Abstract
A liquid chromatographic method was developed for the simultaneous quantification of four major active components in tobacco (Nicotiana tobaccum L.) wastes. Samples were extracted with 70% v/v aqueous methanol, four compounds including chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid and caffeic acid were identified and determined by using LC coupled to electrospray tandem mass spectrometry and LC–UV method, respectively. Separation in LC–UV was on an Alltima C18 column (250 mm × 4.6 mm i.d.; 5 μm) with a mobile phase consisting acetonitrile: ammonium acetate buffer (pH 4.5) (5:95 v/v), at a flow rate of 1.0 mL min−1, detected at 327 nm. Four regression equations showed good linear relationships (r 2 > 0.999) between the peak area of each marker and concentration. The method has good repeatability and precision, the intra-day and inter-day RSD for both retention time and peak area was less than 1.0%. The recoveries, measured at three concentration levels, varied from 96.33 to 101.10%. The LOD (S/N = 3) and LOQ (S/N = 6) were less than 0.010 and 0.795 μg·mL−1, respectively. This assay was successfully applied to the determination of four active compounds in ten samples. The results indicated that the developed assay method was rapid, accurate, reliable and could be readily utilized as a quantitative analysis method for various of tobacco wastes.
Similar content being viewed by others
1 Introduction
Tobacco (Nicotiana tobaccum L.) is widely grown as a non-food cash crop. As new relevant materials showed, China produced about 1840.5 million kg tobacco leaves in 2005 which amounts to 36.5% of the total output, and China has become the largest producing country in the world [1]. Tobacco leaves are mainly used for making cigarettes, cigars and for chewing. Large quantities of tobacco wastes are generated during processing and cigarette making which amounts to 25% of the total tobacco material, and China also has become the largest producing tobacco wastes country in the world. At the same time, disposal of these wastes is a serious problem nowadays, because tobacco wastes are toxic due to the presence of nicotine, and European Union Regulations (EUR) and Italian law classify tobacco wastes as “toxic and hazardous wastes” when the nicotine content exceeds 500 mg kg−1 dw [2]. However, besides nicotine, there are some other biologically important compounds in tobacco wastes, such as phenolic compounds [3], solanesol [4], transgenic proteins [5], and so on. Recently, the chemical compositions of N. tabacum and tobacco wastes have attracted considerable attentions worldwide [3], and tobacco wastes are considered to be a good source of a large number of bioactive substances. Moreover the report of utilization of solanesol from tobacco wastes [6] let us study the synthetic utilization of their active compounds, but not to degrade or discard them. In view of the potential pharmaceutical application, the identification of phenolic compounds from tobacco wastes is therefore of essential importance.
Phenolic compounds exist mostly in the form of glycosides or esters in living plants. They not only have important physiological and some pharmacological activities, but also influence the quality and property of some bio-products [7]. Polyphenol compounds include tannins, coumarins, flavonols, anthocyanins, hydroxylated cyclohexanes and other phenol derivatives, which can affect the aroma of cigarettes, exist in tobacco (N.tabacum L.). Among them, chlorogonic acids and rutin are the most abundant polyphenols [8]. Unfortunately, less attention has been paid to the analysis and separation of phenolic compounds in tobacco wastes.
Chlorogenic acid is the only one compound in the large family, the best known conjugates are those with quinic acid, collectively known as chlorogenic acids (CGAs) or caffeoylquinic acids (CQAs) [9]. Classically, chlorogenic acids (CQAs) are a family of esters and related derivatives formed between certain trans cinnamic acids and (−)-quinic acid (1L-1(OH), 3,4,5-tetrahydroxycyclohexane carboxylic acid), the cinnamic acids most commonly encountered are caffeic, p-coumaric and ferulic [9, 10]. Compounds in this family possess a wide range of biological activities such as antibacterial, antioxidant, hepatocyte protective, antimutagenic, inhibition of HIV-1 RT and activities against human herpes simplex virus and adenoviruses, anti-SARS and anti-AIV(H5N1) activities [11–18]. On the other hand, the tobacco manufacturing process and all the activities which use tobacco often produce tobacco wastes, and of the different solid wastes produced during the process. So, it is difficult to study the composition of CQAs in tobacco wastes. At the same time, profiling the phenolic compounds of tobacco wastes is important to the synthetic utilization of their active compounds.
Recently, LC–MSn has been used to characterize cinnamoylamino acid conjugates and to discriminate between individual isomers of mono-acyl and di-acyl chlorogenic acids [10, 19], and also used to characterize flavanoids [20]. MS fragmentation data have been utilized to develop structure-diagnostic hierarchical keys for the identification of phenolic compounds. In this study, LC–MS was applied to the qualitative profiling of phenolic compounds such as chlorogenic acid (5-CQA), cryptochlorogenic acid (4-CQA), neochlorogenic acid (3-CQA) and caffeic acid (CA), see Fig. 1) in tobacco wastes. To enhance the separation of four compounds, a rapid, accurate and reliable LC–UV method was developed for the quantitative analysis of mark compounds in tobacco wastes samples.
2 Experimental
2.1 Plant Materials
The tobacco wastes were collected from various countries and regions in the world in 2007, and authenticated as the wastes of by Prof. Ding-qiang Lu (College of Life Science and Pharmaceutical Engineering, Nanjing University of Technology). Ten of these waste samples were dried at 60 °C, powered by a herb disintegrator (Qinzhou Sanyang Package Equipment Co.) and then sieved (60 mesh).
2.2 Chemicals and Reagents
LC-grade acetonitrile and acetic acid were purchased from TEDIA Co. (Fairfield, OH, USA); Chlorogenic acid (5-caffecylquinic acid, 5-CQA) and caffeic acid (CA) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP), Beijing, China. Cryptochlorogenic acid (4-CQA) and neochlorogenic acid (3-CQA) were purchased from Chendu Biopurify Phytochemicals (Tianjin, China).
2.3 Instrumentation Equipments
LC–PAD–MS was carried out on a Waters system (Millipore Corp., Milford, MA, USA). The LC equipment comprised a Waters 2695 Separations Module, autosampler with 50 μL loop, and a Waters 2996 Photodiode Array Detector with a light-pipe flow cell (recording at 328, 320, 280, and 254 nm, and scanning from 200 to 400 nm). This was interfaced with a mass spectrometer fitted with MICROMASS Quattro micro TM API source and ESCi TM MULTI-MODE IONIZATION Plus ESI source.
LC–UV was performed using LB-5 pump (Beijing Satellite Manufactory, Beijing, China) with a UV detector (Shimadzu Seisakusho, Kyoto, Japan) and N-2000 workstation (Hangzhou Mingtong S&T, Hangzhou, China).
2.4 LC–ESI–MS–MS and LC–UV Chromatographic Conditions
LC separation was performed on an Alltima C18 (250 × 4.6 mm, 5 μm) column (Alltech, Deerfield, IL, USA). The mobile phase contained solvents A and B, where A was water: acetonitrile: acetic acid (97.5:2:0.5, v/v/v) and B was acetonitrile: acetic acid (99.5:0.5, v/v/v). The linear gradient profile was maintained 2% B in 5 min, from 2 to 5% in 5 min, from 5 to 10% in 20 min, from 10 to 35% in 35 min, from 35 to 100% in 15 min, from 100 to 2% in 5 min, and maintained 2% B in 5 min. The wavelength range of PAD detection was from 200 to 400 nm. The flow-rate was 1 mL min−1 for LC and PAD detection with the column kept at 40 °C. A splitter was connected between the PAD and MS detectors, which reduced the flow rate to 0.2 mL min−1 for MS detection. The electrospray ESI–MS–ES was operated in the negative ion mode with scanning range of m/z 100–800, capillary voltage of 3.0 kV, cone voltage of 30.0 kV and ion source temperature of 120 °C. High purity nitrogen (99.9%) was used as dry gas to evaporate the solvent at a flow rate of 500 L h−1 at 350 °C. Nitrogen was also used as nebuliser gas at 50 psi.
LC–UV separation was performed on an Alltima C18 (250 × 4.6 mm, 5 μm) column (Alltech). The mobile phase contained acetonitrile: ammonium acetate buffer (pH 4.5) (5:95, v/v). The flow-rate was 1 mL min−1 and UV detection with the column kept at 30 °C, detected at 327 nm.
2.5 Preparation of the Stock Solution
The reference standards of the target compounds, i. e., 3-CQA, 4-CQA, 5-CQA and CA were accurately weighed, and their stock solution of 500, 450, 500 and 300 μg mL−1 concentration were prepared by dissolving in methanol, respectively. Then, the stock solutions were diluted to appropriate concentration ranges for the establishment of calibration curves. All solutions were stored at 4 °C.
2.6 Sample Preparation
The powered samples (5.0 g) were extracted assisted by ultrasonic wave with 70% v/v aqueous methanol (125 mL) in an ultrasonic bath at 45 °C for 45 min, to give an extraction yield of more than 70%. The extraction process was repeated two times, and the extract constant a volume of 500 mL. The obtained solutions were centrifuged at 10,000 rpm for 5 min, the supernatants were filtered through a 0.45 μm filter before injection. All samples were determined in triplicate.
3 Results
3.1 Composition of CQAs in Tobacco Wastes
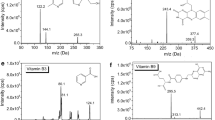
LC chromatographic profiles of the 70% v/v aqueous methanol extract of tobacco wastes detected with negative ion mode ESI–MS and UV at 320 nm, see Fig. 1.
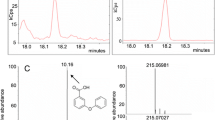
In the methanolic extract of dried tobacco wastes, 3-CQA, 4-CQA, 5-CQA and CA were identified by comparing their retention times, UV (Fig. 1a), ESI–TIC (Fig. 1b), MS spectras and chemical structures (Fig.1c) with those of standard compounds. The structure of 3-CQA, 4-CQA, 5-CQA and CA were determined by MS SCAN model, and by comparing the UV and MS spectra data to those reported in the literature. These results show that CQAs in tobacco wastes are mainly composed of 3-CQA, 4-CQA, 5-CQA and CA.
3.2 Optimization of the Conditions for Preparation of Test Samples
In order to obtain optimal extraction, effect of extraction solvent, method, temperature and time on the extraction yield of active components from tobacco wastes were investigated. The results show that the extraction efficiency was highest when 70% methanol were used as solvent, ultrasonic-assisted extraction (UAE) was found to be more suitable than heat-reflux extraction (HRE) for the preparation of test samples, the optimal extraction temperature and solid–liquid ratio in UAE were 45 °C and 1:25, respectively. The extraction yield of mark compounds was obtained almost 70% within 45 min in single-time extraction. Therefore, the optimal preparation method of four components in tobacco wastes were as follows: test samples were extracted with 70% v/v aqueous methanol by using UAE at 45 °C for 45 min, which process was repeated twice.
3.3 Chromatographic Conditions
To obtain chromatograms with a good separation and resolution of adjacent peaks within a short analysis time, mobile phase, column temperature and flow rate were optimized. The mobile phase containing acid, which could suppress the ionization of phenolic hydroxyl and carboxyl groups was beneficial for good retention and separation of chlorogenic, cryptochlorogenic, neochlorogenic and caffeic acids. Different mobile phase compositions (such as CH3OH–H2O–H3PO4, CH3OH–H2O–HOAc, THF–H2O–H3PO4, CH3OH–H2O–HOAc, CH3CN–H2O–H3PO4, CH3CN–H2O–HOAc and CH3CN–NH4 Ac buffer) were attempted to separate the investigated four components. Acetonitrile:ammonium acetate buffer (pH 4.5) (5:95 v/v) was chosen as the eluting solvent system because efficient separation and desired peak shape could be achieved. Under isocratic elution modes, the four compounds could be separated effectively. The effect of temperature on the separation was investigated in the range of 25–40 °C, 30 °C was found to be optimal. The most suitable flow rate was found to be 1.0 mL min−1. Since their polarity, solubility and other characteristics differ greatly; at least 15 min of elution time were needed for the simultaneous quantification of the four target compounds. On the basis of the UV–VIS spectra of the four components recorded by DAD detection in the range of 190–800 nm, experimental results indicate that four components have the same UV absorption peaks of about 327 nm, so 327 nm was selected for monitoring. Chromatograms of standard mixture (A) and tobacco wastes sample (B) are shown in Fig. 2, peak shape of the four compounds in tobacco wastes were symmetrical. Under the optimized isocratic elution conditions chlorogenic acids and caffeic acid were separated with resolution greater than gradient elusion model. Each component in the samples analyzed was identified by comparing its retention time to that of the respective standard. Retention time for 3-CQA, 5-CQA, 4-CQA and CA were 4.9, 6.2, 7.4 and 11.3 min, respectively (see Fig. 2). Quantification was carried out by integration of the peaks using external standards. As shown in Fig. 2, the LC–UV method was specific for separation of 3-CQA, 5-CQA, 4-CQA and CA in tobacco wastes.
3.4 Linearity, Limits of Detection and Quantification
Calibration curves were obtained by plotting peak areas versus six different concentrations of standard solutions. Response (peak area, Y) was then plotted against concentration (X, μg mL−1) and the six-point calibration curves were found to be linear as least squares regression gave a correlation coefficient. The equations were: neochlorogenic acid (3-CQA) Y = 6.196 × 107 X − 4.453 × 104 (r 2 = 0.9997), chlorogenic acid (5-CQA) Y = 5.841 × 107 X − 4.384 × 104 (r 2 = 0.9996), cryptochlorogenic acid (4-CQA) Y = 5.068 × 107 X − 4.025 × 104 (r 2 = 0.9996), caffeic acid (CA) Y = 8.325 × 107 X − 5.946 × 104 (r 2 = 0.9993), respectively.
The lowest acceptable level of the calibration curve was regarded as the limit of quantification (LOQ). The LOQ of neochlorogenic, chlorogenic, cryptochlorogenic and caffeic acids were 0.719, 0.751, 0.795 and 0.714 μg mL−1, respectively. The limits of detection (LOD; S/N = 3) of neochlorogenic, chlorogenic, cryptochlorogenic and caffeic acids were 0.360, 0.376, 0.398 and 0.357 μg mL−1, respectively. The LOD and LOQ values of the individual compound and the good linearity for each investigated compound indicated that the developed analytical method is precise and sensitive for determining four components in test samples.
3.5 Repeatability, Precision and Stability
The repeatability of the method in the present work was examined by the intra-day and inter-day variability. The intra-day variability was examined on six individual samples within 1 day, and inter-day variability was determined for three different days. The relative standard deviation (RSD) was calculated as a measurement of method repeatability. The results shown in Table 1 indicated that the intra- and inter-day RSD values of the four marker compounds were less than 0.2%, which showed good reproducibility. The precision was evaluated using the results of five replicate injections of the standard solutions containing the four components. The results showed a good precision, and RSDs were less than 0.2%. For the stability test, the same sample solution was analyzed at different times within 48 h at room temperature. The RSD values of the peak areas were all lower than 1.0%, suggesting that it is safe to analyze the sample within 2 days.
3.6 Recovery Test
Standard addition test was performed to determine the accuracy as well as the extraction recovery. In this test, the mixed standard solutions were prepared with three different concentration levels. Four standard solutions of three different concentration levels were added to the sample powder (1.0 g), respectively. The resultant samples were extracted and analyzed with the proposed LC method. The experiments were repeated three times for each level, and the ratio of measured and added amounts was used to calculate the recovery. Results showed that the mean recoveries of 3-CQA, 5-CQA, 4-CQA and CA in samples were 98.2, 101.10, 97.44 and 96.33%, the RSDs were 1.05, 1.57, 1.97 and 2.11% (n = 3), respectively. The data proved the suitability of the extraction method for use with tobacco wastes samples.
3.7 Application
The developed analytical method was successfully applied to the simultaneous determination of 3-CQA, 4-CQA, 5-CQA and CA in ten samples of tobacco wastes which were obtained from various countries and regions in the world, analysis results are listed in Table 2.
As shown in Table 2, the contents of four ingredients varied greatly among the different samples. In the majority of cases, 5-CQA was the main component, whose contents varied from 0.686 to 1.781% in ten samples, with almost 2.6-fold variation. Similar variation could also be found for the other components. The reason for the variation of the contents can be the difference of plant origin, the effect of environment and some other factors, such as season of collection, drying process and storage conditions, etc. Because variations of the marker compounds may influence the quality and potency of the tobacco wastes, it was necessary to develop an effective qualitative and quantitative method to evaluate the quality of tobacco wastes. The assay of one or two constituents only cannot give a complete assessment of the biomass. Thus, in this paper, four major compounds in tobacco wastes were analyzed simultaneously to evaluate its quality.
4 Conclusion
This is the first report on the simultaneous determination of neochlorogenic, chlorogenic, cryptochlorogenic and caffeic acids in tobacco wastes. The developed method was found to be rapid, linear, reliable and accurate. The validation procedure and assay results suggested that this LC–UV method is promising for being used in quality control of tobacco wastes.
References
Zhu X, Zheng F, Cao Z (2006) Acta Tabacaria Sin 12(4):58–64
Marcello CC (1997) Waste Manag Res 15:349–358
Crofcheck C, Loiselle M, Weekley J, Maiti I, Pattanaik S, Bummer PM, Jay M (2003) Biotechnol Prog 19(2):680–682. doi:10.1021/bp025738u
Zhao C, Li C, Zu Y (2007) J Pharm Biomed Anal 44(1):35–40. doi:10.1016/j.jpba.2007.01.021
Howles P, Masoud SA, Blount JW, Rasmussen S, Lamb C, Dixon RA (1999) Curr Plant Sci Biotechnol Agric (Plant Biotechnology and In Vitro Biology in the 21st Century) 36:297–301
Du X, Yuan Q, Zhao J, Li Y (2007) J Chromatogr A 1145(1–2):165–174. doi:10.1016/j.chroma.2007.01.065
Cui H, He C, Zhao G (1999) J Chromatogr A 855(1):171–179. doi:10.1016/S0021-9673(99)00670-6
Jiang HL, He YZ, Zhao HZ, Hu YY (2004) Anal Chim Acta 512(1):111–119. doi:10.1016/j.aca.2004.02.021
Clifford MN, Knight S, Kuhnert N (2005) J Agric Food Chem 53(10):3821–3832. doi:10.1021/jf050046h
Clifford MN, Wu W, Kirkpatrick J, Kuhnert N (2007) J Agric Food Chem 55(3):929–936. doi:10.1021/jf062314x
Laranjinha J, Vierira O, Almeida L, Madeira V (1996) Biochem Pharmacol 51(4):395–402. doi:10.1016/0006-2952(95)02171-X
Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, Schubert G, Below P, Herling AW (1997) Arch Biochem Biophys 339(2):315–322. doi:10.1006/abbi.1996.9874
Kim SS, Lee CK, Kang SS, Jung HA, Choi JS (1997) Arch Pharm Res 20(2):148–154
Borkowski B, Skuza G, Rogoz Z (1999) Herba Pol 45(3):192–198
Plumb GW, Garcia-conesa MT, Kroon PA, Rhodes M, Ridley S, Williamson G (1999) J Sci Food Agric 79(3):390–392. doi:10.1002/(SICI)1097-0010(19990301)79:3<390::AID-JSFA258>3.0.CO;2-0
Morishita H, Ohnishi M (2001) Stud Nat Prod Chem (Bioactive Natural Products (Part F)) 25:919–953
Ramalakshmi K, Rahath KI, Jagan MR (2008) Food Res Int 41(1):96–103. doi:10.1016/j.foodres.2007.10.003
Ma CM, Kully M, Khan JK, Hattori M, Daneshtalab M (2007) Bioorg Med Chem 15(21):6830–6833. doi:10.1016/j.bmc.2007.07.038
Clifford MN, Johnston KL, Knight S, Kuhnert N (2003) J Agric Food Chem 51(10):2900–2911. doi:10.1021/jf026187q
Ma CM, Hattori M, Chen HB, Cai SQ, Daneshtalab M (2008) Phytochem Anal 19(4):294–300. doi:10.1002/pca.1045
Acknowledgment
This work was supported by the National Basic Research Program (2003CB716000) and the National Natural Science Foundation of China (Project No. 20876076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Lu, DQ., Ling, XQ. et al. Simultaneous Determination of Four Active Components in Tobacco Wastes by LC. Chroma 69, 561–566 (2009). https://doi.org/10.1365/s10337-008-0908-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0908-8