Abstract
In transdermal applications of nonsteroidal anti-inflammatory drugs, the rheological and mechanical properties of the dosage form affect the performance of the drug. The aim of this study to develop emulgel and nanostructured lipid carrier NLC-based gel formulations containing ibuprofen, evaluate their mechanical properties, bioadhesive value and ex-vivo rabbit skin permeability. All formulations showed non-Newtonian pseudoplastic behavior and their viscosity values are suitable for topical application. The particle size of the nanostructured lipid carrier system was found to be 468 ± 21 nm, and the encapsulation efficiency was 95.58 ± 0.41%. According to the index of viscosity, consistency, firmness, and cohesiveness values obtained as a result of the back extrusion study, E2 formulation was found to be more suitable for transdermal application. The firmness and work of shear values of the E2 formulation, which has the highest viscosity value, were also found to be the highest and it was chosen as the most suitable formulation in terms of the spreadability test. The work of bioadhesion values of NLC-based gel and IBU-loaded NLC-based gel were found as 0.226 ± 0.028 and 0.181 ± 0.006 mJ/cm2 respectively. The percentages of IBU that penetrated through rabbit skin from the Ibuactive-Cream and the E2 were 87.4 ± 2.11% and 93.4 ± 2.72% after 24 h, respectively. When the penetration of ibuprofen through the skin was evaluated, it was found that the E2 formulation increased penetration due to its lipid and nanoparticle structure. As a result of these findings, it can be said that the NLC-based gel formulation will increase the therapeutic efficacy and will be a good alternative transdermal formulation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ibuprofen (IBU) is a nonsteroidal anti-inflammatory drug (NSAID) administered topically in different dosage forms (e.g., ointments, creams, and gels) or used orally. Like other drugs in this category, it can cause damage to the gastric mucosa and show severe gastrointestinal side effects like gastroduodenal and acute gastrointestinal bleeding [1]. Oral IBU therapy is usually limited because of its short biological half-life, hepatic first-pass metabolism, gastrointestinal side effects, and repeated high multiple dosing [2]. Compared to systemic use, local drug application has advantages such as prevention of side effects and high concentration of the drug in the area where it is required [3]. Therefore, great attention is being paid to developing topical dosage forms of IBU to provide relatively consistent drug levels in the application area for a long time and avoid oral side effects [4, 5].
Penetration of the drug through the dermis is thought to be affected by its ionic state, and it is known that ionized species penetrate less than non-ionized species [6]. Since the skin’s pH is approximately 4.8, most weakly acidic molecules, such as ibuprofen, are in the nonionic state and are suitable for passive diffusion. However, since IBU shows poor water solubility (pH 4.5Solubility: 0.084 mg/L and pH 5.5Solubility: 0.685 mg/L), penetration into the skin is limited [7]. However, it is considered an attractive candidate for transdermal application due to its physicochemical properties, such as suitable partition coefficient (log P: 3.68), low molecular weight (206.29g·mol−1), and short elimination half-life (t1/2: 2–4 h) [8]. Different formulations have been described in the literature for the topical application of IBU [9, 10]. These include skin penetration enhancers, eutectic systems, microemulsions, microspheres, nanostructured lipid carriers, ultrasound-assisted transdermal transport, and ethosomal gel [11,12,13,14,15,16,17,18]. Most of the techniques were reported to enhance the skin penetration for IBU to varying extents.
Emulgels are oil-in-water or water-in-oil type emulsions gelled by mixing with a gelling agent [19]. In an emulsion, drug particles are incorporated into the internal phase, which acts as a drug reservoir, from which the drug is released and absorbed into the skin. Emulgels are a better choice for poorly water-soluble drugs [20]. Therefore, the transdermal activity of Ibuprofen can be increased by preparing the emulgel formulation. Transdermal emulgels have many advantageous properties, such as being greaseless, easily removable, emollient, easily spreadable, non-staining, longer shelf-life, biocompatible, water-soluble, and pleasing appearance [21]. They have high patient acceptability since they possess the advantage of emulsions and gels [22].
Nowadays, one of the other most attractive strategies is nanostructured lipid carriers (NLC) for improving the penetration of drugs through the skin [23]. NLC has been observed as a promising carrier for providing different advantageous properties for transdermal drug delivery and the topical route of administration. This carrier comprises physiologically and biologically degradable lipids that exhibit low cytotoxicity and systemic toxicity. The small size of the lipid particles provides close contact with the stratum corneum and can increase the amount of drug penetrating the mucosa or the skin [24]. NLCs consist of an aqueous phase containing a surfactant or a mixture of surfactants and an unstructured solid lipid matrix composed of solid and liquid lipids. The solid lipids are blended with liquid lipids at a ratio of 70:30 to 99.9:0.1, while the surfactant content is between 1.5%—5% (w/v). These systems have shown great potential as solid matrix lipid nanoparticle carriers for the topical application of poorly soluble active components [25]. Sütő et al. developed an IBU-loaded nanostructured lipid carrier (IBU-NLC) and aimed to increase the skin penetration of ibuprofen to treat diseases such as osteoarthritis. At the end of the studies, the permeability of IBU-NLC gel via excised human skin was significantly greater than that of IBU gel [3].
In transdermal applications, the skin's barrier function causes limited permeability of active substances [26]. In addition, in transdermal applications, the physicochemical properties of the active substance, the chemical structure, and the physical properties of the carrier system also affect the therapeutic effect [27, 28]. A transdermal delivery system must possess favorable properties like good spreadability on the skin, optimum viscosity, easy removal of the product from the packaging, and good adhesive properties within acceptable viscosity are essential [29]. Mechanical and rheological properties are two of the most critical properties of transdermal formulations, and patient acceptability and the appropriate mechanical and rheological properties of the formulations highly influence use. Products with high cohesiveness and hardness could be considered unsatisfactory because they are challenging to apply to the skin. Meanwhile, formulations with very low cohesiveness and adhesiveness characteristics may drip from the tube and the application site. Hence, considering these aspects, the mechanical parameters of the obtained formulation are vital in terms of their technological design [30].
Texture profile analysis (TPA) is the most widely applied and helpful technique for the mechanical properties of pharmaceutical semi-solid formulations to determine interactions between formulation components, complementing rheological information [31, 32]. The TPA mode can extract compressibility, hardness, adhesiveness, elasticity, and cohesiveness from the compression graphs [29, 33]. Considering this information, the system’s mechanical properties and ex-vivo permeability studies should be well evaluated to improve the efficiency of transdermal systems.
Our study aimed to design emulgel and NLC-based gel formulations containing ibuprofen, evaluate their mechanical properties, bioadhesive value, and ex-vivo rabbit skin permeability, and enhance the permeability compared to commercial products. In emulgel and NLC-based formulations developed for this purpose, penetration enhancers (propylene glycol, ethanol, isopropanol, isopropyl myristate, and Tween 80) and lipids (Precirol® ATO 5 and Miglyol® 812) were used in different amounts. These formulations were compared to each other and with the commercial products, gel formulation containing the free drug. In particular, no study has been found in the literature in which the data of TPA, back extrusion, spreadability, and bioadhesion tests performed on transdermal emulgels were evaluated together. In addition, this study is the first to determine the detailed mechanical properties of transdermally applied IBU formulations for spreadability/compressibility, hardness, firmness, adhesiveness, cohesiveness, consistency, and viscosity index, which have not been evaluated in detail in the literature before.
Materials and Methods
Materials
Triethanolamine, NaOH, and propylene glycol (PG) were purchased from Sigma-Aldrich (U.S.A). Isopropyl myristate (IPM), Isopropanol (IP), Ethanol, and Tween 80 (T 80) were purchased from Merck (Germany). Miglyol 812 and Precirol ATO 5 were purchased from Gattefossé (France). The Carbopol® 974 and Carbopol® 934 were supplied from Noveon, Parkoteks Chemical (Turkey). The commercial products were obtained from the Turkish drug market (Neoprofen Gel (NPR J60 14/T02, Pensa Drug, Turkey), Ibuactive Cream (7033034A, Pharmactive Drug, Turkey)). While Neoprofen gel contains 5% ibuprofen, propylene glycol, diethylamine, carbomer (Carbopol®) 940, ethyl alcohol, and deionized water, Ibuactive cream consists of 5% ibuprofen, propylene glycol, isopropyl myristate, glycerol monostearate, polysorbate 60, sorbitan trioleate, lavender essence, and deionized water.
In Vitro Studies
Transdermal Formulations Studies
Preparation of the F1 Gel Formulations
Firstly, 1% and 2% by weight of Carbopol® 934 were dispersed in distilled water at 800 rpm by mixing, and then the system was stirred by adding 10% NaOH until a clear gel (100 g) was formed. To remove air bubbles before application, wait for one night at + 4°C.
Preparation of the F2 Emulgel Formulation
The mixture of Tween 80: Isopropyl myristate: Isopropanol: Propylene glycol (10:5:5:10 w/w) was used for the ibuprofen dissolve. Carbopol® 974 (2% w/w) was also dissolved in distilled water by adding triethanolamine. The water and lipid phases were mixed using Ultra-Turrax (IKA® Eurostar, Germany) to obtain a transdermal emulgel formulation.
Preparation of the F3 and F4 Emulgel Formulations
The ibuprofen was dissolved in a mixture of ethanol and propylene glycol. Carbopol® 934 was dissolved in distilled water by adding triethanolamine and 10% NaOH for F3 and F4 formulations. The water and lipid phases were mixed using Ultra-Turrax to obtain a transdermal emulgel formulation.
All formulations were prepared at room temperature (25°C). The components of the gel and emulgel formulations are given in Table 1.
Preparation of the NLC Dispersion
The lipids were heated to about 60–70°C. The IBU was dissolved in the lipid phase. Tween 80 was dissolved in distilled water for the aqueous phase and heated at the same temperature as the lipid phase. The aqueous phase was mixed with the lipid phase and homogenized for 3 min at 13,500 rpm using an Ultra-Turrax. The pre-emulsion method was used for dispersions of NLC at a 30% amplitude sonication probe (Ultrasonic processor Vibra cell, Sonic & Materials, Newtown, USA) for 5 min. The obtained o/w nanoemulsions were cooled to room temperature [34]. The dispersion of the NLC incorporating the ibuprofen concentration of 5% (w/w) was obtained. The NLC formulation composition is given in Table 1.
Preparation of the NLC-Based Gel
Glycerine was mixed with distilled water. Afterward, Carbopol® 974 was dispersed in the aqueous phase, and triethanolamine was added. The obtained dispersion of the NLC containing IBU was incorporated in this gel with 1.5% (w/w) in a 50:50 ratio. The composition of the NLC-based gel and IBU-loaded NLC-based gel formulation is given in Table 1.
Determination of Viscosity Value
Brookfield DV-III + Rheometer (TC-502, USA) was used for the viscosity measurements at 25.0 ± 0.5°C. Spindle No:52 was used to measure the viscosity. The spindle speed was set as a 10–100% torque value.
Characterization of the Transdermal Formulations
Particle Size, Polydispersity Index, and Zeta Potential of the NLC
The particle size, zeta potential (ZP), and polydispersity index (PDI) of the nanostructured lipid carriers systems were measured at 25°C by a Zetasizer Nano Series (Nono ZS, Malvern Inst., U.K.).
Encapsulation Efficiency (EE) of the NLC
The filtration/centrifugation technique was used for free IBU at 3500 rpm for 60 min (Allegra X-30R, Germany). Firstly, to remove free IBU on the NLC surface, 1 g of NLC dispersion was diluted to 10 mL with pH 7.4 phosphate buffer [35]. Then, the sample was centrifugated. The pH 7.4 phosphate buffer was used for filtrate dilution, and quantification was carried out with a UV spectrophotometer at 222 nm. Equation 1 was used to calculate the encapsulation efficiency (EE).
Morphological Characterization for the NLC
The prepared NLC formulations were analyzed to obtain morphologic images using an optic microscope (Leica DMEP, Germany). The microscopic morphological image of the NLC formulation was evaluated in three stages. First, the IBU-loaded NLC formulation was displayed, and then the formulation was exposed to Ultra-Turrax and probe sonication processes were displayed.
Mechanical Properties of the Transdermal Formulations
Texture Profile Analysis (TPA)
The texture profile analysis (TPA) was carried out with a TA-XT Plus Texture Analyser (Stable MicroSystems, London, UK) with a 5 kg load cell [36, 37]. Samples were placed in a standard bottle with a volume of 25 ml at a height of 8 cm. It was kept in an ultrasonic water bath at 37°C for 20 min to remove air bubbles. All samples were compressed twice at a 15 mm depth at a 2 mm/s rate with an analytical probe of a diameter of 10 mm. The recovery time between two compressions is 15 s [38, 39]. The Texture Exponent 32 Version 5.0.8.0 was used for data analysis. The mechanical parameters of the formulations, such as adhesiveness, fracturability, hardness, cohesiveness, springiness, and resilience, were determined by the force–time graph obtained (Fig. S1).
The adhesiveness value was obtained from the area of the negative part of the curve, and the hardness value was obtained from the maximum force [40]. Hardness is necessary to achieve a given deformation or as the maximum peak force on the first compression cycle. The work required to overcome the attractive force between the probe and the gel surface is expressed in adhesiveness. The rate of the region under the force–time curve produced in the second compression cycle to the region obtained from the first compression cycle, in which consecutive compressions are separated with a given recovery time, is described as cohesiveness (Area 2/Area 1 or Area 5/Area 4 (optional)). Springiness is how fine a formulation physically springs back after it has been allowed to wait for the target wait time between strokes and has been deformed during the first compression. The down-stroke of the second compression is analyzed for the spring back (Distance 2/Distance 1 or Time 2/Time 1 (optional)). Resilience can be measured with a single compression before the waiting period begins with the withdrawal of the first penetration; however, the withdrawal speed should be the same as the compression speed (Area 4/Area 3) [41,42,43,44].
Back Extrusion Studies
A back extrusion test was carried out using a TA-XT Plus Texture Analyser. The analysis is performed with a standard-size back extruder 50 mm in diameter, which is approximately 3/4 full in samples stored at 25°C. The extrusion disk is placed 30 mm above the sample surface and in the center of the containers with a sample height of 40 mm. The disc immerses the sample at a depth of 10 mm at a velocity of 1 mm/s, and the return velocity is 20 mm/s. At this time, the container must be held so it does not go up. Exponent Version 4.0.6.0 (Stable Micro Systems, London, UK) calculates parameters such as viscosity index, consistency, firmness, and cohesiveness (Fig. S2). This analysis will provide new information to the literature, and the mechanical properties of the developed formulations will be evaluated and compared with the existing data [45].
Spreadability
A spreadability test was carried out with a spreadability rig (HDP/SR) using a TA-XT Plus Texture Analyzer, which precisely matched male and female perspex cones. The samples of formulations are filled into female cones at a 90° angle without allowing air bubbles to form in the lower cone. The analysis is performed while the male cone (90° angle) is displaced within 0.5 mm of the base of the female cone. The spreadability is equated to the region under the curve, and the force is evaluated for the test (Fig. S3). The displacement of smaller forces in the female cone indicates smaller values and easier spreadability [46].
Ex-Vivo Bioadhesion Studies
The TA-XT Plus Texture Analyzer with a bioadhesive holder was used for ex-vivo bioadhesion studies. A piece of 2 mm thickness from the inner part of the skin surface of the hairless rabbit was attached to the tip of the probe (P 0.5 Perspex: 12.5 mm) with the help of glue. The prepared formulations and commercial products were applied to the skin, and the instrumental parameters specified were used to evaluate the bioadhesive potential of the transdermal gel formulations. The work of bioadhesion is calculated from the area under the curve of the force-distance plot (Fig. S4). Equation 2 is used to calculate the work of bioadhesion per cm2 (mJ.cm−2) (πr2: the area of the skin surface is in contact with the formulation).
Ex-Vivo Skin Permeation Studies
Ex-vivo skin permeation studies of formulations and commercial products were conducted using a Franz diffusion cell. The hairless rabbit skin was used for permeation studies for IBU. The diffusional cross-sectional area was 1 cm2, and the receptor chamber capacity was 2.5 mL. The 0.5 mL of gel formulations were placed in the donor compartment and 2.5 mL of phosphate buffer (pH 7.4) in the receptor compartment. 5 mL of the aliquots were withdrawn at predetermined time intervals from the receptor phase and with an equal volume of fresh medium to maintain the total volume. The withdrawn samples were analyzed for drug content by UV spectrophotometer at 222 nm. Three parallel studies were performed for each formulation. Equation 3 and Eq. 4 were used to calculate the permeability coefficient and flux, respectively. The calculation of the permeability coefficient in ex-vivo transition is made from the graph of the amount of active substance penetrated through the tissue (μg/cm2) versus time (h). The slope of the linear part in the plotted graph gives the equilibrium flux value (Js). The permeability coefficient (P) is found by dividing this value by the active substance concentration in the donor phase [47].
- P:
-
Permeability coefficient (cm/hour)
- Js:
-
Flux (μg/cm2.hour)
- Cd:
-
Drug concentration in the donor phase (μg/cm3)
- C1-C2:
-
Change in concentration of overtime
IBU Quantification Analysis
IBU was quantified using a UV-spectrophotometer (Cary 60 UV–Vis, Agilent, Germany) for encapsulation efficiency study and ex-vivo quantification analysis. The UV method was developed and validated with linearity, precision, accuracy, and specificity for pH 7.4 phosphate buffer. Detailed information on UV analysis methods and their validation has been given in the Supplementary File.
Results and Discussions
Determination of Viscosity Value
Viscosity indicates the gel's viscoelastic behavior upon applied stress [48]. Viscosity is critical to facilitate the transdermal application of formulations prepared in a carrier gel and control the release profile. Also, this property is an essential indicator for predicting gel behavior in vivo [36].
Carbopols are very high molecular weight polymer derivatives of acrylic acid, which are preferred to change the flow properties in semi-solid and liquid formulations such as gel and suspension [38, 49]. Carbopol forms of a tighter and harder gel with tight bonds between chains.
The rheological behaviors help predict formulation spreadability capacity in the skin [50]. When the viscosity is high, the release of the active ingredient slows down, and the skin stays longer [51]. Prepared formulations should have high viscosity to facilitate transdermal use and low viscosity to delay release [52]. Also, the viscosity is essential in that it enables the flow of the formulation from the container to the application area and its absorption back into the container when the stress is eliminated.
In the viscosity measurements, the viscosity changes with rpm were determined. The formulations' viscosity values are given in Table 2. Incorporating the IBU-loaded NLC into the gels showed increasing viscosity for the NLC-based gel systems. The rheograms (Fig. S5) demonstrated that gel, emulgels, IBU-loaded NLC-based, and NLC-based gels exhibited a non-Newtonian pseudoplastic behavior with an immediate flow after the stress application. This rheologic behavior of formulations showed a shear-thinning characteristic for retention on the skin and ease of application. The obtained viscosity values were found suitable for topical application.
Characterization of the Transdermal Formulations
Particle Size, Polydispersity İndex, and Zeta Potential of the NLC
There are several strategies in the literature to increase the transdermal penetration of IBU. One of them is the preparation of the transdermal formulation, which includes a nanoparticle system. Particle size, distribution, and surface charge are the most important features of a nano-sized drug delivery system designed for transdermal application [53]. In this study, we prepared nanostructured lipid carriers containing IBU and incorporated them into a carrier for transdermal application. The properties of the NLC formulation that may affect the penetration of IBU through the skin are presented in Table 3.
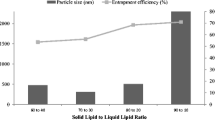
In this study, two different processes were used during the mixing of the oil phase and the water phase while preparing the NLC dispersion. These processes are Ultra-turrax and probe sonication processes. It was observed that the size, distribution, and zeta potential of the particles in the NLC dispersion changed depending on these processes. Similar results were also available in the literature [54, 55]. While larger particles were obtained with the Ultra-turrax process, a reduction in particle size was observed after the probe sonication process. The difference in the size of particles containing IBU is quite dramatic. The particle size of IBU-NLC formed by Ultra-turrax treatment was 2969 ± 785 nm, whereas the particle size of IBU-NLC formed by probe sonication was 468 ± 21 nm. The probe sonication process reduced the particle size approximately six times compared to the Ultra-turrax process. The lower particle size is acceptable for transdermal application [56, 57].
A PDI of less than 0.5 indicates the particle size is uniform [58]. The PDI values of the NLC-IBU after the Ultra-turrax and probe sonication were lower than 0.5 (Table 3), meaning they were monodispersity (Fig. S6).
The zeta potential shows the charge of the nanoparticles in a media and permits the evaluation of the degree of repulsion between charged particles. The surface charges are important for the skin penetration of formulation in transdermal delivery. Its high zeta potential can prevent the formation of nanoparticles by electrical repulsion [59]. It is acceptable that values higher than ± 30 mV electrically stabilize the dispersion [34]. This value was around ± 30 mV, indicating good physical stability. In this study, the obtained zeta potential value was found -10.60 ± 0.31 for after-probe sonication, low. However, the Brownian behavior of the NLC will be decreased, limiting the possibility of particle aggregation since the NLC dispersions will be loaded in a semi-solid carrier [34].
Encapsulation Efficiency (EE) of the NLC
The amount of the free IBU present in the aqueous phase was analyzed for the EE. The EE of the NLC was 95.58 ± 0.41%. The lipids used may have increased the solubility of IBU and thus increased EE [34, 60]. Previous studies have stated that NLCs exhibit high entrapment efficiency due to their structure's lipid components [61]. Similar results were obtained regarding loading efficiency from NLC studies containing different IBUs [3, 62].
Morphological Characterization for the NLC
The particles were successfully formed. Their shape morphology was confirmed in the optic microscope images, which are shown in Fig. S7 (a and b). The nanoparticles were spherical, as in other literature studies [63, 64].
Mechanical Properties of the Transdermal Formulations
Texture Profile Analysis (TPA)
Topical dosage forms such as gel, emulgel, and cream should have sufficient mechanical properties for ease of application and effectiveness. Texture analysis is used to predict the structure of gels during application and their behavior under physiological conditions. If the formulation is too hard and cohesive, it is not easy to apply, or if the adhesiveness and cohesiveness are low, it will drip on the tube or application site [65]. Values such as spreadability/compressibility, cohesiveness, and hardness of developed formulations and commercial products can be determined by TPA [66]. To compare the mechanical properties of the transdermal formulations developed in this study, tissue profile analyses were performed at room temperature.
The adhesiveness, which indicates the topical residence time of the formulations, is related to the broke of the cohesive bonds and depends on the viscosity of the formulation [67]. The TPA results are given in Table 4 and Fig. S8. When the adhesion values of the transdermal formulations were compared, it was observed that the adhesion values of the emulgel formulations were considerably lower than the NLC-based gel formulations. The adhesive value increases depending on the increase in viscosity. Accordingly, it was found that the E2 formulation with the highest viscosity value had the highest adhesive value. The IBU-loaded NLC formulation (E2) showed the best adhesiveness property. When the adhesion values of the commercial products were examined, we saw that Neoprofen gel had low adhesive values, whereas Ibuactive Cream had high adhesion values. While the maximum adhesion value (-484.3 ± 58.9 g.sec) was obtained in the E2 NLC-based gel formulation, the adhesion value of Neoprofen gel was obtained as -417.3 ± 17.0 g.sec.
The force required the deformation of the gels provided by hardness [68]. The gels must have optimum hardness values for proper therapeutic effect [69, 70]. When the hardness is low, the retention time of the gel in the application area is reduced. However, the gel of suitable hardness can easily come out of its packaging, reducing the work required for application [39]. The hardness of the E2 formulation was found to be the highest because it displayed the highest viscosity. There is a direct proportionality between the viscosity and texture parameters of the formulations.
The cohesiveness values of the IBU-loaded transdermal formulations were between 0.72 to 1.44. The highest value obtained was for F2 emulgel formulation, and the lowest was for Ibuactive Cream. Cohesiveness shows the integrity of the product and its ability to hold together [71]. The reason can be interpreted as the polymeric network structure formed by Carbopol® 974 increases the cohesiveness value.
The springiness is a textural parameter related to the sample's elasticity [72]. The lowest value of the springiness parameter was observed in the F3 formulation and was found to be similar to the other formulations. The springiness value affects the application efficiency of transdermal formulations. The high springiness value shows that additional force is needed to apply the formulations. When the springiness values of the transdermal formulations developed in this study were examined, it was observed that all formulations had suitable values for transdermal application [73].
When the resilience value of the formulations was compared, the highest value was obtained from the F2 emulgel (1.19) formulation. Resilience is a measure of the elastic recovery of the sample [74]. For this reason, a higher resilience value was observed in formulations with a higher proportion of polymeric material.
When the textural properties of gel and emulgel formulations were evaluated, a change was observed depending on the type and amount of polymer used. The textural properties of the formulations using Carbopol® 974 polymer were superior; therefore, the carrier system was preferred for NLC. In addition, it was concluded that the textural properties of the transdermal formulation containing IBU developed in this study were affected by rheological properties such as viscosity.
Back Extrusion Studies
The viscosity index, consistency, firmness, and cohesiveness defined the formulation’s texture features. The higher the measured value, the firmer the sample, and the maximum force is a measure of firmness [75]. The area of the negative part expresses the viscosity index, while the cohesiveness is the value at which the negative force is maximum [76, 77]. The results of back extrusion studies are shown in Table 5 and Fig. 1a. The results obtained from the texture analysis showed that the parameters of the developed formulations generally enhanced with the augment in viscosity. The formulation density determines the consistency value, and the higher the density, the higher the consistency value [45]. The results obtained from the hardness analysis show a similar profile to the results of this analysis. E2 (IBU-loaded NLC-based gel) formulation gave the highest consistency and hardness values. Determination of the ability to reconstruct the gel after the application is expressed by cohesiveness. The gel has high cohesiveness, which provides complete structural recovery after the application, and increases the effectiveness in the application area [40]. The cohesiveness results of the gel, emulgels, and NLC-based gel formulations differed. The lowest cohesiveness value belongs to F3. The cohesiveness value of transdermal formulations containing IBU and market preparations are listed as follows, from largest to smallest: E2 > Ibuactive Cream > E1 > F1 > Neoprofen Gel > F2 > F4 > F3. This data shows that the cohesiveness of the NLC-based gel formulation has been greatly enhanced by the transport inside the NLC. Thus, the E2 formulation was more suitable than the other formulations.
The E2 formulation (IBU-loaded NLC-based gel) developed in this study was more suitable for transdermal application than other formulations regarding mechanical properties.
Spreadability
Spreadability values of gels are crucial for even application to the skin and patient compliance. It is considered optimum properties for a gel to spread slowly and show high spreadability [78]. The firmness of the gel is generally involved in the spreadability, and the firmness necessary to deform a sample is a determinant of spreadability [79]. The results of the spreadability are given in Table 6 and Fig. 1b. The formulations were found to be homogeneous and smooth, and consistency was suitable for the spreadability. The formulations' spreadability is derived from the character of its more fundamental, i.e., viscosity [80]. When evaluated from this point of view, we observed that the F1 formulation, which has the highest viscosity among transdermal formulations and market products containing IBU, has the highest hardness and shear values. We determined that the order of viscosity and spreadability values were the same among the Ibuactive Cream, E1, and E2 formulations. The firmness and work of shear values of the E2 formulation, which has the highest viscosity value, were also found to be the highest.
Ex-Vivo Bioadhesion Studies
In this study, bioadhesive emulgel and NLC-based gel for administering transdermal medications have been developed to enhance the local effects of IBU. The adhesion of the mucosal surface because of contact with another surface is defined as bioadhesion [68]. The bioadhesion of gel is a significant factor for the higher benefit of the developed preparation.
Results of bioadhesion tests for prepared formulations are shown in Table 7 and Fig. S9. Table 7 shows that the emulgels and IBU-loaded NLC-based gel showed good bioadhesive properties. Among the emulgel formulations, the F2 formulation showed the best work of bioadhesion. Since the F2 formulation contains 2% Carbopol® 974 in its composition, it was thought to have a higher work of bioadhesion than other formulations and market products. The differences in bioadhesive parameters were significant (p < 0.05). Carbopol® 974 is generally a polymer with excellent bioadhesive properties [49]. When the value of the work of bioadhesion of F3 and F4 formulations containing Carbopol® 934 were compared, it was observed that the enhancement in the polymer proportion significantly increased the work of bioadhesion. The work of bioadhesion values of NLC-based gel and IBU-loaded NLC-based gel were 0.226 ± 0.028 and 0.181 ± 0.006 mJ/cm2, respectively. It was observed that loading IBU on NLC-based gels significantly (p < 0.05) decreased the bioadhesion value.
Ex-Vivo Skin Permeation Studies
The effectiveness of transdermal drug delivery systems differs significantly in their capacity to penetrate a drug through the skin. For ex-vivo penetration studies in this work, the emulgels, NLC-based gel, and a commercial product (Neoprofen Gel and Ibuactive Cream 5%) were compared regarding the skin penetration profiles. Figure 2 depicts the penetration profiles of drugs from transdermal IBU formulations. The penetration profiles were examined; it was observed that the diffusion of IBU from the formulation was the lowest in the F4 emulsion. It was found that the E2 formulation showed good IBU penetration properties, and the drug was diffused 66% at 12 h. The IBU% that penetrated from the Ibuactive Cream and the IBU-loaded NLC-based gel (E2) were 87.4 ± 2.11% and 93.4 ± 2.72% after 24 h, respectively. When the amounts of IBU diffused through the skin after 24 h were compared, no significant differences were observed among the other formulations except the F4 formulation (p > 0.05).
The flux values of NLC-based gel E2 formulation and market product Ibuactive Cream were significantly higher than the flux values of emulgel formulations and Neoprofen Gel (p < 0.05) (Table 8). The nanoparticle structure may be effective in the passage of the skin's lipid bilayer and facilitate skin diffusion, which is why NLC-based gels increase the passage of ibuprofen. The literature has stated that nanostructured lipid carrier systems play a positive role in the passage of active molecules through the skin [81]. Because IBU has good solubility in alkaline solvents such as NaOH, some market products (Neoprofen Gel 5%) have used NaOH as a solubility enhancer [82]. To increase the dermal permeability of IBU, carrier systems consisting of lipid particles have been used frequently [83,84,85]. Drugs administered using lipid-structured NLCs are more advantageous than polymer-based systems since they resemble the lipid structure of membranes in vivo. In addition, the fact that lipids are biocompatible and biodegradable causes them to be preferred [86].
IBU is readily and almost completely absorbed after oral administration. However, less than 10% of the dose is excreted unchanged, and more than 60% is excreted in the urine within 24 h [87]. It is still unclear how oral ibuprofen reaches the local site of action with sub-therapeutic plasma levels, and also limited data are available on the pharmacokinetics (dermatokinetic) of topical administration of IBU [88].
One key advantage of topical ibuprofen use is its decreased absorption and systemic distribution compared to oral ibuprofen [89]. In a study by Patel et al. [87], Berner et al. reported that a group of patients applied 8 g of gel to the entire knee circumference three times daily for three days. This study examined different samples (e.g., subcutaneous tissue, quadriceps tendon, musculus vastus medialis, and joint capsule). Blood samples were taken before using the gel and before the tissue was removed. Drug concentrations found in muscle (20.32 ± 3.47 g/ml) were much higher than those in subcutaneous tissue (9.13 ± 2.07 g/ml). For this study, plasma IBU concentrations were 0.1 g/ml at about 10 h, i.e., drug concentrations in the tissue were 100 times higher than for the plasma. Although ex-vivo permeation studies on prepared formulations are informative, performing in vivo dermatokinetic studies is important to measure drug penetration into various skin layers following topical application.
Topical administration of IBU could effectively avoid the side effects of oral administration and achieve the desired penetration. Gel formulations improved permeation efficiency combined with emulsion and NLC-based formulations. IBU-loaded NLC-based formulation significantly enhanced ibuprofen permeability from gel formulation, demonstrating the synergy between gel composition and the small size of the lipid particles provided on drug permeation. A similar effect, but to a lesser extent, was observed with the emulgel-based formulations. When evaluating TPA, back extrusion, and spreadability results, we can see that the IBU-loaded NLC-based gel (E2) formulation is superior to the market product. We think that this superiority in mechanical properties of the IBU-loaded NLC-based gel (E2) formulation will result in a product that is better accepted by the patient. In our study, we can observe the difference made by the mechanical properties used to evaluate transdermal semisolid preparations. This difference will also be new information for the literature.
Conclusions
In this study, NLC-based gels and emulgels containing IBU were successfully prepared. As a transdermal formulation, the mechanical properties of NLC-based gel formulation (E2) have been found suitable for application to the skin. The mechanical properties of IBU-containing emulgels and NLC-based gels were evaluated in detail, and new specifications were presented in the literature. Viscosity, TPA profile, spreadability, and bioadhesion results have confirmed that our formulations are comparable to marketed topical IBU gel and cream. When the penetration of ibuprofen through the skin was evaluated, it was found that the E2 formulation increased penetration due to its lipid and nanoparticle structure. As a result of these findings, the NLC-based gel formulation will increase therapeutic efficacy and be a good alternative to transdermal formulation. It is considered to carry out skin tolerance studies of the formulations developed in further studies.
References
Rhee YS, Chang SY, Park CW, et al. Optimization of ibuprofen gel formulations using experimental design technique for enhanced transdermal penetration. Int J Pharm. 2008;364(1):14–20. https://doi.org/10.1016/j.ijpharm.2008.07.029.
Wu H, Deng Z, Zhou B, et al. Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. J Mol Liq. 2019;283:399–409. https://doi.org/10.1016/j.molliq.2019.03.046.
Sütő B, Berkó S, Kozma G, et al. Development of ibuprofen-loaded nanostructured lipid carrier-based gels: characterization and investigation of in vitro and in vivo penetration through the skin. Int J Nanomedicine. 2016;11:1201–12. https://doi.org/10.2147/IJN.S99198.
Muktadir A, Babar A, Cutie AJ, et al. Medicament release from ointment bases: III. Ibuprofen: in vitro release and in-vivo absorption in rabbits. Drug Dev Ind Pharm. 1986;12:2521–40. https://doi.org/10.3109/03639048609063197.
Babar A, Solanki UD, Cutie AJ, et al. Piroxicam release fromdermatological bases: in vitro studies using cellulose membrane and hairless mouse skin. Drug Dev Ind Pharm. 1990;16:523–40. https://doi.org/10.3109/03639049009114900.
Hadgraft J, Valenta C. pH, pKa and dermal delivery. Int J Pharm. 2000;200(2):243–7. https://doi.org/10.1016/S0378-5173(00)00402-6.
Bolla PK, Clark BA, Juluri A, et al. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics. 2020;12(2):151. https://doi.org/10.3390/pharmaceutics12020151.
Djekic L, Martinovic M, Stepanović-Petrović R, et al. Formulation of hydrogel-thickened nonionic microemulsions with enhanced percutaneous delivery of ibuprofen assessed in vivo in rats. Eur J Pharm Sci. 2016;92:255–65. https://doi.org/10.1016/j.ejps.2016.05.005.
Takahashi A, Suzuki S, Kawasaki N, et al. Percutaneous absorption of non-steroidal antiinflammatory drugs from in situ gelling xyloglucan formulations in rats. Int J Pharm. 2002;246:179–86. https://doi.org/10.1016/S0378-5173(02)00394-0.
Chen H, Chang X, Du D, et al. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 2006;315:52–8. https://doi.org/10.1016/j.ijpharm.2006.02.015.
Brucks R, Nanavaty M, Jung D, et al. The effect of ultrasound on the in vitro penetration of ibuprofen through human epidermis. Pharm Res. 1989;6:697–701. https://doi.org/10.1023/a:1015938522673.
Stott PW, Williams AC, Barry BW. Transdermal delivery from eutectic systems: enhanced permeation of a model drug İbuprofen. J Control Release. 1998;50:297–308. https://doi.org/10.1016/S0168-3659(97)00153-3.
Bolourtchian N, Karimi K, Aboofazeli R. Preparation and characterization of ibuprofen microspheres. J Microencapsul. 2005;22:529–38. https://doi.org/10.1080/02652040500161941.
Watkinson RM, Guy RH, Hadgraft J, et al. Optimisation of Cosolventconcentration for topical drug delivery – II: influence of propylene glycol on ibuprofen permeation. Skin Pharmacol Physiol. 2009;22:225–30. https://doi.org/10.1159/000231528.
Shumilov M, Bercovich R, Duchi S, et al. Ibuprofen transdermal ethosomal gel: characterization and efficiency in animal models. J Biomed Nanotechnol. 2010;6:569–76. https://doi.org/10.1166/jbn.2010.1153.
Abdel-Mottaleb MMA, Neumann D, Lamprecht A. Lipid nanocapsules for dermal application: a comparative study of lipid-based versus polymer-based nanocarriers. Eur J Pharm Biopharm. 2011;79:36–42. https://doi.org/10.1016/j.ejpb.2011.04.009.
Watkinson RM, Guy RH, Oliveira G, et al. Optimisation of cosolvent concentration for topical drug delivery III – influence of lipophilic vehicles on ibuprofen permeation. Skin Pharmacol Physiol. 2011;24:22–6. https://doi.org/10.1159/000315139.
Ren Q, Deng C, Meng U, et al. In vitro, ex vivo, and in vivo evaluation of the effect of saturated fat acid chain length on the transdermal behavior of ibuprofen-loaded microemulsions. J Pharm Sci. 2014;103:1680–91. https://doi.org/10.1002/jps.23958.
Pakhare AV, Deshmane SV, Deshmane SS, et al. Design and Development of Emulgel Preparation Containing Diclofenac Potassium. Asian J Pharm. 2017;11(4):S712. https://doi.org/10.22377/ajp.v11i04.1699.
Sah SK, Badola A, Nayak BK. Emulgel: Magnifying the application of topical drug delivery. Indian J Pharm Biol Res. 2017;5(1):25–33. https://doi.org/10.30750/ijpbr.5.1.4.
Jain A, Gautam SP, Gupta Y, et al. Development and Characterization of Ketoconazole Emulgel for Topical Drug Delivery. Der Pharmacia Sinica. 2010;1(3):221–31.
Lathiyare KB, Suresh PK, Jain V. Development and İn vitro characterization of piroxicam loaded emulgel for topical Delivery. Int J Pharm Pharm Res. 2015;2(3):18–32.
Alam S, Aslam M, Khan A, et al. Nanostructured lipid carriers of pioglitazone for transdermal application: from experimental design to bioactivity detail. Drug Deliv. 2016;23(2):601–9. https://doi.org/10.3109/10717544.2014.923958.
Joshi M, Patravale V. Nanostructured lipid carrier (NLC) based gel of celecoxib. Int J Pharm. 2008;346:124–32. https://doi.org/10.1016/j.ijpharm.2007.05.060.
Beloqui A, Solinís MA, Rodríguez-Gascón A, et al. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine NBM. 2016;12:143–61. https://doi.org/10.1016/j.nano.2015.09.004.
Elias PM, Wakefield JS. Skin Barrier Function. In: Krutmann J, Humbert P, editors. Nutrition for Healthy Skin. Berlin, Heidelberg: Springer; 2010. https://doi.org/10.1007/978-3-642-12264-4_4.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today. 2000;3(9):318–26. https://doi.org/10.1016/S1461-5347(00)00295-9.
Müller-Goymann CC. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur J Pharm Biopharm. 2004;58(2):343–56. https://doi.org/10.1016/j.ejpb.2004.03.028.
Sita VG, Vavia P. Bromocriptine nanoemulsion-loaded transdermal gel: optimization using factorial design, in vitro and in vivo evaluation. AAPS PharmSciTech. 2020;21(3):80. https://doi.org/10.1208/s12249-020-1620-8.
Kumar S, Prasad M, Rao R. Topical delivery of clobetasol propionate loaded nanosponge hydrogel for effective treatment of psoriasis: Formulation, physicochemical characterization, antipsoriatic potential and biochemical estimation. Mater Sci Eng C. 2021;119:111605. https://doi.org/10.1016/j.msec.2020.111605.
Carvalho FC, Calixto G, Hatakeyama IN, Luz GM, Gremião MPD, Chorilli M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev Ind Pharm. 2013;39(11):1750–7. https://doi.org/10.3109/03639045.2012.734510.
Felippim EC, Marcato PD, Maia Campos PMBG. Development of Photoprotective Formulations Containing Nanostructured Lipid Carriers: Sun Protection Factor, Physical-Mechanical and Sensorial Properties. AAPS PharmSciTech. 2020;21:311. https://doi.org/10.1208/s12249-020-01858-y.
Vitorino C, Alves L, Antunes FE, Sousa JJ, Pais AA. Design of a dual nanostructured lipid carrier formulation based on physicochemical, rheological, and mechanical properties. J Nanopart Res. 2013;15:1993. https://doi.org/10.1007/s11051-013-1993-7.
Marques AC, Rocha AI, Leal P, et al. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Inter J Pharm. 2017;533:455–62. https://doi.org/10.1016/j.ijpharm.2017.04.025.
Araujo J, Gonzalez-Mira E, Egea MA, et al. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393:167–75. https://doi.org/10.1016/j.ijpharm.2010.03.034.
Jones DS, Woolfson AD, Brown AF. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int J Pharm. 1997;151(2):223–33. https://doi.org/10.1016/S0378-5173(97)04904-1.
Cevher E, Taha MAM, Orlu M, et al. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 2008;15:57–67. https://doi.org/10.1080/10717540701829234.
Tuğcu-Demiröz F, Acartürk F, Özkul A. Preparation and characterization of bioadhesive controlled-release gels of cidofovir for vaginal delivery. J Biomater Sci Polym Ed. 2015;26(17):1237–55. https://doi.org/10.1080/09205063.2015.1082808.
Kaplan M, Tuğcu-Demiröz F, Vural İ, et al. Development and characterization of gels and liposomes containing ovalbumin for nasal delivery. J Drug Deliv Sci Tech. 2018;44:108–17. https://doi.org/10.1016/j.jddst.2017.12.006.
Bansal K, Rawat MK, Jain A, et al. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS PharmSciTech. 2009;10(3):716–23. https://doi.org/10.1208/s12249-009-9260-z.
Texture Technologies, 2003. Textural profile analysis explained and annotated. http://www.texturetechnologies.com/texture_profile_analysis.html. Accessed 27.05.2022.
Hurler J, Engesland A, Kermany BP, et al. Improved Texture Analysis for Hydrogel Characterization: Gel cohesiveness, adhesiveness, and hardness. J App Poly Sci. 2012;125:180–8. https://doi.org/10.1002/app.35414.
Toksoy MO, Tuğcu-Demiröz F, Tırnaksız F. Evaluation of Rheological and Textural Properties of Toothpastes. FABAD J Pharm Sci. 2013;38(3):135–41.
Tuğcu-Demiroz F. Development of in situ poloxamer-chitosan hydrogels for vaginal drug delivery of benzydamine hydrochloride: Textural, mucoadhesive and in vitro release properties. Marmara Pharm J. 2017;21(4):762–70. https://doi.org/10.12991/mpj.2017.3.
Tuğcu-Demiröz F. Vaginal delivery of benzydamine hydrochloride through liposomes dispersed in mucoadhesive gels. Chem Pharm Bull. 2017;65:660–7. https://doi.org/10.1248/cpb.c17-00133.
Glibowski P, Zarzycki P, Krzepkowska M. The rheological and instrumental textural properties of selected table fats. Int J Food Prop. 2008;11(3):678–86. https://doi.org/10.1080/10942910701622599.
Timur B, Yılmaz Usta D, Teksin ZS. Investigation of the effect of colloidal structures formed during lipolysis of lipid-based formulation on exemestane permeability using the in vitro lipolysis-permeation model. J Drug Deliv Sci Tech. 2022;77:103797. https://doi.org/10.1016/j.jddst.2022.103797.
Chellathurai BJ, Anburose R, Alyami MH, et al. Development of a polyherbal topical gel for the treatment of acne. Gels. 2023;9(2):163. https://doi.org/10.3390/gels9020163.
Bonacucina G, Martelli S, Palmieri GF. Rheological mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int J Pharm. 2004;282:115–30. https://doi.org/10.1016/j.ijpharm.2004.06.012.
Estanqueiro M, Amaral MH, Sousa Lobo JM. Comparison between sensory and instrumental characterization of topical formulations: impact of thickening agents. Int J Cosmet Sci. 2016;38:389–98. https://doi.org/10.1111/ics.12302.
Sivaraman A, Ganti SS, Nguyen HX, et al. Development and evaluation of a polyvinyl alcohol based topical gel. J Drug Deliv Sci Tech. 2017;39:210–6. https://doi.org/10.1016/j.jddst.2017.03.021.
Oktay AN, Ilbasmis-Tamer S, Han S, et al. Preparation and in vitro/in vivo evaluation of flurbiprofen nanosuspension-based gel for dermal application. Eur J Pharm Sci. 2020;155:105548. https://doi.org/10.1016/j.ejps.2020.105548.
Amasya G, Inal O, Sengel-Turk CT. SLN enriched hydrogels for dermal application: Full factorial design study to estimate the relationship between composition and mechanical properties. Chem Phys Lipids. 2020;228:104889. https://doi.org/10.1016/j.chemphyslip.2020.104889.
Teeranachaideekul V, Müller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—Effects of formulation parameters on physicochemical stability. Int J Pharm. 2007;340(1–2):198–206. https://doi.org/10.1016/j.ijpharm.2007.03.022.
Nnamani PO, Hansen S, Windbergs M, et al. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int J Pharm. 2014;477(1–2):208–17. https://doi.org/10.1016/j.ijpharm.2014.10.004.
Allen LV Jr. Transdermals: The skin as part of a drug delivery system. Int J Pharm Compd. 2011;15(4):308.
Nnamani PO, Ugwu AA, Nnadi OH, et al. Formulation and evaluation of transdermal nanogel for delivery of artemether. Drug Deliv Transl Res. 2021;11(4):1655–74. https://doi.org/10.1007/s13346-021-00951-4.
Yılmaz Usta D, Olgac S, Timur B, et al. Development and pharmacokinetic evaluation of Neusilin® US2-based S-SNEDDS tablets for bosentan: Fasted and fed states bioavailability, IVIS® real-time biodistribution, and ex-vivo imaging. Int J Pharm. 2023;643:123219. https://doi.org/10.1016/j.ijpharm.2023.123219.
Kesharwani R, Sachan A, Singh S, et al. Formulation and evaluation of solid lipid nanoparticle (SLN) based topical gel of etoricoxib. Int J Appl Pharm Sci Res. 2016;6(10):124–31. https://doi.org/10.7324/JAPS.2016.601017.
Lobão H, Frigerio P, Fonseca C, et al. New thermoresponsive eyedrop formulation containing ibuprofen loaded-nanostructured lipid carriers (NLC): development, characterization and biocompatibility studies. Curr Drug Deliv. 2016;13:953–70. https://doi.org/10.2174/1567201813666151111143434.
Czajkowska-Kośnik A, Szekalska M, Winnicka K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol Rep. 2019;71(1):156–66. https://doi.org/10.1016/j.pharep.2018.10.008.
Khan D, Qindeel M, Ahmed N, et al. Development of an intelligent, stimuli-responsive transdermal system for efficient delivery of Ibuprofen against rheumatoid arthritis. Int J Pharm. 2021;610:121242. https://doi.org/10.1016/j.ijpharm.2021.121242.
Aslam M, Aqil M, Ahad A, et al. Application of Box-Behnken design for preparation of glibenclamide loaded lipid based nanoparticles: Optimization, in vitro skin permeation, drug release and in vivo pharmacokinetic study. J Mol Liq. 2016;219:897–908. https://doi.org/10.1016/j.molliq.2016.03.069.
Chen H, Wang Y, Zhai Y, et al. Development of a ropivacaine-loaded nanostructured lipid carrier formulation for transdermal delivery. Colloids Surf A Physicochem Eng Asp. 2015;465:130–6. https://doi.org/10.1016/j.colsurfa.2014.10.046.
Pandey SS, Maulvi FA, Patel PS, et al. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: In vitro and in vivo studies. Colloids Surf B Biointerfaces. 2020;186:110681. https://doi.org/10.1016/j.colsurfb.2019.110681.
Jones DS, Lawlor MS, Woolfson AD. Examination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-comaleic anhydride) and poly(vinylpyrrolidone): rheological and mathematical interpretation of textural parameters. J Pharm Sci. 2002;91:2090–101. https://doi.org/10.1002/jps.10195.
Bonacucina G, Cespi M, Misici-Falzi M, et al. Rheological adhesive and release characterisation of semisolid Carbopol/tetraglycol systems. Int J Pharm. 2006;307(2):129–40. https://doi.org/10.1016/j.ijpharm.2005.09.034.
Tuğcu-Demiröz F, Acartürk F, Erdoğan D. Development of long-acting bioadhesive vaginal gels of oxybutynin: formulation, in vitro and in vivo evaluations. Int J Pharm. 2013;457:25–39. https://doi.org/10.1016/j.ijpharm.2013.09.003.
Lehr CM, Bouwstra JA, Schacht EH, et al. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–8. https://doi.org/10.1016/0378-5173(92)90353-4.
Grabovac V, Guggi D, Bernkop-Schnürch A. Comparison of the mucoadhesive properties of various polymers. Adv Drug Deliv Rev. 2005;57:1713–23. https://doi.org/10.1016/j.addr.2005.07.006.
Radočaj OF, Dimić EB, Vujasinović VB. Optimization of the texture of fat-based spread containing hull-less pumpkin (Cucurbita pepo L.) seed press-cake. Acta Period Technol. 2011;42:131–43. https://doi.org/10.2298/APT1142131R.
Chandra MV, Shamasundar BA. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int J Food Prop. 2015;18(3):572–84. https://doi.org/10.1080/10942912.2013.845787.
Gokhale JP, Mahajan HS, Surana SJ. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed Pharmacother. 2019;112:108622. https://doi.org/10.1016/j.biopha.2019.108622.
Fermin BC, Hahm TS, Radinsky JA, et al. Effect of proline and glutamine on the functional properties of wheat dough in winter wheat varieties. J Food Sci. 2005;70(4):E273–8. https://doi.org/10.1111/j.1365-2621.2005.tb07183.x.
Nasaruddin F, Chin NL, Yusof YA. Effect of processing on instrumental textural properties of traditional Dodol using back extrusion. Int J Food Prop. 2012;15(3):495–506. https://doi.org/10.1080/10942912.2010.491932.
De Vuyst L, Zamfir M, Mozzi F, et al. Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. Int Dairy J. 2003;13:707–17. https://doi.org/10.1016/S0958-6946(03)00105-5.
Singh K, Kaur H, Hari Kumar SL. Design and development of sustained release injectable in situ gel of cytarabine. Pharmacophore. 2013;4(6):252–67.
Ahad A, Aqil M, Ali A. Investigation of antihypertensive activity of carbopol valsartan transdermal gel containing 1. 8-cineole. Int J Biol Macramol. 2014;64:144–9. https://doi.org/10.1016/j.ijbiomac.2013.11.018.
Sunita P, Palaniswamy M. A bio-inspired approach of formulation and evaluation of Aegle marmelos fruit extract mediated silver nanoparticle gel and comparison of its antibacterial activity with antiseptic cream. Eur J Integr Med. 2020;33:101025. https://doi.org/10.1016/j.eujim.2019.101025.
Pande V, Patel S, Patil V, et al. Design expert assisted formulation of topical bioadhesive gel of sertaconazole nitrate. Adv Pharm Bull. 2014;4(2):121. https://doi.org/10.5681/apb.2014.019.
Espinosa-Olivares MA, Delgado-Buenrostro NL, Chirino YI, et al. Nanostructured lipid carriers loaded with curcuminoids: Physicochemical characterization, in vitro release, ex vivo skin penetration, stability and antioxidant activity. Eur J Pharm Sci. 2020;155:105533. https://doi.org/10.1016/j.ejps.2020.105533.
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101. https://doi.org/10.1016/j.addr.2012.09.021.
Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. https://doi.org/10.1016/S0939-6411(00)00087-4.
Larrañeta E, Stewart S, Ervine M, et al. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J Funct Biomater. 2018;9(1):13. https://doi.org/10.3390/jfb9010013.
Mishra V, Bansal KK, Verma A, et al. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics. 2018;10(4):191. https://doi.org/10.3390/pharmaceutics10040191.
Salvi VR, Pawar P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J Drug Deliv Sci Technol. 2019;51:255–67. https://doi.org/10.1016/j.jddst.2019.02.017.
Patel A, Bell M, O’Connor C, et al. Delivery of ibuprofen to the skin. Int J Pharm. 2013;457(1):9–13. https://doi.org/10.1016/j.ijpharm.2013.09.019.
Yang F, Wang W, Zhou J, et al. Transdermal delivery of IBU-PLGA nanoparticles with dissolving microneedle array patch. J Drug Deliv Sci Tech. 2024;95:105528. https://doi.org/10.1016/j.jddst.2024.105528.
Manoukian MAC, Migdal CW, Tembhekar AR, et al. Topical administration of ibuprofen for injured athletes: considerations, formulations, and comparison to oral delivery. Sports Med-Open. 2017;3:36. https://doi.org/10.1186/s40798-017-0103-2.
Acknowledgements
We want to thank Sanovel (Turkey) for the gift samples of Ibuprofen.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
Duygu YILMAZ USTA: conceptualization, methodology, writing-original draft preparation. Zeynep Safak TEKSIN: supervision, writing-reviewing, and editing. Fatmanur TUGCU-DEMIROZ: corresponding author, supervision, writing-reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Nisarg Modi, Yousuf Mohammed, and Lakshmi Raghavan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yılmaz Usta, D., Teksin, Z.S. & Tugcu-Demiroz, F. Evaluation of Emulgel and Nanostructured Lipid Carrier-Based Gel Formulations for Transdermal Administration of Ibuprofen: Characterization, Mechanical Properties, and Ex-Vivo Skin Permeation. AAPS PharmSciTech 25, 124 (2024). https://doi.org/10.1208/s12249-024-02831-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02831-9