Abstract
Each year an estimated 120 million episodes of pneumonia occur in children younger than 5 years of age, resulting in one million deaths globally. Within this age group the lungs are still developing by increasing alveoli numbers and airway dimensions. Pneumonia during this critical developmental period may therefore adversely affect the lung’s structure and function, with increased risk of subsequent chronic lung disease. However, there are few longitudinal studies of pneumonia in otherwise healthy children that extend into adulthood to help address this important question. Birth cohort, longitudinal, case-control and retrospective studies have reported restrictive and obstructive lung function deficits, asthma, bronchiectasis, and chronic obstructive pulmonary disease. In particular, severe hospitalised pneumonia had the greatest risk for long-term sequelae. Most studies, however, were limited by incomplete follow-up, some reliance upon parental recall, risk of diagnostic misclassification, and potential confounders such as nutrition, social deprivation, and pre-existing small airways or lungs. More long-term studies measuring lung function shortly after birth are needed to help disentangle the complex relationships between pneumonia and later chronic lung disease, while also addressing host responses, types of infection, and potential confounding variables. Meanwhile, parents of young children with pneumonia need to be advised about the importance of symptom resolution, post-pneumonia. In addition, paying attention to factors associated with optimising lung growth such as good nutrition, minimising exposure to air pollution, avoiding cigarette smoke, and decreasing the risk of preventable infections through good hygiene and having their children fully vaccinated should be emphasised. Finally, in the developing world and for disadvantaged communities in developed countries, public health policies leading to good quality housing and heating, hygiene, education, and improving socio-economic status are also essential.
Similar content being viewed by others
1. Introduction
1.1 The burden of pneumonia
Pneumonia is the greatest cause worldwide of childhood morbidity and mortality [1]. The most important respiratory pathogens implicated in severe and fatal cases of childhood pneumonia are Streptococcus pneumoniae [2], Haemophilus influenzae type b [3], respiratory syncytial virus (RSV) [4], and seasonal influenza virus [5], three of which are already targeted by vaccines that are licensed currently. In 2010, there were an estimated 120 million episodes of clinically diagnosed pneumonia involving children younger than 5 years of age globally, including 14 million with severe disease requiring hospital treatment [6]. Of greater concern, in 2013 as many as one million children died from pneumonia, accounting for 15% of all fatalities in this age group [7]. Most cases (>98%) of pneumonia and pneumonia-related deaths (>99%) are in developing countries, occurring mostly out of hospital and in the first year of life [6]. Nevertheless, pneumonia is also important in developed countries, and prior to the introduction of pneumococcal conjugate vaccines there were an estimated 2.6 million cases of pneumonia annually in children aged younger than 5 years, including 1.5 million requiring hospitalisation, and 3,000 deaths [8]. However, despite high vaccine uptake, disadvantaged indigenous children living in these affluent nations still have amongst the highest rates of pneumonia recorded [9,10]. In addition to the usual recognised risk factors for pneumonia, such as household overcrowding, low birthweight and exposure to inhaled environmental toxicants, indigenous children living in remote communities also experience early and heavy nasopharyngeal colonisation by pneumococcal serotypes not included in conjugate vaccines [11].
1.2 Clinical gaps
Despite the high prevalence of pneumonia, including its global clinical and economic importance, many gaps remain in our understanding and management of pneumonia in young children. These gaps, recently summarised in several reviews [12–14], include the long-term effects of pneumonia and its potential role in adult-focused pulmonary disorders [15]. This information is important, for not only managing individual patients, but also for understanding the true burden of disease, including the contribution of early childhood pneumonia to the estimated 200 million people with chronic obstructive pulmonary disease (COPD) and the 235 million people with asthma worldwide [16]. Only then can the full benefit of interventions, such as duration and type of antibiotics or the introduction of new vaccines into national immunisation programmes, be understood. In this review, we focus on the long-term (>6 months) consequences of pneumonia in children and we exclude studies that relate primarily to wheezing illness in children.
2. Pneumonia in young children and subsequent adult lung disease
Throughout the last three decades evidence has emerged suggesting that adult-onset chronic pulmonary disorders are likely to have their origins in early life. Here we review the evidence of the published data.
There are two published systematic reviews on the long-term impact of childhood pneumonia [17,18], with one review [17] also including studies relating to bronchiectasis. As the authors used different search terms, there were both similarities and differences between the two reviews. Nevertheless, the Thomson et al [17] review involved two additional prospective studies [19,20] on radiographically confirmed pneumonia.
In the studies included in the review by Edmonds et al [18], a total of 722 children from 13 studies (10 hospital, 3 community-based birth cohorts) were followed for sequelae, 61% of whom were younger than 2 years of age at the time of their pneumonia, and none had any identifiable pre-existing risk factors for chronic pulmonary disorders. Radiographic confirmation of pneumonia was available only for the 379 (52%) children included in the hospital-based studies. The median length of follow-up was 10.8 (interquartile range [IQR] 2.1–17.0) years and overall there was a median 34% (IQR 12%–45%) attrition rate of study subjects. Seventy-seven cases (10.4%; 95% confidence interval [CI] 5.4%–15.4%) were found to have major sequelae involving restrictive lung disease, obstructive lung disease or bronchiectasis. Meta-analysis showed the risk of one of these complications was higher in hospitalised (13.6%; 95% CI 6.2%–21.1%) than non-hospitalised children (5.5%; 95% CI 2.8%–8.3%), whilst among the pathogens, adenovirus pneumonia was associated with the highest risk (54.8%; 95% CI 39.2%–70.5%) of developing one of these major sequelae. Of the sequelae, restrictive lung disease was the most commonly detected (5.5%; 95% CI 2.5%–10.2%). Bronchiectasis, either alone (0.9%; 95% CI 0.07%–8.7%) or in combination with restrictive lung disease (1.2%; 95% CI 0.05%–7.7%), was found only in those who had been hospitalised for pneumonia and obstructive lung disease in those with a history of severe adenovirus pneumonia (2.8%; 95% CI 0.18%–6.4%). Nevertheless, in this review lung function tests suggesting airway obstruction were also reported in up to one-third of 62 South African children 1–7 years after their pneumonia [21] and in 14 of 18 (78%) 7–8-year-old children with a history of chlamydial pneumonia in early infancy [22]. Although children younger than 2 years of age had a greater risk of sequelae than those aged 2–5 years at the time of their pneumonia, this did not reach statistical significance (13.4%; 95% CI 4.5%–22.3% vs 8.7%; 95% CI 3.1%–14.3% respectively, odds ratio [OR] 1.22 [95% CI 0.21–7.1]). Furthermore, in the multivariable model only hospitalisation remained a major risk factor for sequelae (OR3.65 [95%CI 1.96–6.8]).
Here, we summarise studies identified from these reviews and our own search with a focus on prospective and case-control studies. Relevant studies were identified by searching the PubMed database using the terms ‘children’ and ‘pneumonia’ and ‘long term’, respectively in their titles or abstracts without language restrictions. Studies published prior to 13 April 2015 were included.
2.1 Prospective longitudinal and case-control studies
These data are summarised in Tables 1 and 2. Three large follow-up, community-based, birth cohort studies from the United Kingdom (UK) assessing sequelae in non-hospitalised children with clinically diagnosed pneumonia were identified. The extracted information for two birth cohort studies was from health visitor records of infants born between 1920 and 1930 in Hertfordshire County, England (males only) [23], and between 1921 and 1935 in Saint Andrews, Scotland, respectively [24]. The surviving participants from both cohorts were evaluated when they were aged in their 50s and 60s. The third community-based birth cohort study was from the 1958 British National Child Development Study where, at the age 7-year follow-up interview, parents were asked if their child ever had pneumonia [25]. Subjects in this study were assessed at 34 to 35 years of age. Each of these three birth cohort studies reported that pneumonia in early childhood was associated with restrictive lung function.
In contrast, two other birth cohorts [26,27] indicated pneumonia in early childhood was associated with an obstructive rather than restrictive lung function deficit (Table 1). The first study was from Derbyshire County, England, where records for babies born between 1917 and 1922 were preserved [26]. These records also included their respiratory health, documented by health visitors in the first 2 years of life. Overall, 618 of the 1,909 (32%) men and women known to have been born in the six chosen districts at that time, now aged in their mid-60s and 70s, completed health questionnaires and underwent spirometry. After adjusting for age, height, smoking, and asthma, the 13 men with pneumonia diagnosed before age 2 years had significantly lower forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV1/FVC values than the 315 men without a history of pneumonia. The second report was from the Tucson Children’s Respiratory Study, United States of America, (USA) where 1,246 unselected healthy infants were enrolled from 1980 to 1984 and followed into adulthood [27,35]. It reported on those with a history of clinically diagnosed and radiographically confirmed pneumonia during the first 3 years of life [27]. In more than half of the original 66 cases, pneumonia was associated with respiratory viruses (mainly RSV) and in almost two-thirds wheezing was present. None were hospitalised. Forty-four (67%) of these children were followed as adults into their mid-to-late 20s and as a group they had persistently impaired lung function with decreased FEV1/FVC ratios and reduced mid-expiratory flow rates indicating small airway obstruction, which was only partially corrected by bronchodilators. Compared to those without pneumonia, these young adults had a two-fold increased risk of active, physician-diagnosed asthma (OR 1.95 [95% CI 1.11–3.44]).
Other birth cohort studies (Table 1) have also reported reduced lung function in adults following childhood lower respiratory tract illnesses. The Newcastle Thousand Families Study, UK, involved 1,142 infants born in 1947 and their lower respiratory illnesses were recorded by health visitors during the first 5 years of life [30]. Of the 252 (22%) who were later recruited from this birth cohort into a nested case-control study in 1961 and underwent spirometry at age 14-years, 122 returned for additional assessments at 49–51 years of age (Table 2). Those with a history of lower respiratory illnesses before age 5-years had significantly lower FEV1 recordings than their healthy peers at age 14-years and they also demonstrated a greater decline in FEV1 between 14 and 50 years of age. Finally, the Barry Caerphilly Growth Study undertaken in Barry and Caerphilly, Wales, UK, reported the results of spirometry performed on 679 of the original 951 (71%) 25-year-old subjects born in these two towns between 1972 and 1974 and from whom research personnel had gathered information on upper and lower respiratory tract infections during 14 home visits from birth until the subjects reached 5 years of age [28]. The major findings from this study were that lower respiratory tract infections during the first year of life were associated with decreased lung function, involving all lung function parameters except FVC, suggesting these children were at increased risk of airway obstruction. Indeed, a dose-response effect was observed and the authors estimated that a two-fold increase in lower respiratory tract infections during the first year of life was associated with a decrease in FEV1 equal to that seen with cigarette smoking for 10–17 pack-years.
2.2 Retrospective studies
The review by Edmonds and colleagues [18] identified seven retrospective studies published between 1971 and 2000 that reported outcomes for specific pathogens: adenovirus [36,37], Mycoplasma pneumoniae [38–40], Chlamydia trachomatis [22], and Staphylococcus aureus [41]. However, these data cannot be extrapolated to ‘general pneumonic illnesses’ because of the likely high selection bias inherent in such retrospective studies. Moreover, none of the studies had searched systematically for other co-existing respiratory pathogens that may also have contributed to the child’s clinical presentation and long-term outcomes.
Many other retrospective studies relating childhood respiratory outcomes to poorer adult respiratory health have been published and these include population-based studies, such as that of Barker and Osmond [42], which concluded that there was strong evidence of a direct causal link between acute lower respiratory infection in early childhood and chronic bronchitis in adult life. Furthermore, this study suggested that infection in early childhood had a greater influence than cigarette smoking in determining the geographical distribution of chronic bronchitis with national time trends reflecting the influence of both factors [42]. Most retrospective studies are consistent with the longitudinal studies’ findings that early childhood lung infections are associated with future lung disease or poorer lung function in adults [17,18,26–29].
2.3 Studies focused on bronchiectasis only
The rarer complication of bronchiectasis is characterised by chronic wet or productive cough, periodic infectious exacerbations, and irreversible bronchial dilation and wall thickening seen on either plain radiographs or computed tomography of the chest [43]. It normally has multiple aetiologies, including prior severe infections, immune deficiencies, and primary ciliary dyskinesia, and its pathogenesis remains poorly understood. However, bronchiectasis in children from indigenous communities and developing countries is associated with preterm delivery and episodes of early and recurrent pneumonia during infancy [44–47], while 30–60% of adults with recently diagnosed bronchiectasis report repeated lower respiratory tract infections during childhood [48–50]. It is hypothesised that severe pneumonia, including that resulting occasionally from adenovirus outbreaks, can damage ciliated airway epithelium in the growing lung, impairing airway clearance defences and setting up a cycle of repeated or persistent infection and inflammation involving airway infiltration by activated neutrophils and CD4+ T-lymphocytes, followed by degradation of bronchial wall supporting structures, bronchial dilatation, and ultimately bronchiectasis [51]. Nevertheless, studies that identified severe respiratory infections during childhood as risk factors for bronchiectasis were also retrospective and relied upon patient recall or involved highly specific patient groups [48–50]. Readers are referred to the review by Thomson et al [17] that included studies specifically relating to bronchiectasis post-pneumonia.
3. Possible explanations for long-term effects
3.1 Early lung growth and development
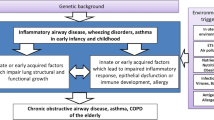
During normal fetal development and the first 3–4 years of rapid post-natal growth, the developing lungs are most vulnerable to injury by infectious and non-infectious insults, including reprogramming by various gene—environment interactions [52,53]. It is during the early post-natal period, when new alveoli are still forming and post-natal lung growth is most rapid, that the developing lungs are most susceptible to the long-term effects of pneumonia. Lung development occurs over five phases, the first four of which are in utero, and all five phases can be influenced by maternal, genetic, and environmental factors, including infection in the critical post-natal growth and alveolarisation period [54,55]. Within the developing embryo the lung buds first appear between 4 and 7 weeks, the conducting airways are established by 17 weeks and the respiratory zone, which includes the respiratory bronchioles and alveolar ducts, is fully developed by 27-weeks gestation. Alveolar sacs first emerge, followed by alveoli, after 28 weeks and at full term 100–150 million alveoli have formed. After birth the alveoli continue to multiply reaching the estimated adult number of 300–600 million by at least 3–4 years of age. The alveoli also increase in volume and the airways keep growing, doubling their size by the end of adolescence in females and the mid-twenties for males [55]. During the intrauterine period and the first few years of life various environmental insults to the developing lung may adversely impact upon future respiratory health. For example, preterm delivery can interrupt lung development with subsequent maturation occurring out of phase compared with those born at full term, and the resulting airways are smaller with fewer alveoli and potential negative influences upon lung function [52,56].
Respiratory infections with consequent inflammatory responses may interrupt the critical alveolarisation phase of lung development, restricting alveolar numbers and/or size and leading to often mild, but impaired lung growth. The association between pneumonia and obstructive lung disease is possibly through similar mechanisms to those leading to bronchiectasis, which is an obstructive lung disease. Lung infections at the peak periods of somatic lung growth (a normal newborn has only approximately 5% of a healthy adult’s alveolar surface area [57]) may also alter the programming of lung development at a local or systemic level. The effects of early infection, especially viral lower respiratory tract infections, upon lung growth, programming and future lung function disease types are beyond the scope of this review and readers are referred to the respiratory literature [55,56,58].
3.2 Other confounders and effects
In addition to the effects of lower respiratory infections, maternal smoking, and other maternal factors, nutrition, and allergic sensitisation can have potential negative influences upon lung function [52,56,59,60]. Of the above factors, solid experimental evidence on the impact of poor nutrition [61,62] and tobacco smoking in animals [63–65] support the association studies in human literature where infants who are poorly nourished or exposed to tobacco smoke (both in utero and in early life) are more likely to have lung infections and are also independently more likely to have adult lung disease and/or poorer lung function [53,55,56]. In animal studies these effects are reversible (at least partially) since improvement in nutrition early in life results in better adult lung development [66]. Pre- and post-natal tobacco exposure have independent long-term pulmonary effects [67,68] and cessation of tobacco exposure improves the accelerated decline in lung function [69]. While it is beyond the scope of this review to elaborate upon these factors (see reviews [60,66,67]), readers should be cognisant of the importance of these issues. The potential reversibility of impaired lung growth and function also highlights the opportunities for future intervenions.
An alternative interpretation is that, irrespective of external factors, lung function from infancy tracks throughout childhood into adult life meaning those predisposed to COPD, bronchiectasis, and other adult lung dysfunction are destined to do so, with pneumonia simply being a biomarker for those with underlying abnormal lung function and growth. Consequently, it follows that no interventions will alter the future development of adult lung disease. This rather nihilistic view is, however, unduly pessimistic. Although randomised controlled trial evidence in humans is not available (and likely never will be), there are many biological reasons why this opinion is incorrect. Examples include marked improvements in survival and lung function seen in patients with cystic fibrosis over the last two decades [70], the reversibility (at least partially) seen in animal studies described above [60,66], and the reduction in mortality from pneumonia through improved case management [7]. Indeed, it is highly likely that the interactions are complex since the host’s respiratory microbiome, immune responses, epigenetic influences, and their physical environment are being recognised increasingly as playing a role in the human phenotypes of health and disease [71]. As shown recently for RSV in unselected term infants [72], both possibilities may not be mutually exclusive. Thus infants with smaller airways and/or reduced numbers of alveoli at birth could have greater numbers of lower respiratory tract infections, including pneumonia, during the critical post-natal period and these infections might also lead to further disruption of alveolar multiplication and/or airway growth and development.
At this point it is also worth noting that recent studies report up to 40% of adults with a chronic productive cough and ‘difficult to control’ asthma may have radiographic evidence of underlying bronchiectasis [73], while 29–58% of those with severe COPD also have bronchiectasis as either a direct complication of COPD or part of an overlap syndrome [74,75]. These reports highlight the complex relationships observed between early childhood respiratory infections and chronic airway disorders in adults. There may be at least two different processes operating in these circumstances producing either functional (COPD) and/or structural (bronchiectasis) defects. A clue to the presence of these mechanisms was provided by a recent prospective study of respiratory exacerbations in infants with cystic fibrosis detected by a newborn screening program [76]. The overall exacerbation rate in the first 2 years of life was negatively associated with FEV1 at 5 years of age, suggesting airway remodelling, while only the severe 20% of exacerbations requiring hospitalisation were associated with structural airway injury causing bronchiectasis at age 5-years.
3.3 Case ascertainment issues
An important limitation for many studies was the reliance upon parental reporting of symptoms and that some of the cases may have had bronchiolitis, virus-induced wheezing or asthma rather than pneumonia. Even when the diagnosis of pneumonia is supported by radiographic evidence it can still be difficult to differentiate between patchy pulmonary infiltrates and localised atelectasis from mucous plugs in non-hospitalised children with moderate, and often viral, lower respiratory tract illnesses [27]. Radiographic abnormalities (including alveolar consolidation) are often present in infants with moderate-to-severe bronchiolitis [77] and many of the studies relating lower respiratory infections to long-term outcomes do not report on, or do not exclude, presence of wheeze in the study population. The respiratory physiology of wheeze in infants, which is more likely to occur when abnormally small airways and/or atopic sensitisation are present, is probably going to be different to that of pneumonia, which is primarily a parenchymal illness. Consequently, as these diseases have different underlying pathologies and pathogenetic mechanisms, they are also very likely to have different long-term outcomes [59,78,79].
4. Studies strengths and weaknesses
The major strengths of the community-based birth cohort studies are the recruitment of unselected subjects and their longitudinal nature spanning several decades, which allowed prospectively collected infant and early childhood exposure data to be analysed with those describing adult respiratory outcomes [23–28,30]. Important limitations though are losses to follow-up, which included those who had died shortly after discharge from hospital [32] and from chronic pulmonary disorders, such as COPD in the older aged cohorts [23,42], as well as those who were too unwell to attend or to perform satisfactory lung function tests. One study assessed only men [23], while those lost to follow-up were usually socioeconomically disadvantaged and may have been at greater risk of respiratory morbidity, even if they had never smoked [80,81]. The early birth cohorts were also conducted before antibiotics were available. Thus the nature or likelihood of injury to the developing lung during alveolarisation may differ whether or not cases of bacterial pneumonia received antibiotics.
Despite the birth cohort studies being prospective, the respiratory illness data were generally collected by visiting healthcare workers, usually some weeks or months later, and in one study up to 7 years after the event [25]. Consequently, the diagnosis of pneumonia often relied upon parental recall and in only one of these community-based studies was the diagnosis of pneumonia made by a paediatrician and confirmed radiographically [27]. Moreover, most studies provided little information on the number of pneumonia episodes experienced at an individual level, limiting our capacity to determine if a single episode in an infant or young child is associated with impaired lung function in later adult life or if instead pneumonia needs to be severe or recurrent to have such effects. In addition, the subjects in some studies were asked to self-report some potentially important confounding variables, such as gestation at delivery, maternal smoking, and occupational exposures to environmental toxicants. As discussed above, an unknown proportion of these episodes may have been from bronchiolitis, virus-induced wheeze, or asthma rather than pneumonia. Thus, these studies suffered from varying degrees of potential selection, gender and recall bias, as well as diagnostic misclassification. Also, as with any observational studies, the possibility of residual unmeasured confounding remains an issue.
The hospital-based studies reported in the systematic review [18] involved small numbers of subjects (20–190), of whom 19–59% were followed for a mean of 7.8 (range 1.5–17) years. All those reporting upon a single respiratory pathogen (S. aureus, M. pneumoniae and C. trachomatis) were retrospective. Each study was limited by incomplete data collection, failure to use sensitive diagnostic techniques to exclude co-infection by other respiratory pathogens, likely referral bias, and differential patient follow-up where the potential existed for children with symptoms to be more likely to be brought back for review. Finally, other major limitations were the lack of baseline clinical and lung function data prior to the episode of pneumonia in all but one study [27] and the lack of data on potential confounders and the effect of interventions provided.
5. Conclusions and future directions
It is widely recognised that pneumonia in young children causes a considerable worldwide burden of mortality and short-term morbidity in survivors. What is less well known is that infectious insults to the rapidly growing and still developing lungs in the first 1–3 years of life are independently associated with an increased risk of impaired lung function in adulthood. The risks appear greatest for those whose illness is of sufficient severity to warrant treatment in hospital. The long-term effects associated with early childhood pneumonia include restrictive or obstructive lung function deficits and an increased risk of adult asthma, non-smoking related COPD, and bronchiectasis. The studies underpinning these observations do however have important limitations. They are a mixture of prospective and retrospective studies, involving both community- and hospital-based populations experiencing illness of varying severity, with incomplete follow-up and opportunities for sampling and recall bias, and diagnostic misclassification of bronchitis, bronchiolitis, viral-induced wheezing and asthma as pneumonia. Most important of all is that most studies do not have prior lung function data for their pneumonia cases and subsequent impairments in lung function might simply reflect pre-existing abnormalities in already susceptible infants and young children.
More high-quality, long-term studies are needed, ideally involving birth cohorts from both developing and developed countries with antenatal recruitment and, whenever feasible, measurements of lung function should be conducted shortly after birth. To minimise the risk of misclassification, standardised diagnostic criteria for pneumonia should also be used. Such studies are challenging and expensive to perform, but can help focus future research, while helping to guide clinical practice and child public health policies. A promising start has been made in South Africa with the Drakenstein Child Health Study [82]. Although, by relying solely upon the World Health Organization clinical definitions of pneumonia many of the cases will have lower airway, rather than alveolar involvement, and thus several of the questions raised in this review will not be addressed by this birth cohort study. The underlying airway cellular, immunological, and microbiological mechanisms associated with chronic pulmonary disorders originating in the antenatal and early childhood periods are also important, but beyond the scope of this review and are discussed elsewhere [54–59,83]. However, gaining an understanding of these mechanisms is vital for developing effective therapeutic interventions to either prevent or reverse lifelong injury to the developing lungs. Meanwhile, for clinicians it is important to recognise that young children with pneumonia are at risk of chronic pulmonary disease as adults, irrespective whether this is a direct result of their infection or that susceptibility to pneumonia itself is a marker for potential underlying deficits in lung function. Either way, a careful history should be taken for each patient, recording details of the pregnancy, nature and timing of the birth, maternal and household tobacco smoking history, exposure to indoor (e.g. biomass fuels) and outdoor air pollution, prior history of respiratory symptoms, especially chronic cough, wheezing or breathing difficulties, previous respiratory illness episodes, their nutritional state, immunisation record and personal and family histories of atopy, asthma or other chronic lung diseases. The parents and caregivers should be counselled about the possibility of adult-onset lung disease in the child and advised accordingly of the importance of avoiding active and passive smoking, indoor and outdoor air pollution, future occupational inhalant exposures, and ensuring that all recommended vaccines are received in a timely manner. Public health interventions, such as appropriate housing, household heating and hygiene, reducing barriers to accessing healthcare and immunisation services, promoting education, and improving socio-economic status are also important [29,84], especially in developing countries and the economically disadvantaged in developed countries (e.g. indigenous populations in Australia, New Zealand, Canada, and the USA with high burdens of lung disease [85]).
Finally, it is essential that the complex relationships between pre-existing lung impairment, early childhood pneumonia, and chronic pulmonary disorders in adults are disentangled and better understood so that policy makers can make informed decisions about public health interventions to help stem the tide of chronic lung disease in adults. As an example, it will be necessary to take into account that vaccines against common respiratory pathogens may have longer-term benefits than simply protecting against episodes of acute pneumonia. By 2030, COPD is predicted to become the third leading cause of deaths globally [86], and with almost half of the cases now occurring in adults who have never smoked, the roles played by exposure to other inhaled environmental toxicants and lower respiratory tract infections during early childhood need to be highlighted, investigated, and managed at both an individual and population level [87].
References
Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS et al.; Severe Acute Lower Respiratory Infections Working Group. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013;381:1380–90. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(12)61901-1. PMID:23369797
O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N et al.; Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009;374:893–902. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(09)61204-6. PMID:19748398
Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N et al.; Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009;374:903–11. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(09)61203-4. PMID:19748399
Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–55. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(10)60206-1. PMID:20399493
Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011;378:1917–30. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(11)61051-9. PMID:22078723
Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381:1405–16. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(13)60222-6. PMID:23582727
Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(14)61698-6. PMID:25280870
Madhi SA, De Wals P, Grijalva CG, Grimwood K, Grossman R, Ishiwada N et al. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J 2013;32:e119–27. PMID:23099423
O’Grady KA, Taylor-Thomson DM, Chang AB, Torzillo PJ, Morris PS, Mackenzie GA et al. Rates of radiologically confirmed pneumonia as defined by the World Health Organization in Northern Territory Indigenous children. Med J Aust 2010;192:592–5. PMID:20477736
Kovesi T. Respiratory disease in Canadian First Nations and Inuit children. Paediatr Child Health (Oxford) 2012;17:376–80. PMID:23904781
O’Grady KA, Lee KJ, Carlin JB, Torzillo PJ, Chang AB, Mulholland EK et al. Increased risk of hospitalization for acute lower respiratory tract infection among Australian indigenous infants 5–23 months of age following pneumococcal vaccination: a cohort study. Clin Infect Dis 2010;50:970–8. PMID:20192729 https://doi.org/www.dx.doi.org/10.1086/651079
Chang AB, Ooi MH, Perera D, Grimwood K. Improving the Diagnosis, Management, and Outcomes of Children with Pneumonia: Where are the Gaps? Front Pediatr 2013;1:29. https://doi.org/www.dx.doi.org/10.3389/fped.2013.00029. PMID:24400275
Prayle A, Atkinson M, Smyth A. Pneumonia in the developed world. Paediatr Respir Rev 2011;12:60–9. https://doi.org/www.dx.doi.org/10.1016/j.prrv.2010.09.012. PMID:21172677
Lassi ZS, Das JK, Haider SW, Salam RA, Qazi SA, Bhutta ZA. Systematic review on antibiotic therapy for pneumonia in children between 2 and 59 months of age. Arch Dis Child 2014;99:687–93. https://doi.org/www.dx.doi.org/10.1136/archdischild-2013-304023. PMID:24431417
Henderson AJ. The child is father of the man: the importance of early life influences on lung development. Thorax 2014;69:976–7. https://doi.org/www.dx.doi.org/10.1136/thoraxjnl-2014-205752. PMID:25052576
Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014;11:404–6. https://doi.org/www.dx.doi.org/10.1513/AnnalsATS.201311-405PS. PMID:24673696
Thomson M, Myer L, Zar HJ. The impact of pneumonia on development of chronic respiratory illness in childhood. Pediatr Allergy Immunol Pulmonol. 2010;23:279–90. https://doi.org/www.dx.doi.org/10.1089/ped.2010.0056
Edmond K, Scott S, Korczak V, Ward C, Sanderson C, Theodoratou E et al. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS ONE 2012;7:e31239. https://doi.org/www.dx.doi.org/10.1371/journal.pone.0031239. PMID:22384005
Eastham KM, Hammal DM, Parker L, Spencer DA. A follow-up study of children hospitalised with community-acquired pneumonia. Arch Dis Child 2008;93:755–9. https://doi.org/www.dx.doi.org/10.1136/adc.2007.128900. PMID:18381341
Kycler Z, Breborowicz A, Olejniczak K, Alkiewicz J, Bugaj U. Respiratory problems in children after severe pneumonia. Pneumonol Alergol Pol 2001;69:167–73. PMID:11575000
Wesley AG. Prolonged after-effects of pneumonia in children. S Afr Med J 1991;79:73–6. PMID:1989091
Weiss SG, Newcomb RW, Beem MO. Pulmonary assessment of children after chlamydial pneumonia of infancy. J Pediatr 1986;108:659–64. https://doi.org/www.dx.doi.org/10.1016/S0022-3476(86)81037-X. PMID:3701509
Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991;303:671–5. https://doi.org/www.dx.doi.org/10.1136/bmj.303.6804.671. PMID:1912913
Shaheen SO, Sterne JA, Tucker JS, Florey CD. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax 1998;53:549–53. https://doi.org/www.dx.doi.org/10.1136/thx.53.7.549. PMID:9797752
Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med 1998;338:581–7. https://doi.org/www.dx.doi.org/10.1056/NEJM199802263380904. PMID:9475765
Shaheen SO, Barker DJ, Shiell AW, Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med 1994;149:616–9. https://doi.org/www.dx.doi.org/10.1164/ajrccm.149.3.8118627. PMID:8118627
Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics 2015;135:607–16. https://doi.org/www.dx.doi.org/10.1542/peds.2014-3060. PMID:25733757
Lopez Bernal JA, Upton MN, Henderson AJ, Dedman D, McCarthy A, Davey Smith G et al. Lower respiratory tract infection in the first year of life is associated with worse lung function in adult life: prospective results from the Barry Caerphilly Growth study. Ann Epidemiol 2013;23:422–7. https://doi.org/www.dx.doi.org/10.1016/j.annepidem.2013.05.006. PMID:23790346
Colley JR, Douglas JW, Reid DD. Respiratory disease in young adults: influence of early childhood lower respiratory tract illness, social class, air pollution, and smoking. BMJ 1973;3:195–8. https://doi.org/www.dx.doi.org/10.1136/bmj.3.5873.195. PMID:4718835
Tennant PW, Gibson GJ, Parker L, Pearce MS. Childhood respiratory illness and lung function at ages 14 and 50 years: childhood respiratory illness and lung function. Chest 2010;137:146–55. https://doi.org/www.dx.doi.org/10.1378/chest.09-0352. PMID:19581355
Mok JY, Simpson H. Outcome for acute bronchitis, bronchiolitis, and pneumonia in infancy. Arch Dis Child 1984;59:306–9. https://doi.org/www.dx.doi.org/10.1136/adc.59.4.306. PMID:6721555
Puchalski Ritchie LM, Howie SR, Arenovich T, Cheung YB, Weber M, Moore S et al. Long-term morbidity from severe pneumonia in early childhood in The Gambia, West Africa: a follow-up study. Int J Tuberc Lung Dis 2009;13:527–32. PMID:19335961
Piippo-Savolainen E, Remes S, Kannisto S, Korhonen K, Korppi M. Asthma and lung function 20 years after wheezing in infancy: results from a prospective follow-up study. Arch Pediatr Adolesc Med 2004;158:1070–6. https://doi.org/www.dx.doi.org/10.1001/archpedi.158.11.1070. PMID:15520345
Chang AB, Masel JP, Boyce NC, Torzillo PJ. Respiratory morbidity in central Australian Aboriginal children with alveolar lobar abnormalities. Med J Aust 2003;178:490–4. PMID:12741934
Castro-Rodríguez JA, Holberg CJ, Wright AL, Halonen M, Taussig LM, Morgan WJ et al. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med 1999;159:1891–7. https://doi.org/www.dx.doi.org/10.1164/ajrccm.159.6.9811035. PMID:10351936
Similä S, Linna O, Lanning P, Heikkinen E, Ala-Houhala M. Chronic lung damage caused by adenovirus type 7: a ten-year follow-up study. Chest 1981;80:127–31. https://doi.org/www.dx.doi.org/10.1378/chest.80.2.127. PMID:6265156
Sly PD, Soto-Quiros ME, Landau LI, Hudson I, Newton-John H. Factors predisposing to abnormal pulmonary function after adenovirus type 7 pneumonia. Arch Dis Child 1984;59:935–9. https://doi.org/www.dx.doi.org/10.1136/adc.59.10.935. PMID:6093715
Sabato AR, Martin AJ, Marmion BP, Kok TW, Cooper DM. Mycoplasma pneumoniae: acute illness, antibiotics, and subsequent pulmonary function. Arch Dis Child 1984;59:1034–7. https://doi.org/www.dx.doi.org/10.1136/adc.59.11.1034. PMID:6508338
Kim CK, Chung CY, Kim JS, Kim WS, Park Y, Koh YY. Late abnormal findings on high-resolution computed tomography after Mycoplasma pneumonia. Pediatrics 2000;105:372–8. https://doi.org/www.dx.doi.org/10.1542/peds.105.2.372. PMID:10654958
Mok JY, Inglis JM, Simpson H. Mycoplasma pneumoniae infection. A retrospective review of 103 hospitalised children. Acta Paediatr Scand 1979;68:833–9. https://doi.org/www.dx.doi.org/10.1111/j.1651-2227.1979.tb08220.x. PMID:539406
Ceruti E, Contreras J, Neira M. Staphylococcal pneumonia in childhood. Long-term follow-up including pulmonary function studies. Am J Dis Child 1971;122:386–92. https://doi.org/www.dx.doi.org/10.1001/archpedi.1971.02110050056004. PMID:5129527
Barker DJ, Osmond C. Childhood respiratory infection and adult chronic bronchitis in England and Wales. Br Med J (Clin Res Ed) 1986;293:12715. PMID:3096461 https://doi.org/www.dx.doi.org/10.1136/bmj.293.6557.1271
Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA et al.; extended voting group. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med J Aust 2015;202:21–3. https://doi.org/www.dx.doi.org/10.5694/mjac14.00287. PMID:25588439
Singleton R, Morris A, Redding G, Poll J, Holck P, Martinez P et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol 2000;29:182–7. PMID:10686038
Valery PC, Torzillo PJ, Mulholland K, Boyce NC, Purdie DM, Chang AB. Hospital-based case-control study of bronchiectasis in indigenous children in Central Australia. Pediatr Infect Dis J 2004;23:902–8. https://doi.org/www.dx.doi.org/10.1097/01.inf.0000142508.33623.2f. PMID:15602188
Singleton RJ, Valery PC, Morris P, Byrnes CA, Grimwood K, Redding G et al. Indigenous children from three countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol 2014;49:189–200. https://doi.org/www.dx.doi.org/10.1002/ppul.22763. PMID:23401398
Brower KS, Del Vecchio MT, Aronoff SC. The etiologies of non-CF bronchiectasis in childhood: a systematic review of 989 subjects. BMC Pediatr 2014;14:4. https://doi.org/www.dx.doi.org/10.1186/s12887-014-0299-y. PMID:25492164
Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000;162:1277–84. https://doi.org/www.dx.doi.org/10.1164/ajrccm.162.4.9906120. PMID:11029331
King PT, Holdsworth SR, Farmer M, Freezer N, Villanueva E, Holmes PW. Phenotypes of adult bronchiectasis: onset of productive cough in childhood and adulthood. COPD 2009;6:130–6. https://doi.org/www.dx.doi.org/10.1080/15412550902766934. PMID:19378226
Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respir Med 2007;101:1163–70. https://doi.org/www.dx.doi.org/10.1016/j.rmed.2006.11.008. PMID:17223027
Dagli E. Non cystic fibrosis bronchiectasis. Paediatr Respir Rev 2000;1:64–70. https://doi.org/www.dx.doi.org/10.1053/prrv.2000.0011. PMID:16263448
Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010;65:14–20. https://doi.org/www.dx.doi.org/10.1136/thx.2008.112136. PMID:19729360
Britt RD Jr, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Expert Rev Respir Med 2013;7:515–31. https://doi.org/www.dx.doi.org/10.1586/17476348.2013.838020. PMID:24090092
Smith LJ, McKay KO, van Asperen PP, Selvadurai H, Fitzgerald DA. Normal development of the lung and premature birth. Paediatr Respir Rev 2010;11:135–42. https://doi.org/www.dx.doi.org/10.1016/j.prrv.2009.12.006. PMID:20692626
Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med 2013;1:728–42. https://doi.org/www.dx.doi.org/10.1016/S2213-2600(13)70118-8. PMID:24429276
Carraro S, Scheltema N, Bont L, Baraldi E. Early-life origins of chronic respiratory diseases: understanding and promoting healthy ageing. Eur Respir J 2014;44:1682–96. https://doi.org/www.dx.doi.org/10.1183/09031936.00084114. PMID:25323240
Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Hum Dev 1986;13:1–11. https://doi.org/www.dx.doi.org/10.1016/0378-3782(86)90092-7. PMID:3956418
Fuchs O, von Mutius E. Prenatal and childhood infections: implications for the development and treatment of childhood asthma. Lancet Respir Med 2013;1:743–54. https://doi.org/www.dx.doi.org/10.1016/S2213-2600(13)70145-0. PMID:24429277
Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J 2015;45:774–89. https://doi.org/www.dx.doi.org/10.1183/09031936.00062714. PMID:25359340
Sahebjami H. Nutrition and lung structure and function. Exp Lung Res 1993;19:105–24. https://doi.org/www.dx.doi.org/10.3109/01902149309031714. PMID:8467757
Sahebjami H, MacGee J. Effects of starvation on lung mechanics and biochemistry in young and old rats. J Appl Physiol (1985) 1985;58:778–84. PMID:3156843
Maritz G, Probyn M, De Matteo R, Snibson K, Harding R. Lung parenchyma at maturity is influenced by postnatal growth but not by moderate preterm birth in sheep. Neonatology 2008;93:28–35. https://doi.org/www.dx.doi.org/10.1159/000105522. PMID:17630495
Collins MH, Moessinger AC, Kleinerman J, Bassi J, Rosso P, Collins AM et al. Fetal lung hypoplasia associated with maternal smoking: a morphometric analysis. Pediatr Res 1985;19:408–12. https://doi.org/www.dx.doi.org/10.1203/00006450-198519040-00018. PMID:4000765
Maritz GS, Morley CJ, Harding R. Early developmental origins of impaired lung structure and function. Early Hum Dev 2005;81:763–71. https://doi.org/www.dx.doi.org/10.1016/j.earlhumdev.2005.07.002. PMID:16081227
Blacquière MJ, Timens W, Melgert BN, Geerlings M, Postma DS, Hylkema MN. Maternal smoking during pregnancy induces airway remodelling in mice offspring. Eur Respir J 2009;33:1133–40. https://doi.org/www.dx.doi.org/10.1183/09031936.00129608. PMID:19129273
Shaheen S, Barker DJ. Early lung growth and chronic airflow obstruction. Thorax 1994;49:533–6. https://doi.org/www.dx.doi.org/10.1136/thx.49.6.533. PMID:8016787
U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006
DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics 2004;113 Suppl:1007–15. PMID:15060193
Willemse BW, Postma DS, Timens W, ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J 2004;23:464–76. https://doi.org/www.dx.doi.org/10.1183/09031936.04.00012704. PMID:15065840
Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA et al. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J 2015;45:670–9. https://doi.org/www.dx.doi.org/10.1183/09031936.00119714. PMID:25395034
Holt PG. The mechanism or mechanisms driving atopic asthma initiation: The infant respiratory microbiome moves to center stage. J Allergy Clin Immunol 2015;136:15–22. PMID:26145983 https://doi.org/www.dx.doi.org/10.1016/j.jaci.2015.05.011
Zomer-Kooijker K, Uiterwaal CS, van der Gugten AC, Wilbrink B, Bont LJ, van der Ent CK. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. Eur Respir J 2014;44:666–74. https://doi.org/www.dx.doi.org/10.1183/09031936.00009314. PMID:24993909
Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest 2009;136:1521–8. https://doi.org/www.dx.doi.org/10.1378/chest.09-0174. PMID:19542254
O’Donnell AE. Bronchiectasis in patients with COPD: a distinct COPD phenotype? Chest 2011;140:1107–8. https://doi.org/www.dx.doi.org/10.1378/chest.11-1484. PMID:22045871
Hurst JR, Elborn JS, De Soyza A; BRONCH-UK Consortium. COPD-bronchiectasis overlap syndrome. Eur Respir J 2015;45:310–3. https://doi.org/www.dx.doi.org/10.1183/09031936.00170014. PMID:25653262
Byrnes CA, Vidmar S, Cheney JL, Carlin JB, Armstrong DS, Cooper PJ et al.; ACFBAL Study Investigators. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax 2013;68:643–51. https://doi.org/www.dx.doi.org/10.1136/thoraxjnl-2012-202342. PMID:23345574
Carsin A, Gorincour G, Bresson V, Oudyi M, David M, Mancini J et al. Chest radiographs in infants hospitalized for acute bronchiolitis: real information or just irradiation?. Arch Pediatr 2012;19:1308–15. https://doi.org/www.dx.doi.org/10.1016/j.arcped.2012.09.019. PMID:23141565
Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax 2014;69:805–10. https://doi.org/www.dx.doi.org/10.1136/thoraxjnl-2013-204815. PMID:24646659
Kusel MM, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J 2012;39:876–82. https://doi.org/www.dx.doi.org/10.1183/09031936.00193310. PMID:21920891
Ore T, Ireland P. Chronic obstructive pulmonary disease hospitalisations and mortality in Victoria: analysis of variations by socioeconomic status [Epub ahead of print]. Aust N Z J Public Health 2015
Lee SJ, Kim SW, Kong KA, Ryu YJ, Lee JH, Chang JH. Risk factors for chronic obstructive pulmonary disease among never-smokers in Korea. Int J Chron Obstruct Pulmon Dis 2015;10:497–506. https://doi.org/www.dx.doi.org/10.2147/COPD.S77662. PMID:25784796
le Roux DM, Myer L, Nicol MP, Zar HJ. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: the Drakenstein Child Health Study. Lancet Glob Health 2015;3:e95–103. https://doi.org/www.dx.doi.org/10.1016/S2214-109X(14)70360-2. PMID:25617203
Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med 2015;191:34–44. https://doi.org/www.dx.doi.org/10.1164/rccm.201405-0901PP. PMID:25369458
Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol 2014;49:430–4. https://doi.org/www.dx.doi.org/10.1002/ppul.23030. PMID:24610581
Chang AB, Marsh RL, Upham JW, Hoffman LR, Smith-Vaughan H, Holt D et al. Toward making inroads in reducing the disparity of lung health in Australian Indigenous and New Zealand Maori children. Front Pediatr 2015;3:9. https://doi.org/www.dx.doi.org/10.3389/fped.2015.00009. PMID:25741502
World Health Organization. Chronic respiratory diseases. Burden of COPD. https://doi.org/www.who.int/respiratory/copd/burden/en; accessed 24th April, 2015.
Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009;374:733–43. https://doi.org/www.dx.doi.org/10.1016/S0140-6736(09)61303-9. PMID:19716966
Funding: AC is funded by an Australian National Health and Medical Research Council (NHMRC) practitioner fellowship (Grant 1058213). KG and AC are funded by a NHMRC Centre of Research Excellence grant (1040830) on lung health in Indigenous children. The funders had no role in study design, collection and analysis of data, decision to publish, or writing of the manuscript.
Competing interests: KG has been a member of advisory boards on pneumonia, otitis media, and pneumococcal conjugate vaccines for GlaxoSmithKline (GSK) Biologicals. AC has received an investigator-driven grant from GSK evaluating the impact of a vaccine on the lower airways of children.
Provenance and peer review: Commissioned; externally peer reviewed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions: All authors met ICMJE authorship criteria. KG, AC generated and designed the research plan. KG, AC contributed equally to the writing of the first draft of the manuscript and writing of the manuscript. KG, AC agree with the manuscript results and conclusions. KG, AC approved the final version of the manuscript. Citation: Grimwood K and Chang AB. Long-term effects of pneumonia in young children. pneumonia 2015;6:101–114
Rights and permissions
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
About this article
Cite this article
Grimwood, K., Chang, A.B. Long-term effects of pneumonia in young children. Pneumonia 6, 101–114 (2015). https://doi.org/10.15172/pneu.2015.6/671
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.15172/pneu.2015.6/671