Abstract
Background
Preoperative staging of patients with colorectal carcinoma (CRC) has the potential benefit of altering treatment options when metastases are present. The clinical value of chest computed tomography (CT) in staging remains unclear.
Materials and Methods
All patients who undergo colorectal surgery in our hospital are prospectively registered, including patient, treatment, and histopathological characteristics; outcome; and follow-up. Since January 2007, routine preoperative staging CT of chest and abdomen for patients with CRC has been performed as part of our regional guidelines. In this observational cohort study, an analysis on outcome was done after inclusion of 200 consecutive patients.
Results
Synchronous metastases were present in 60 patients (30%). Staging chest CT revealed pulmonary metastases in 6 patients, with 1 false positive finding. In 50 patients indeterminate lesions were seen on chest CT (25%). These were diagnosed during follow-up as true metastases (n = 8), bronchus carcinoma (n = 2), benign lesions (n = 25), and remaining unknown (n = 15). Ultimately, synchronous pulmonary metastases were diagnosed in 13 patients (7%), in 6 patients confined to the lung (3%). In none of the patients the treatment plan for the primary tumor was changed based on the staging chest CT.
Conclusion
The low incidence of pulmonary metastases and minimal consequences for the treatment plan limits the clinical value of routine staging chest CT before operation. It has several disadvantages such as costs, radiation exposure, and prolonged uncertainty because of the frequent finding of indeterminate lesions. Based on this study, a routine staging chest CT in CRC patients is not advocated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preoperative staging of patients with colorectal carcinoma (CRC) has the potential benefit of altering treatment options when metastases are found. Synchronous metastases are usually detected in the liver, lung, and peritoneal cavity. Staging with abdominal computed tomography (CT) for liver metastases has resulted in various new approaches aimed at increasing the chance of curative treatment. It seems a logical next step to apply this approach on synchronous pulmonary metastases as well. Preoperative staging with CT of chest and abdomen in a routine “one-stop shop” setting also has a logistical advantage, saving time for both patient and physician. The clinical benefit of a staging chest CT, however, has been controversial. There are few studies describing the outcome and clinical relevance of a staging chest CT.1–5 The main problem of staging with chest CT lies in the frequent finding of indeterminate lesions (20%–30%). These lesions are usually difficult to determine and seldom malignant (10%–20%).1,3 The main advice is not to delay treatment of the primary tumor or of liver metastases when indeterminate lesions are found.2,3,6 The outcome can be debated, because in most of these studies the number of patients was limited or the studies were carried out more then 10 years ago. Accurate staging is increasingly important in the oncological multidisciplinary treatment plan of CRC. Rapid technical advancements and increasing knowledge resulting from histopathological correlation studies enhance the determination of pulmonary anomalies on chest CT.7,8 For these reasons, routine staging with abdominal and chest CT was decided upon as a part of our regional guidelines for colorectal cancer. The aim of this study was to analyze the outcome and clinical benefit of routine staging with chest CT after inclusion of a consecutive series of 200 patients with colorectal cancer.
Methods
The Medical Spectrum Twente is a large teaching hospital in the Eastern part of the Netherlands that functions as a referral center for liver and lung surgery. All patients operated on in our hospital for CRC are prospectively registered in a database designed for colorectal surgery, including patient, treatment, and histopathological characteristics; outcome; and follow-up. Since January 2007, routine preoperative staging CT of chest and abdomen for patients with CRC has been performed as part of our regional CRC guidelines, when feasible. An analysis on benefit was intended after inclusion of 200 patients with staging CT of chest and abdomen as a prospective observational cohort study.
All patients with colon and rectal cancer presented to our department, also those with an urgent or acute presentation, were included. When a staging CT scan could not be performed before operation because of acute circumstances, the scan was done within 1 month after the operation. Patients with rectal cancer at 0–10 cm from the anal verge were additionally staged with a pelvic MRI for estimation of the local invasion (cTN stage). In the cases of cT4 tumors or cT3 tumors with a distance < 1 mm from the mesorectal fascia, a long schedule of chemoradiation consisting of 25 × 2 Gy combined with oral capecitabine was given followed by surgery 6–8 weeks later. In the cases of cT3 tumors with a distance of >1 mm from the mesorectal fascia, a short schedule radiotherapy consisting of 5 × 5 Gy was given, followed by surgery the following week. Both according to the Dutch guidelines for rectal cancer.
A CT scan of chest and abdomen was performed on a 16- and 64-slice scanner (Toshiba Aquillion 16 and 64) after intravenous contrast injection (visipaque 320, 90 mL, 3 mL/s.), in the portal venous phase, with a slice thickness of 1 mm and a reconstruction of 0.8 mm. Lesions were evaluated on density, number of lesions, morphology, localization, and size. The lesions found on chest CT were defined by the radiologists as benign, malignant, or indeterminate. Indeterminate lesions are defined as lesions seen on chest CT that could not be judged by the radiologist as either benign or malignant.
Follow-up was done for all patients when feasible and consisted of 3-monthly visits with carcinoembryonic antigen (CEA) measurements, according to the national guidelines. Indeterminate pulmonary lesions were reevaluated during follow-up, with consideration of individual patients’ circumstances. The definitive diagnosis of pulmonary metastases was based on imaging (aspect and growth rate on CT and/or PET scanning) or histological confirmation. Indeterminate pulmonary lesions were considered benign when there were no signs of malignant growth on repeat chest CT and no increase in CEA after at least 1 year of follow-up.
Results
The 200 patients were included between January 2007 and August 2008 (Table 1). Elective procedures were done on 164 patients (81%). Urgent or acute procedures were done on 36 patients (19%), of whom 16 patients had a staging CT after the surgical procedure. Findings during follow-up till July 2009 were taken into the analysis.
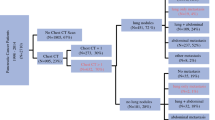
Synchronous metastases in the liver, lung and/or peritoneal cavity were found in 60 patients of the study group (30%) (Table 2). Pulmonary metastases were diagnosed on the initial chest CT in 6 patients (3%). In 1 of these 6 patients the pulmonary lesion turned out to be benign after resection. Indeterminate lesions were seen on 50 staging chest CTs (25%) (Fig. 1). Additional diagnostic procedures done during follow-up for indeterminate lesions were a repeat chest CT scan in 29 patients, a PET scan in 11 patients, and a bronchoscopy and percutaneous needle biopsy in 4 patients (Table 3). In 2 patients the percutaneous needle biopsy resulted in a small pneumothorax; in 1 of 4 patients a definite histological diagnosis could be made. In 8 patients the indeterminate lesions were diagnosed as pulmonary metastases (16%), in 2 patients as primary bronchus carcinoma, in 25 patients as benign lesions, and in 15 patients no diagnosis was made (Fig. 1). The time to final diagnosis of the indeterminate lesions took 3 months up to 1 year after resection of the primary tumor.
Outcome of staging chest CT in patients with CRC. All numbers refer to the number of patients. Reasons: No evaluation, in these patients the absence of presence of pulmonary metastases would have no consequences for (further) treatment, such as with incurable metastases on other locations or the wish of the patients to receive no further treatment. Deceased, patients who died during hospital stay or within 3 months after discharge. LTFU, lost to follow-up, both patients were referred to other hospitals for reasons other then the indeterminate pulmonary lesions
Ultimately, 13 of the 200 study patients had synchronous pulmonary metastases (7%) (Tables 2 and 4). In 6 patients the metastases were confined to the lung (3%). Of these 6 patients, 4 had no mesenterial lymph node metastases at the primary tumor site. The prevalence of synchronous lung metastases was higher in patients with rectal cancer (0–15 cm from the anal verge) (7 of 71, 10%) than in patients with colon cancer (6 of 129, 5%). Three patients with rectal tumors who had neoadjuvant treatment (radiation or chemoradiation) turned out to have lung metastases (3 of 40, 8%) (Table 4). From the 47 patients with liver metastases, 6 patients also had lung metastases (13%). In 2 of 6 patients who had a conventional chest x-ray as well, the metastasis were visible on chest x-ray.
In none of the patients with either lung metastases or indeterminate lesions was the neoadjuvant or operative plan for the primary tumor changed. In 2 patients, intended curative metastasectomy of true pulmonary metastases was done; both patients had recurrent disease within 10 months after resection.
Discussion
As in preceding studies, this study shows the limited clinical value of a routine preoperative staging chest CT in colorectal cancer patients. The incidence of lung metastases is low (7%), especially those that are confined to the lung (3%). Only in 5 patients were lung metastases diagnosed before treatment of the primary tumor (2.5%). Indeterminate lesions on chest CT are frequently found (in 25% of patients), and discrimination between benign and malignant lesions is often difficult. Only a minority turn out to be metastases (16%). Staging with chest CT did not result in a change of the treatment plan for the primary tumor, nor did it result in curative treatment of pulmonary metastases. It does cause diagnostic dilemmas and prolonged uncertainty and requires resources.
The clinical relevance of staging on distant metastases is highly dependent on the consequences for the treatment plan. Colorectal surgery knows a considerable morbidity and mortality, which can be reduced when the presence of incurable disease is diagnosed before the operation. For asymptomatic patients with widespread metastases, only chemotherapeutic treatment might be preferable. Also in symptomatic patients with incurable disease, treatment alternatives such as stenting for impending bowel obstruction, limited surgery with only colostomy, or radiation therapy in bleeding rectal tumors might be better alternatives. Curable metastases found on the staging CT can result in alternative strategies such as neoadjuvant chemotherapy, a “liver-first” approach, or simultaneous resections. Intended curative resection of synchronous liver metastases can be done in an estimated 25%–40% of patients.9,10 Consequently, accurate staging before resection of colorectal cancer has become a requirement for optimal oncological treatment. Abdominal CT is a very reliable diagnostic tool for liver metastases and has proven to be better than noncontrast-enhanced ultrasound.9,11,12 However, where accurate staging of the liver has resulted in changes in treatment plans and possible favorable outcome, this does not seem to be true for pulmonary staging in this study. Several reasons were found that contribute to the suggested difference between synchronous liver and lung metastases. The incidence of lung metastases is much lower (7%), and the curative options for synchronous lung metastases are very limited or may be even nonexistent.13–15 This might be due to the bad prognosis of synchronous pulmonary metastases as an expression of tumor behavior, as has been suggested by other authors.13–15 Metastases confined to the lung are rare (3%) and limits the value of a routine staging chest CT when no metastases are found on the staging abdominal CT.
Small nodules are often seen on chest CT and are usually benign, as was also found by Kronawitter et al. and Brent et al.1,3 The difficulty with these lesions is that they are usually too small (between 0.5 and 1 cm) to evaluate on radiological characteristics such as density and shape. Determination of the nature of these lesions has proven to be rather difficult. PET scanning has the same disadvantage of a limited discriminative capacity for small lesions. Percutaneous needle biopsies for small lesions can be technically difficult and are prone to sampling errors; when the histological result does not show a malignancy, a metastasis is still not excluded. It can, however, be harmful, because of the risk of pneumothorax and bleeding complications. In this study, it took several months up to 1 year after resection of the primary tumor before the indeterminate lesions could be classified. Our definition of a benign lesion, which is no growth and no serum-CEA increase after 1 year, however, is a mere assumption and not definitive proof that the lesion is truly benign. On the other hand, the duration and absence of clinically relevant disease does imply there is no need to try to find these lesions before treatment of the primary tumor. Staging with chest CT during follow-up might be a better alternative.
The diagnostic difficulties of findings on chest CT are also reflected in literature. The reported incidence of synchronous pulmonary metastases of colorectal cancer varies largely, from 3% to 18%.1,3,4,6 This is likely influenced by the applied definition of pulmonary metastases, which is usually a derived definition because of a lack of obtainable histological proof. In this study, neither the “gold” standard for false or true metastases was available. Because of the criteria used for true metastases and study design, the real incidence might be slightly underestimated. It is, however, a close approximation of the number of patients with clinically relevant lung metastases in a nonselected population.
We agree with previous authors that small lesions on chest CT (< 1 cm) should be considered of limited clinical relevance and ignored in choosing the initial treatment plan.1–3,6 The finding of large pulmonary metastases might change the treatment plan, but these kinds of metastases are rare and often visible on chest x-ray. Few studies compared staging chest CT with chest x-ray. Two studies compared a negative chest x-ray with outcome on chest CT in preoperative staging, concluding that a staging chest CT had little to no additional value.1,6 One other study compared findings on staging chest x-ray with chest CT.2 This study showed that in 4 of 7 patients the pulmonary metastases were visible on chest x-ray. This number is comparable to the 2 of 6 visible metastases on chest x-ray in this study.
Concerning the change of treatment plan in cases of resectable liver metastases, pulmonary staging with chest CT seems the best, most sensitive option to exclude extrahepatic disease. Evaluation of consequences for hepatic metastasectomy was not the aim of this study, and the patient group is too small to draw conclusions; in the 5 patients with synchronous liver and lung metastases, the liver metastases were not resectable. Povoski et al. did study this specific patient group and concluded that chest CT had only minimally improved detection of malignant lesions of the lung over chest x-ray.6
It is reasonable to argue that selected patients might benefit from a staging chest CT instead of chest x-ray, for instance, patients with compromised health who are about to undergo major surgical procedures for hepatic or peritoneal metastases or patients who will receive neoadjuvant treatment for rectal cancer. The latter group would especially benefit because pulmonary metastases seem to occur more often in rectal cancer than in colon cancer and the operative procedure might be more extensive. In our study, the proportion of patients with rectal cancer, especially located 0–10 cm from the anal verge, is too small to draw straightforward conclusions for this specific group but deserves further study. It is, however, likely the same problem with indeterminate lesions on chest CT will occur. Future studies on imaging techniques with a higher discriminative capacity for small pulmonary nodules remain required to improve staging in high-risk patient groups.
Relevant disadvantages other than the diagnostic difficulties of routine chest CT are the significant radiation exposure and costs; routine staging with CT of the chest together with CT of the abdomen is twice as expensive and induces twice as much radiation exposure. When indeterminate lesions are seen, it may further cause a prolonged period of uncertainty, unnecessary anxiety for patients and relatives and possibly lead to a delay in neoadjuvant, surgical, and adjuvant treatment. The need for additional diagnostic procedures such as repeat chest CTs with a summative radiation exposure, PET scanning, or percutaneous needle biopsies, further increases costs and possible harm to the patient.
To conclude, in our opinion routine staging of colorectal cancer patients with chest CT is not advocated based on these study results. We believe that defining high-risk patient groups and predictive factors for pulmonary metastases followed by either immediate staging in selected patients or “delayed” staging with chest CT during follow-up might be a better and less expensive alternative to identify patients with pulmonary metastases.
References
Kronawitter U, Kemeny NE, Heelan R, Fata F, Fong Y. Evaluation of chest computed tomography in the staging of patients with potentially resectable liver metastasis from colorectal carcinoma. Cancer. 1999;86:229–35.
McIntosh J, Sylvester PA, Virjee J, Callaway M, Thomas MG. Pulmonary staging in colorectal cancer—is computerised tomography the answer? Ann R Coll Surg Engl. 2005;87:331–3.
Brent A, Talbot R, Coyne J, Nash G. Should indeterminate lung lesions reported on staging CT scans influence the management of patients with colorectal cancer? Colorectal Dis. 2007;9:816–8.
Kirke R, Rajesh A, Verma R, Bankart MJG. Rectal cancer: Incidence of pulmonary metastases on thoracic CT and correlation with T staging. J Comput Assist Tomogr. 2007;31:569–71.
Kosmider S, Stella DL, Field K, Moore M, Ananda S, Oakman C, et al. Preoperative investigations for metastatic staging of colon and rectal cancer across multiple centres—What is current practice? Colorectal Dis. 2009;11:592–600.
Povoski SP, Fong Y, Sgouros SC, Kemeny NE, Downey RJ, Blumgart LH. Role of chest CT in patients with negative chest x-rays referred for hepatic colorectal metastases. Ann Surg Oncol. 1998;5:9–15.
Kawaguchi T, Kusumoto M, Maeshima A, Tateishi U, Suzuki K, Moriyama N. High resolution Computed tomography appearances of surgically resected pulmonary metastases from colorectal cancer, with histopathologic correlation. Radiat Med. 2005;23:418–26.
Yoon HE, Fukuhara K, Michiura T, Takada M, Imakita M, Nonaka K, et al. Pulmonary nodules 10 mm or less in diameter with ground-glass opacity component detected by high resolution computed tomography have a high possibility of malignancy. Jpn J Thorac Cardiovasc Surg. 2005;53:22–8.
Valls C, Andia E, Sanchez A, Guma A, Figueras J, Torras J, Serrano T. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology. 2001;218:55–60.
Kobayashi H. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicentre study. Surgery. 2007;141:67–75.
SE Engelen, Beets GL, Beets-Tan RGH. Role of preoperative local and distant staging in rectal cancer. Onkologie. 2007;30:141–5.
Bentrem DJ, DeMatteo RP, Blumgart LH. Surgical therapy for metastatic disease to the liver. Annu Rev Med. 2005;56:139–56.
Lin BR, TC Chang, YC Lee, PH Lee, KJ Chang, JT Liang. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol. 2009;16:1026–32.
Treasure T. Pulmonary metastasectomy: a common practice based on weak evidence. Ann R Coll Surg Engl. 2007;89:744–8.
Rena O, Casadio C, Viano F, Cristofori R, Ruffini E, Filosso PL, et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg. 2002;21:906–12.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Grossmann, I., Avenarius, J.K.A., Mastboom, W.J.B. et al. Preoperative Staging with Chest CT in Patients with Colorectal Carcinoma: Not as a Routine Procedure. Ann Surg Oncol 17, 2045–2050 (2010). https://doi.org/10.1245/s10434-010-0962-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-0962-y