may constitute a new axis that can be targeted therapeutically to reduce or eliminate the burden of human breast cancer.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Miyoshi Y, Murase K, Saito M, Oh K. (2010) Prediction of hormone sensitivity for breast cancers. Breast Cancer. 17:86–91.
Sasano H, Nagasaki S, Miki Y, Suzuki T. (2009) New developments in intracrinology of human breast cancer: estrogen sulfatase and sulfotrans-ferase. Ann. N. Y. Acad. Sci. 1155:76–9.
Wang T, You Q, Huang FS, Xiang H. (2009) Recent advances in selective estrogen receptor modulators for breast cancer. Mini Rev. Med. Chem. 9:1191–201.
Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. (2012) Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 38:698–707.
de la Vega M, Diaz-Canton E, Alvarez RH. (2012) Novel targeted agents for the treatment of advanced breast cancer. Future Med. Chem. 4:893–914.
Hong DS, et al. (2013) Targeting the molecular chaperone heat shock protein 90 (HSP90): lessons learned and future directions. Cancer Treat. Rev. 39:375–87.
Pearl LH. (2005) Hsp90 and Cdc37: a chaperone cancer conspiracy. Curr. Opin. Genet. Dev. 15:55–61.
Garcia-Carbonero R, Carnero A, Paz-Ares L. (2013) Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 14:e358–69.
Karnitz LM, Felts SJ. (2007) Cdc37 regulation of the kinome: when to hold ‘em and when to fold ‘em. Sci. STKE. 2007:pe22.
Smith JR, Clarke PA, de Billy E, Workman P. (2009) Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene. 28:157–69.
Manning BD, Cantley LC. (2007) AKT/PKB signaling: navigating downstream. Cell. 129:1261–74.
Yoon MK, Mitrea DM, Ou L, Kriwacki RW. (2012) Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem. Soc. Trans. 40:981–8.
Kocher O, Comella N, Tognazzi K, Brown LF. (1998) Identification and partial characterization of PDZK1: a novel protein containing PDZ interaction domains. Lab. Invest. 78:117–25.
Kocher O, et al. (2003) Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J. Biol. Chem. 278:52820–5.
Ghosh MG, Thompson DA, Weigel RJ. (2000) PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 60:6367–75.
Dunbier AK, et al. (2010) Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J. Clin. Oncol. 28:1161–7.
Kim H, et al. (2013) PDZK1 is a novel factor in breast cancer that is indirectly regulated by estrogen through IGF-1R and promotes estrogen-mediated growth. Mol. Med. 19:253–62.
Zerfaoui M, et al. (2010) Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J. Immunol. 185:1894–902.
Flowers JL, et al. (1986) Use of monoclonal antie-strogen receptor antibody to evaluate estrogen receptor content in fine needle aspiration breast biopsies. Ann. Surg. 203:250–4.
Freudenberg JA, et al. (2009) The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp. Mol. Pathol. 87:1–11.
Renoir JM, Marsaud V, Lazennec G. (2013) Estrogen receptor signaling as a target for novel breast cancer therapeutics. Biochem. Pharmacol. 85:449–65.
Moulder SL. (2010) Does the PI3K pathway play a role in basal breast cancer? Clin. Breast Cancer. 10 (Suppl. 3):S66–71.
Agelaki S, et al. (2009) Caveolin-1 regulates EGFR signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol. Ther. 8:1470–7.
Basso AD, et al. (2002) Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 277:39858–66.
Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. (2003) Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 63:2139–44.
Panaretou B, et al. (2002) Activation of the AT-Pase activity of hsp90 by the stress-regulated cochaperone aha1. Mol. Cell. 10:1307–18.
Barrett T, et al. (2007) NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res. 35:D760–5.
Boersma BJ, et al. (2008) A stromal gene signature associated with inflammatory breast cancer. Int. J. Cancer. 122:1324–32.
Iwamoto T, et al. (2011) Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer. J. Natl. Cancer Inst. 103:264–72.
Kocher O, Krieger M. (2009) Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr. Opin. Lipidol. 20:236–41.
Hu S, et al. (2009) Systematic analysis of a simple adaptor protein PDZK1: ligand identification, interaction and functional prediction of complex. Cell. Physiol. Biochem. 24:231–42.
Yu Q, et al. (2006) Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 9:23–32.
Wang H, et al. (2008) IGFBP2 and IGFBP5 overexpression correlates with the lymph node metastasis in T1 breast carcinomas. Breast J. 14:261–7.
Kocher O, et al. (2008) Influence of PDZK1 on lipoprotein metabolism and atherosclerosis. Biochim. Biophys. Acta. 1782:310–6.
Inoue J, et al. (2004) Overexpression of PDZK1 within the 1q12-q22 amplicon is likely to be associated with drug-resistance phenotype in multiple myeloma. Am. J. Pathol. 165:71–81.
Acknowledgments
This work was supported in part by grant RSG-116608 from the American Cancer Society and grant HL072889 from the National Institutes of Health, as well as funds from the Louisiana Cancer Research Consortium (New Orleans, LA, USA) to AH Boulares.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (https://doi.org/creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Kim, H., Abd Elmageed, Z.Y., Davis, C. et al. Correlation between PDZK1, Cdc37, Akt and Breast Cancer Malignancy: The Role of PDZK1 in Cell Growth through Akt Stabilization by Increasing and Interacting with Cdc37. Mol Med 20, 270–279 (2014). https://doi.org/10.2119/molmed.2013.00166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2013.00166