Abstract
Ketoconazole (KCZ) nanoemulgel containing permeation enhancer was formulated as a vehicle for transungual drug delivery, and its efficacy to inhibit the growth of onychomycotic dermatophytes was investigated in vitro. Different components of oil-in-water nanoemulsions were moderately agitated by classical titration method and passed through a high-pressure homogenizer to formulate various nanoemulsions, which were further identified by constructing pseudo-ternary phase diagrams. Stress-stability testing was carried out for the nanoemulsions, and those that passed these tests were characterized for mean droplet size, zeta potential, morphology, pH, refractive index, viscosity and transmittance. Mean droplet size and zeta potential of the optimized nanoemulsion (NE3) were found to be 77.52 ± 0.92 nm (polydispersity index (PDI) = 0.128 ± 0.035) and −5.44 ± 0.67 mV, respectively. Optimized nanoemulsion was converted into nanoemulgel (NEG1) with 1% (w/w) of gelling agent (Carbopol® Ultrez 21) and 1%–2% (v/v) thioglycolic acid as permeation enhancer, and evaluated for pH, viscosity, spreadability, extrudability, tensile strength and bio-adhesion measurement. In vitro cumulative drug released at the end of 24 h from NE3, NEG1 and drug suspension were found to be 98.87 ± 1.29, 84.42 ± 2.78% and 54.86 ± 2.19%, respectively. Ex vivo transungual permeation values for KCZ through goat hooves from NE3, NEG1 and drug suspension were found to be 62.49 ± 2.98, 77.54 ± 2.88% and 38.54 ± 2.54%, respectively, in 24 h. The antifungal effect of NEG1 on Trichophyton rubrum and Candida albicans showed a significant (p < 0.05) zone of inhibition as compared to drug solution. Skin irritation and histopathology studies on rat skin showed the safe topical use and enhanced permeation of formulated nanoemulgel.
Similar content being viewed by others
INTRODUCTION
Onychomycosis accounts for about 50% of the nail diseases and mainly occurs in the elderly. It is a fungal infection, which causes nails’ thickening, splitting, roughening, discolouration, etc. More than 80% of onychomycosis is caused by dermatophytes, such as Trichophyton rubrum (T. rubrum) and Trichophyton mentagrophytes, whereas approximately 5%–17% are caused by yeast, for which Candida albicans (C. albicans) is majorly (>70%) responsible (1–3). There are four major clinical presentations of onychomycosis: distal subungual, proximal subungual, superficial and total dystrophic. Various antifungal drugs are being used for the treatment of onychomycosis, but they have one or more lacunae, for example, terbinafine and itraconazole are not licensed in the UK for children (4). Terbinafine is also associated with idiosyncratic liver and skin reactions (4). US Food and Drug Administration (US FDA) has not approved fluconazole for the treatment of onychomycosis in the USA because its dose and duration of treatment are not clear (3,4). Griseofulvin is a disappointing drug in toenail disease having a cure rate of only 30%–40% (3). On the other hand, ketoconazole (KCZ), an imidazole-class antifungal drug, is used orally in the treatment of chronic mucocutaneous fungal infections of nails and skin, having a cure rate of 50% to 70% in fingernail and 15%–30% in toenail infections (3). It is also used in the treatment of various systemic fungal infections, such as candidiasis and blastomycosis (5). It acts by blocking the synthesis of ergosterol, an essential component of the fungal cell (4,6). Several formulations in different topical preparations containing 2% KCZ (for example, solution, shampoo, cream, gel and lotion for the purpose of local dermatological therapy) are available in the market. However, all these formulations either have less contact time with the targeted area (for example, solution and lotion) or less permeation through the skin (for example, cream and gel). By enhancing the topical permeability of KCZ, it can be used for the effective treatment of onychomycosis with fewer side effects.
Nanoemulsions or sub-micron emulsions (droplet size, 20–200 nm) are used as vehicles for the delivery of active pharmaceutical ingredients (API) due to their high kinetic stability and approaching thermodynamic stability (7). Nanoemulsions, developed by high-energy emulsification methods, such as high-pressure homogenizers and high-shear motionless mixers, offer several industrial advantages in terms of controlled and uniform droplet size distribution (8). Unlike microemulsions, which require a high surfactant concentration, nanoemulsions are formulated with reasonable surfactant concentration for the efficient topical delivery of API owing to their small droplet size, large surface area and low surface tension. They allow rapid penetration of lipophilic actives and thus improving efficacy and minimizing side effects by reducing the dose. Nanoemulsions may be used as a substitute for other less-stable lipidic nanocarriers (for example, liposomes and vesicles) to improve transdermal permeation of many drugs over the conventional topical formulations (9–12). Due to improved physical stability, and their non-toxic and non-irritant nature, they can be employed in topical drug delivery systems, providing greater absorption of solubilized lipophilic drugs. Apart from that, they can be formulated into a variety of formulation types, such as foams, creams, liquids, sprays and gels (13). There are many topical formulations, like nail lacquers (14–17), creams (18–20), ointments (21,22), bio-adhesive patch (23) and solutions (24,25), which are being used for the treatment of nail infections, but gel is being preferred because it fulfils the required dermatological properties, such as thixotropy, greaselessness, emollient and non-staining nature, ease in spreadability, etc. Gel can be defined as a semisolid system, which consists of dispersion of particles with interpenetration of liquid. There is a formation of a three-dimensional structure, having a two-phase system with the dispersion of inorganic/organic particles in the continuous phase (26).
Nanoemulsions can be converted into gel, a system designated as ‘nanoemulgel’, which prolongs the contact time of the formulation with the applied surface, in contrast to nanoemulsion, which has a tendency to flow and run off. Apart from that, for most of the lipophilic drugs (like KCZ, in this case), which cannot be incorporated directly into an aqueous gel base due to solubility limitation, an emulgel can provide ease of formulation and improve the therapeutic effectiveness of the existing drugs by incorporating drug molecules in the oil phase of o/w emulsion and uniformly dispersing them in aqueous phase containing the gel base. Thus, better stability and sustained release of a drug can be attained by simply incorporating the drug-containing o/w nanoemulsion into a gel base. Nanoemulgel can be applied easily with a lower risk of inflammation or adverse reactions (27). Previously, various antifungal drugs have been incorporated into nanoemulgels for the treatment of onychomycosis, such as terbinafine (3,15,28), miconazole nitrate (26), fluconazole (14), butenafine (18), ciclopirox olamine (19), etc.
The goal of our research was to formulate a topical nanoemulgel of KCZ with desirable characteristics and enhanced transungual permeability through the nail (by using a permeation enhancer, thioglycolic acid) for the effective treatment of onychomycosis. The optimized nanoemulgels were evaluated by skin irritation study, histopathological analysis and ex vivo antifungal activity, along with stability studies for its safe and efficacious topical use.
MATERIALS AND METHODS
Materials
Ketoconazole (KCZ), Cremophor EL and isopropyl myristate were kindly supplied by Sigma-Aldrich (St. Louis, MO, USA). Labrafac™ Lipophile WL1349 (medium-chain triglycerides), Labarafac PG, Plurol® oleique and Transcutol P were obtained as a gift sample from Gattesfosse (Saint Priest, Cedex, France). Miglyol 810N was purchased from Cremer Oleo Division (Hamburg, Germany). Polysorbate 20 and 80 (Tween 20 and 80), polyethylene glycol 200 and 400 (PEG 200 and 400), thioglycolic acid and formaldehyde were purchased from Thomas Baker Chemicals (Mumbai, India). Glycerol, potassium dihydrogen phosphate, dimethyl sulfoxide (DMSO) and sodium hydroxide pellets were purchased from S.D. Fine Chemicals Pvt. Ltd. (Mumbai, India). Carbopol® Ultrez 21 polymer was obtained as a gift sample from Lubrizol India Pvt. Ltd. (Mumbai, India) and 95% 2-amino-2-methyl-1-propanol solution (AMP-95) was supplied by Angus Chemie GmbH (Germany). Ethanol was purchased from Changshu Yangyuan Chemicals (China). Water for UPLC analysis was obtained from Loba Chemie Pvt. Ltd. (Mumbai, India). Strains of C. albicans (MTCC No. 4748) and T. rubrum (MTCC No. 7859) were purchased from the Institute of Microbial Technology (IMTECH, Chandigarh, India). Antifungal activity was performed at our institutional hospital (H.A.H. Centenary Hospital, New Delhi, India). All other chemicals used in the study were of analytical grade.
Analytical Method Development
KCZ was analysed by an ultra-performance liquid chromatography (UPLC) system (S.No. F09 UPB 920 M; Model Code# UPB; Waters, MA, USA) equipped with a binary solvent delivery system, an autosampler, column manager and a photodiode array (PDA) detector (Synapt, Waters, Manchester, UK). Waters ACQUITY UPLC BEH C18 (2.1 mm × 100 mm; 1.7 μm) column was used for the chromatographic separation. The mobile phase was composed of 0.1% (w/v) orthophosphoric acid in water: acetonitrile (55:45 (v/v)) adjusted to flow at a rate of 0.5 mL/min. All the samples were filtered through a 0.22-μm nylon syringe filter before analysis.
Formulation Development of Nanoemulsion
Screening of Components
The criterion for screening of components was the solubility of KCZ in different oils, surfactants and co-surfactants (Table I). An excess amount of drug was added to 5 mL of the selected oil, surfactant and co-surfactant in a glass vial, sealed and stored upright. The samples were vortexed and maintained at 37 ± 1°C for 72 h in a shaking incubator (Shellab, USA) to facilitate solubilization. To remove the undissolved drug, samples were centrifuged (MX-305, Tomy, Japan) at 3000 rpm for 15 min. The supernatant was filtered through a 0.22-μm nylon syringe filter. The solubility of KCZ was determined by the developed analytical method, as described in ‘Analytical Method Development’ section.
Preparation of Drug-Loaded Nanoemulsion
Aqueous titration method, followed by high-pressure homogenization (HPH), was used for the preparation of nanoemulsions. Construction of pseudo-ternary phase diagrams was done for the determination of concentration range of different components (29). Surfactant and co-surfactant (S:CoS) were mixed in different weight ratios (1:0, 1:1, 1:2, 2:1, 3:1, 1:3 and 4:1), either in increasing concentration ratios of surfactant or co-surfactant, with respect to each other (30). Under moderate agitation on a vortex mixer (Abdos Labtech Pvt. Ltd., India), different weight ratios of oil and Smix (S:CoS) were diluted dropwise with the aqueous phase to form the crude nanoemulsion. These nanoemulsions were passed through a high-pressure homogenizer (Nano DeBEE, BEE International Inc., MA, USA) by optimizing its process variables (operating pressure and number of cycles) to get a desirable mean droplet size of less than 200 nm. Later, the product was visually assessed on the basis of clarity, transparency and flow ability, and then finally categorized as nanoemulsions. Nanoemulsions were represented on the ternary-phase diagrams with one axis of aqueous phase, the second of oil phase and the third of Smix at fixed-weight ratios. The drug-loaded nanoemulsions were prepared by dissolving 2% (w/v) of KCZ in the mixture of oil and Smix. The developed formulations were then subjected to different stress-stability tests.
Stress-Stability Studies
Heating-Cooling Cycle
The formulations were subjected to heating-cooling cycles (n = 3) over a temperature range of 4–45°C, stored at each temperature condition for 48 h and observed for any physical instability in the form of phase separation, flocculation or precipitation (31).
Centrifugation
The selected nanoemulsion formulations were centrifuged (MX-305, Tomy, Japan) at 3500 rpm for 30 min to rule out the metastable systems, and observed for any changes in homogeneity of nanoemulsions (31).
Freeze-Thaw Cycle
Nanoemulsion formulations were kept between temperatures, −21°C and +25°C, for three cycles for a duration of 48 h, and examined for changes in homogeneity of nanoemulsions (31).
Characterization of Nanoemulsion
Droplet Size
For understanding or determining the behaviour of nanoemulsions, mean droplet size is very important. It was determined by using Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK) based on the principle of photon correlation spectroscopy, which analyses fluctuation in light scattering due to Brownian motion of particles. Light scattering was monitored at 25°C at a scattering angle of 90°. The nanoemulsion (1–1.5 mL) was transferred to a disposable polystyrene cuvette with the help of a micropipette, and the mean droplet size was determined in triplicate.
Zeta Potential
Zeta potential is the electric potential which exists at the hydrodynamic plane of shear of a particle. It was measured by applying an electric field across the dispersion. Nanoemulsions were placed in clear disposable zeta cells, and zeta potential, which indicates the surface charge of the developed nanoemulsions, was measured by Zetasizer (Malvern Instruments, Malvern, UK) at 25°C, in triplicate.
Morphology
Morphology of the nanoemulsions was studied using transmission electron microscopy (TEM) (TECNAI G2 (200 kV) HR-TEM from FEI company, Holland) at All India Institute of Medical Sciences (AIIMS), New Delhi, India. One drop of the diluted aqueous nanoemulsion was placed on a fomvar-coated copper grid and stained with a drop of 2% aqueous solution of phosphotungstic acid (PTA), and dried before examining under the electron microscope.
pH, Refractive Index, Viscosity and Transmittance
The pH values for nanoemulsions were determined at 25°C by a calibrated pH meter (Mettler Toledo, Langacher, Switzerland). Refractive indices (RI) of the prepared nanoemulsions were determined by using Abbe refractometer. The viscosity of nanoemulsions was measured using rheometer (MCR101, Rheoplus, Anton Paar India Pvt. Ltd., India), equipped with a cone-plate-type measuring system. The rheological studies were carried out at variable shear rates ranging from 1 to 100 s−1, and a rheogram was developed (viscosity vs. rate of shear). Transmittance was observed by using UV spectrophotometer (UV 1601, Shimadzu, Japan) at 630 nm. One millilitre of nanoemulsion formulation was taken in a test tube, and ethanolic dilution was analysed at 630 nm, in triplicate.
Stability Studies of Nanoemulsion
Nanoemulsions were assessed at accelerated condition of storage by varying temperature and humidity, as per ICH guidelines Q1A (R2) (2003) (32). Nanoemulsions were placed in 5-mL glass vials, sealed and stored upright. Physical and chemical stabilities of nanoemulsion were evaluated for 3 months by storing them at three different temperature and humidity conditions (25 ± 2°C/60 ± 5% relative humidity (RH), 40 ± 2°C/65 ± 5% RH and 60 ± 2°C/75 ± 5% RH) and then characterized at specific time intervals for various parameters (33). The nanoemulsions were also visually observed for any signs of turbidity, phase separation, coalescence, etc.
Preparation of Nanoemulgel
Different formulations of nanoemulgel were prepared by the addition of a gelling agent (0.5% to 1.5% (w/v)) and penetration enhancer (1.0% to 2.0% (v/v) thioglycolic acid). The gel base was prepared by dispersing the gelling agent (Carbopol® Ultrez 21 polymer) in purified water, with constant stirring at moderate speed using a magnetic stirrer, and then the optimized nanoemulsion was incorporated into the gel base. The prepared nanoemulgel formulations were inspected visually for their colour, appearance and consistency. Viscosity of nanoemulgel formulations was determined by the above-described method (‘pH, Refractive Index, Viscosity and Transmittance’ section).
Evaluation of Nanoemulgel
Spreadability Study
Spreadability was determined by using an apparatus suggested by Mutimer et al. (34). There was a wooden block and a pulley attached to it at one end. On the basis of ‘Slip’ and ‘Drag’ characteristics of nanoemulgel, spreadability measurement was done. An excess of nanoemulgel (≈2 g) was placed between two uniform slides placed on the block, where one glass slide was fixed and another was attached to a pulley. On the top of the two slides, a 1-kg weight was placed for 5 min to provide a uniform film of the nanoemulgel between the slides. The time taken by the upper slide to move on the application of weight to it through the pulley was noted, and spreadability was calculated by using the following formula, in triplicate:
where
- S :
-
Spreadability
- M :
-
Weight applied to upper slide
- L :
-
Length of glass slide
- T :
-
Time taken to separate the slides completely from each other.
Extrudability Study
It is a test to measure the force required to extrude the gel from the tube. On the application of weight, the amount of gel extruded from the aluminium tube was determined. The nanoemulgel extruded should be at least 0.5 cm ribbon in 10 s (28). The higher the quantity of gel extruded, the better is the extrudability. The extrudability of each formulation was measured, in triplicate, and calculated by using the formula:
where
- E :
-
Extrudability
- M :
-
Applied weight to extrude gel from tube
- A :
-
Area
Tensile Strength Measurement
Tensile strength of the nanoemulgel was determined by using TA.XT2 texture analyzer (Stable Micro Systems, Goldalming, UK). The mechanical properties have been assessed using the texture analyzer with a 5 kg-loaded cell. Data acquisition and mathematical analysis were done by using Texture Expert® software (35,36). Consistency, cohesiveness and firmness were determined for the prepared nanoemulgels using the recommended specifications.
Bio-adhesion Measurement
Bio-adhesion measurement was done by means of a tensile test, which indicates the gel’s bio-adhesive potential. The maximum force required for detaching a piece of goat hooves from nanoemulgel, after an initial period of contact, was determined (36,37). The force involved in the process was measured by texture analyzer. The average maximum positive force (adhesiveness) was determined for the developed nanoemulgels.
Stability Studies of Nanoemulgel
Prepared nanoemulgels (≈5 g) were packed in aluminium collapsible tubes and kept for stability studies at 25 ± 2°C/60 ± 5% RH, 40 ± 2°C/65 ± 5% RH and 60 ± 2°C/75 ± 5% RH for a period of 3 months, as per ICH guidelines (32,38). At an interval of 15 days, samples were withdrawn and evaluated for physical appearance, pH, viscosity, spreadability, extrudability and texture analyses.
In Vitro Drug Release Study
In vitro drug release study was performed using dialysis membrane-60 (average diameter; 15.9 mm, average flat width; 25.27 mm, Himedia®, India) which was activated for about 1 h in a solvent system of phosphate buffer (pH 7.4) and ethanol (3:1). About 1 g of nanoemulgel and appropriate amount of nanoemulsion and aqueous drug suspension (containing the same equivalent amount of drug as contained in 1 g nanoemulgel) were placed on the artificial membrane, and the receptor compartment was filled with solvent system (10 mL). The whole assembly was maintained at 37 ± 1°C, and the speed of stirring was kept constant (100 rpm) for 24 h (39). Then, the aliquot of drug samples of about 1 mL were withdrawn at pre-determined time intervals, filtered through a 0.22-μm membrane filter and replaced with an equal volume of a fresh solvent. The analysis was done by the analytical method, as described in ‘Analytical Method Development’ section. Release kinetics modelling was done by putting the in vitro release data into the zero-order, first-order and Higuchi release kinetics model(s), and the model showing highest value of correlation coefficient (R 2) was considered to be the best-fit model.
Transungual Permeation Study
Hooves from freshly slaughtered goat, free of connective and cartilaginous tissues, were taken from the local slaughterhouse and soaked in distilled water for 24 h. From the lower part of the hooves, a section of about 1 mm thickness was cut. The hoof membrane was placed carefully on the Franz diffusion cell of 10-mL capacity, and the surface area available for permeation was 1.23 cm2. About 1 g of nanoemulgel and appropriate amount of nanoemulsion and aqueous drug suspension (containing the same equivalent amount of drug as contained in 1-g nanoemulgel) were applied evenly on the surface of the membrane. The receptor compartment was filled with solvent (phosphate buffer, pH 7.4; and ethanol in the ratio of 3:1), and the whole assembly was maintained at 37 ± 1°C with constant stirring (100 rpm) for 24 h. Then, an aliquot of drug sample, about 1 mL, was taken after a time interval of 1 h, which was replaced by the fresh solvent. The analysis was done by the analytical method as described in ‘Analytical Method Development’ section.
Antifungal Activity
Disk diffusion susceptibility method was used to determine the antifungal activity of drug alone and drug-loaded nanoemulgel against T. rubrum and C. albicans by slight modification of the previous method (40). Stock solutions of KCZ and its nanoemulgel were prepared in DMSO and distilled water, respectively. Appropriate dilutions (25 μg/mL) from both stock solutions were pipetted onto sterile blank disks (Sigma-Aldrich), and after complete soaking, disks were dried and stored at 4°C for further use within 2 weeks. Both the strains (T. rubrum and C. albicans) were maintained and cultivated in petri dishes containing Sabouraud dextrose agar (SDA) medium. After incubating and sub-culturing for 15 days at 28°C, three to five colonies of both strains were suspended in 5 mL of sterile saline solution (0.9% (w/v)), transferred to a sterile tube and mixed thoroughly on a vortex mixer for 2 min. The suspension was allowed to stand for 10 min for the sedimentation of hyphal fragments, and from the supernatant, inoculums were prepared by adjusting to a 0.5 McFarland turbidity standard (106 CFU/mL) by using a haemocytometer. One-millilitre inoculum, from a suspension of 104 CFU/mL, was spread on the surface of SDA plates with a sterile loop. Disks containing drug and nanoemulgel were then inserted into inoculated plate by using sterile forceps. Triplicate sets of plates were incubated at 28°C and zone diameters (where growth abruptly decreased) were measured after 24 and 48 h to estimate marked reduction in colony sizes.
Skin Irritation Study
The protocol was approved by the Institutional Animal Ethics Committee with approval number 1120. Three groups (n = 3) of male Wistar-strain rats (180–200 g) were used in the study. Positive control (0.8% (v/v) aqueous solution of formalin was used as standard irritant), nanoemulgel and negative control (no application) were applied (n = 3) on the dorsal side (2 cm2) of properly shaven skin of rat. The formulation was removed after 24 h and examined for any signs of erythema and edema (38,41). Undesirable skin changes, i.e. change in colour and change in skin morphology, were visually checked for a period of 24 h. The resulting reactions were compared and scored against control group (n = 3).
Histopathological Study
After a 24-h observation period of skin irritation study, as described in the previous section, rats were sacrificed and excised skin samples from nanoemulgel-treated and negative control (no application) groups were taken. Each specimen was immediately immersed in 10% (v/v) formalin solution buffered with phosphate buffer saline (pH 7.4), dehydrated in graded concentrations of ethanol, immersed in xylene and then embedded in paraffin block for fixing. Five-micrometre thick sections of skin were cut using a microtome and stained with haematoxylin and eosin. The samples were then observed for gross histopathology under an inverted microscope (ix71, Olympus Corporation, Japan) at ×10 magnification.
Statistical Analysis
Plotting of all graphs and processing of all the experimental data were done by using Microsoft Excel 2007 software, and presented as mean ± standard deviation (S.D.). The Student-Newman-Keuls multiple comparison test (Software: GraphPad InStat v. 3.06) was used to find the statistical significance. A value of p < 0.05 was considered statistically significant.
Results
Analytical Method Development
Retention time of KCZ was found to be 2.7 min at a detection wavelength of 223 nm employing a mobile phase of 0.1% (w/v) orthophosphoric acid in water/acetonitrile (55:45 (v/v)). The assay was linear (r 2 = 0.999) in the concentration range of 2–200 ppm, with a detection limit of 5 ng/mL and a quantification limit of 15 ng/mL. The percentage recovery was found to be in the range of 95% to 100%.
Formulation Development of Nanoemulsion
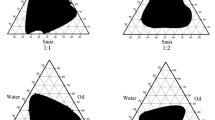
All excipients for the formulation were selected from among the “Generally-Recognized-as-Safe” (GRAS) category (Table I). Labrafac™ Lipophile WL1349 was selected as the oil phase due to the highest solubility of KCZ (5.4 ± 0.21 mg/mL) as compared to other experimental oils. Tween 80 and PEG 400 were used as the surfactant and co-surfactant, respectively, since they showed the maximum drug solubility, i.e. 5.4 ± 0.56 mg/mL and 5.2 ± 0.12 mg/mL, respectively (Table I). Four phase diagrams were prepared with the 1:2, 2:1, 3:1 and 4:1 weight ratios of Smix (Tween 80: PEG 400), as shown in Fig. 1. Oil phase and Smix were then mixed in the weight ratios of 1:9 to 9:1. Sixteen different combinations (1:9, 1:8, 1:7, 1:6, 1:5, 2:8 (1:4), 1:3.5, 1:3, 3:7 (1:2.3), 1:2, 4:6 (1:1.5), 5:5 (1:1), 6:4 (1:0.7), 7:3 (1:0.43), 8:2(1:0.25) and 9:1 (1:0.1)) of oil and Smix were chosen, so that maximum ratios could be covered for the study. About 2% (w/v) drug was loaded in the formulation by solubilizing the drug in oil and Smix phase. Aqueous phase was added in the range of 5%–95% (v/v) of the total volume of nanoemulsion at 5% interval to form the crude nanoemulsions, which were later passed through high-pressure homogenizer to get more uniform-sized droplets. The prepared nanoemulsion formulations were then subjected to stress-stability testing. After subjecting to heating-cooling cycle, centrifugation and freeze-thaw cycle, those formulations which passed the test (Table II), and showed stability, were further characterized. We selected one nanoemulsion (NE1, NE2, NE3 and NE4) each from the above-mentioned experimental ratios of Smix. Through titration method, the mean droplet sizes for the formulations NE1, NE2, NE3 and NE4 were found to be 835.6 ± 3.28, 760.7 ± 5.28, 665.3 ± 5.29 and 651.0 ± 5.20 nm, respectively (Table III, C0). After running 3 cycles of high pressure homogenization at 10,000 psi, mean droplet size was effectively reduced to 242.6 ± 2.73, 215.0 ± 4.98, 201.0 ± 2.92 and 193.3 ± 2.19 nm for NE1, NE2, NE3 and NE4, respectively. On increasing the number of cycles to 4, no further considerable size reduction was observed. The same trend in size reduction was observed up to 2 cycles at 20,000 psi, after which no major changes in size reduction were achieved. So, we optimized the mean droplet sizes for the formulations NE1, NE2, NE3 and NE4 as 126.6 ± 0.65 nm, 98.06 ± 0.46 nm, 77.52 ± 0.92 nm and 63.46 ± 4.22 nm, respectively (Fig. 2a), having polydispersity index (PDI) values of 0.121 ± 0.046, 0.192 ± 0.023, 0.128 ± 0.035 and 0.357 ± 0.044, respectively.
Characterization of Nanoemulsion
Zeta potential values for nanoemulsion formulations NE1, NE2, NE3 and NE4 were found to be −1.58 ± 0.21, −2.75 ± 0.43, −5.44 ± 0.67 and −7.68 ± 0.35 mV, respectively (Fig. 2b). Nanoemulsions were in the nanometric size range (≈90–120 nm), as observed by TEM (Fig. 3), which is in corroboration with the zeta sizer measurements. The pH values of the nanoemulsion formulations, NE1, NE2, NE3 and NE4, were found to be in the range of 6.2 ± 0.34 to 6.5 ± 0.23 (Table IV). Refractive indices of nanoemulsion formulations NE1, NE2, NE3 and NE4 were found to be in the range of 1.43 ± 0.12 to 1.46 ± 0.45 (Table IV). Viscosity of nanoemulsion formulations, measured at a shear rate of 100 s−1, were found to be in the range of 14.00 ± 0.21 to 24.33 ± 1.44 cP, as expected for o/w nanoemulsions because of higher aqueous content (>45% (v/v)). Viscosity was also measured at different rates of shear ranging from 1 to 100 s−1, and a rheogram was developed to know the thixotropic behaviour of nanoemulsion (Fig. 4). Transmission test of nanoemulsion formulations was performed and transmittance values of formulations were found to be in the range of 98%–99% (Table IV). It showed that formulations were clear and transparent. After the evaluation of nanoemulsion formulations, optimized formulation (NE3) was selected for the stability studies and further formulated into a nanoemulgel.
Stability Studies of Nanoemulsion
The stability studies revealed that during the storage period of 3 months at 25 ± 2°C/60 ± 5% RH, 40 ± 2°C/65 ± 5% RH and 60 ± 2°C/75 ± 5% RH, optimized nanoemulsion (NE3) showed very negligible change in mean droplet size, zeta potential, PDI, pH, RI, viscosity and transmittance (Table V). No phase separation and flocculation were observed, proving its stable nature. At the end of 3 months, the drug content was assayed and was found to be >95% of the initial drug added, which shows the chemical stability of the system during the storage period.
Preparation of Nanoemulgel
Nanoemulgel was prepared by adding NE3 into overnight-soaked gelling agent (Carbopol® Ultrez 21) at 0.5% (w/v) (NEG0.5), 1.0% (w/v) (NEG1) and 1.5% (w/v) (NEG1.5) with continuous stirring at 500 rpm (42–44). The final formulation also contained glycerine as humectant, methyl paraben as a preservative, thioglycolic acid as a penetration enhancer (45) and amino-methyl propanol (AMP-95) to maintain the pH (6–6.5).
Evaluation of Nanoemulgel
The pH values for NEG0.5, NEG1 and NEG1.5 were found to be in the range of 6.4 ± 0.24 to 6.6 ± 0.29 (Table VI). Viscosities for NEG0.5, NEG1 and NEG1.5 were found to be 750 ± 5.34, 1142 ± 10.33 and 1200 ± 12.56 cP, respectively. Spreadability values for NEG0.5, NEG1 and NEG1.5 were found to be 5.6 ± 0.12, 3.5 ± 0.22 and 2.5 ± 0.44 cm, respectively (Table VI). Extrudabilities of NEG0.5, NEG1 and NEG1.5 were found to be 1.2 ± 0.44, 1.4 ± 0.56 and 1.5 ± 0.34 cm, respectively, in 10 s on applying a weight of 400 g (Table VI). Texture analysis of NEG0.5, NEG1 and NEG1.5 formulations was performed, and the determined parameters, such as firmness, consistency and cohesiveness of the formulation, are presented in Table VI. The negative area of the graph, which is a measure of the total resistance to withdrawal force, indicates sample consistency. The bio-adhesiveness values of the prepared nanoemulgel formulations, NEG0.5, NEG1 and NEG1.5, are given in Table VI. On assessing spreadability, extrudability, texture profile and bio-adhesiveness values of nanoemulgel formulations, NEG1 was optimized and selected for further studies. The drug content in NEG1 was found to be 0.81 ± 0.04% (w/w). NEG1 was found to be stable during the 3-month storage period at different conditions of temperature and humidity. There was no change in physical appearance, pH, viscosity and other rheological properties of the formulation (data not shown). During this storage period, the drug content was assayed and was found to be >98% of the initial drug loaded (0.81 ± 0.04% (w/w) of gel base), confirming the chemical stability of the system.
In Vitro Drug Release Study
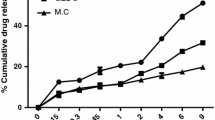
In vitro release study was performed to compare the release of KCZ from NE3, NEG1 and aqueous drug suspension. Comparative in vitro release studies showed that NE3, NEG1 and aqueous drug suspension released 98.87 ± 1.29, 84.42 ± 2.78% and 54.86 ± 2.19% cumulative drug in 24 h, as shown in Fig. 5. Various kinetic models were applied to the nanoemulgel formulation to determine release mechanism of the formulation.
Transungual permeation study
Ex vivo permeation studies were carried out using goat hoof membrane (section of goat hoof). Permeation of drug from NE3, NEG1 and aqueous drug suspension was found to be 62.49 ± 2.98, 77.54 ± 2.88 and 38.54 ± 2.54%, respectively, in 24 h (Fig. 6).
Antifungal Activity
In vitro antifungal activity revealed that during 24 h of incubation period, KCZ solution (in DMSO) and NEG1 exhibited similar antifungal effect on T. rubrum and C. albicans (Table VII). However, due to the small droplet size of NEG1, the drug slowly diffused into the medium and showed a significant (p < 0.05) area of zone of inhibition (28.3 ± 0.9 mm for T. rubrum and 28.1 ± 0.8 mm for C. albicans) after 48 h of incubation, as compared to drug solution (22.4 ± 1.4 mm for T. rubrum, and 23.5 ± 1.3 mm for C. albicans), which had already completely diffused into the medium within the first 24 h. The zone of inhibition of DMSO (as it is antifungal in nature) alone was also determined to compare the actual antifungal activity of KCZ and its formulation, NEG1 (Table VII).
Skin Irritation Study
The scores of skin irritation study (erythema and edema) on rat skin after 24 h post treatment of positive control, NEG1 and negative control are represented in Table VIII.
Histopathological Study
Histopathological analysis was done to observe any pathological changes in rat skin during the application of NEG1. Histopathological images of negative control skin (Fig. 7a) were compared with the images of NEG1-treated sample (Fig. 7b) to evaluate any epidermal and sub-epidermal cellular changes.
Discussion
For the development of an appropriate nanoemulsion, the selection of a suitable oil, surfactant and co-surfactant is very crucial. On the basis of solubility studies of KCZ in different oils, surfactants and co-surfactants, Labrafac™ Lipophile WL1349 was selected as the oil phase, whereas a non-ionic surfactant, Tween 80, was selected as surfactant for KCZ nanoemulsions as they have the least toxicity and irritant potential as compared to ionic surfactants, and having low critical micelle concentration (46), PEG 400 was selected as the co-surfactant. Co-surfactant (PEG 400) plays a very decisive role during nanoemulsion formulation by decreasing the interfacial stress, leading to interfacial film flexibility. It can be observed that at Smix ratio of 1:0 (Tween 80 used alone without PEG 400), higher concentration of surfactant was required for the stabilization of minimum amount of oil phase. When the concentration of co-surfactant in the Smix was increased from 1:1 to 1:2 (NE1; Fig. 1a), an area of nanoemulsion in the phase diagram was observed to be increased, but on further increasing the concentration to 1:3, the nanoemulsion area decreased which showed phase separation during the storage period of 24 h at room temperature. When surfactant (Tween 80) concentration was increased with respect to co-surfactant (PEG 400) in the Smix to a ratio of 2:1 (NE2; Fig. 1b), the nanoemulsion area was increased. The area of nanoemulsion was further increased on increasing the Smix ratio to 3:1 (NE3; Fig. 1c), due to stabilization of maximum amount of oil (Fig. 1c). But, on further increasing the Smix ratio to 4:1 (NE4; Fig. 1d), the nanoemulsion area was found to be slightly decreased. Thus, maximum nanoemulsion areas were observed in the Smix ratios of 2:1 and 3:1.
Nanoemulsions are kinetically stable systems and are formed by using a particular concentration of various components, with no sign of phase separation, creaming or cracking under various stress conditions. The most important feature of nanoemulsions is the mean droplet size, which must be in the nanometric range. The influence of process variables, i.e. operating pressure (psi) and number of homogenization cycles, to achieve the mean droplet size of less than 200 nm, was studied. The results showed that the mean droplet size had an inverse relationship with both the homogenization pressure and number of cycles. On increasing the number of cycles to 4 at 10,000 psi, we did not get mean droplet size less than 200 nm. But at 20,000 psi, with the run of 2 cycles, a mean droplet size range of approximately 200 nm was achieved. So, we optimized the variables of HPH on the basis of minimum pressure and number of cycles to get droplets of size less than 200 nm. Hence, 2 cycles of HPH at 20,000 psi pressure were selected as the optimized variables. The increase in droplet size was observed in NE1, which might be due to the expansion of the interfacial film by the co-surfactant leading to a larger droplet size, whereas decrease in droplet size in NE2 and NE3 might be due to a higher percentage of surfactant in Smix (30). But in the case of NE4, undoubtedly, the average droplet size was reduced, but PDI of NE4 was found to be a little higher, i.e. 0.357 ± 0.044, due to the presence of two differently sized peaks (Fig. 2a), which might be either due to instability caused by heat generation in situ while running 3 cycles of HPH at 20,000 psi, as nanoemulsions are thermodynamically not stable, or formation of surfactant micelles (47).
Nanoemulsions developed with non-ionic surfactants were stabilized mainly via steric mechanism, although an electrostatic mechanism cannot be ruled out. The origin of negative zeta potential of nanoemulsions might be due to preferential adsorption or desorption of electrolyte ions on the surface (47). On increasing the concentration of Tween 80, an increase in negative value of zeta potential might be associated with the presence of impurities, i.e. peroxides, free fatty acids, etc. (48,49). Transmission electron microscopy (TEM) study revealed the droplet morphology of the developed nanoemulsion. Nanoemulsion droplets were observed to be discrete, round in outline and dark in appearance in the photomicrographs. pH values of nanoemulsions were found to be favourable for topical application. Refractive index (RI) is the measure of the speed of light through a substance. The harder it is for light to travel through a medium (i.e., the denser is a medium), the higher is the value of RI. Thus, RI indicates the isotropic nature of the formulation. As the concentration of oil was increased in the formulation, RI was observed to be increased in Table IV (50). The obtained rheogram (viscosity vs. rate of shear) confirmed non-Newtonian, pseudo-plastic (shear thinning) behaviour of optimized nanoemulsion (Fig. 4). On the basis of the highest area of zone of nanoemulsification from pseudo-ternary phase diagram, and optimized results of pH, RI, viscosity and transparent visual aspect, with mean droplet size below 200 nm, we selected NE3 for gel transformation, especially for its topical application by conferring a pleasant skin feel (51,52).
Among different nanoemulgels, NEG0.5 was not geared up well with respect to visual appearance and viscosity, whereas NEG1.5 was too viscous and turbid in appearance. Nanoemulgel, NEG1, was found to be a creamy and viscous preparation having a smooth homogeneous texture, glossy appearance and no sign of phase separation. Viscosity values of NEG0.5, NEG1 and NEG1.5 suggested increase in viscosity with increase in the concentration of the gelling agent (Table VI) (26). The values of spreadability indicated that the nanoemulgel was easily spreadable by an application of a small amount of shear. On increasing the concentration of gelling agent, decrease in spreadability was observed (14,53). The more the gel is extruded, the better was the extrudability; hence, less amount of force was required to extrude the gel easily. The higher the value of firmness, the thicker is the consistency of a sample. Adhesiveness of nanoemulgel was found to be increased on increasing the concentration of Carbopol® Ultrez 21 (54,55). On the basis of the above evaluation parameters, NEG1 was optimized and selected for further studies.
Cumulative drug released from NE3 was higher as compared to drug suspension in 24 h due to its nanometric droplet size, which provides high-specific surface area for solubilization, consequently enhancing the dissolution rate and solubility (29,30). The three-dimensional rigid structure of NEG1 showed sustained release of drug as compared to NE3, which released almost all drug within the prescribed period. It was found that the NEG1 followed the Higuchi model with an R 2 value of 0.936. Cumulative drug permeated from NEG1 through goat hoof was more than NE3 and drug suspension due to the presence of permeation enhancer thioglycolic acid in the final formulation (nanoemulgel), which cleaves the disulphide bonds between the keratin molecules and increases the partitioning of the drug molecule (16). As the drug was poorly aqueous and soluble, we choose DMSO as the solvent for dissolving the drug for determining the antifungal activity in vitro. DMSO itself possesses antifungal activity. So, to determine the actual antifungal activity of the drug, we have carried out the antifungal activity of DMSO alone, by taking the same equivalent vehicle (DMSO) as taken for drug solution, as described in the ‘Antifungal Activity’ section. Actual in vitro antifungal activity (after subtracting the antifungal activity value of DMSO) revealed that during 48 h of incubation period, nanoemulgel of KCZ showed a significant (p < 0.05) area of zone of inhibition as compared to KCZ solution in DMSO against T. rubrum and C. albicans due to the small droplet size and sustained diffusion into the medium.
Skin irritation study conducted on rats showed that during the 24-h observation period, no evidence of irritation (erythema and edema) was observed on visual inspection after the application of nanoemulgel on rat skin. Nanoemulgel (NEG1) showed a skin irritation score (erythema and edema) of <2, which is considered to be non-irritant (no skin irritation) according to Draize et al. (56). Thus, the developed formulation was non-sensitizing and safe for topical use. In the histopathological analysis of rat skin, the microstructure of normal skin (Fig. 7a) showed a tight and intact multilayer structure, whereas the morphology of NEG1-treated skin (Fig. 7b) showed some disruptions of the stratum corneum layer with increased intercellular gap. As stratum corneum plays a major role in preventing the permeation of drugs, its disruption may enhance the permeation of applied formulation through the skin, suitable for its topical delivery (41).
Conclusion
In the present research work, KCZ-loaded nanoemulsion was developed by employing Labrafac™ Lipophile WL 1349 as the oil phase and Tween 80 and PEG 400 as the surfactant and co-surfactant, respectively. Optimized nanoemulsion (NE3) was formulated into nanoemulgel (NEG1) with desirable viscosity, spreadability, extrudability, texture and bio-adhesion, as compared to drug suspension for topical application. In vitro release and ex vivo permeation study of the optimized nanoemulgel (NEG1) showed better efficacies, as compared to drug suspension. The optimized nanoemulgel (NEG1) showed enhanced in vitro antifungal activity on T. rubrum and C. albicans as compared to drug solution in DMSO and thus could be a promising formulation to reduce the symptoms of and cure onychomycosis faster than the conventional therapies. Further, skin irritation study showed the safe use of nanoemulgel for topical applications. Thus, nanoemulgel is a safe, effective and promising formulation for the topical treatment of onychomycosis.
References
Campbell AW, Anyanwu EC, Morad M. Evaluation of the drug treatment and persistence of onychomycosis. Sci World J. 2004;4:760–77.
Järv H, Naaber P, Kaur S, Eisen M, Silm H. Toenail onychomycosis in Estonia. Mycoses. 2004;47(1–2):57–61.
Elewski BE, Hay RJ. Update on the management of onychomycosis: highlights of the Third Annual International Summit on Cutaneous Antifungal Therapy. Clin Infect Dis. 1996;23(2):305–13.
Roberts D, Taylor W, Boyle J. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148(3):402–10.
Bhattacharyya A, Bajpai M. Development and oral bioavailability of self emulsifying formulation of ketoconazole. Int J Pharm Sci Nanotechnol. 2013;5:1858–65.
Lazardi K, Urbina J, De Souza W. Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1990;34(11):2097–105.
Capek I. Degradation of kinetically-stable o/w emulsions. Adv Colloid Interf Sci. 2004;107(2):125–55.
Seekkuarachchi IN, Tanaka K, Kumazawa H. Formation and charaterization of submicrometer oil-in-water (O/W) emulsions, using high-energy emulsification. Ind Eng Chem Res. 2006;45(1):372–90.
Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280(1):241–51.
Alam MS, Ali MS, Alam N, Siddiqui MR, Shamim M, Safhi M. In vivo study of clobetasol propionate loaded nanoemulsion for topical application in psoriasis and atopic dermatitis. Drug Invent Today. 2013;5(1):8–12.
Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotechnol. 2008;6(8):11.
Pratap SB, Brajesh K, Jain S, Kausar S. Development and characterization of a nanoemulsion gel formulation for transdermal delivery of carvedilol. Int J Drug Dev Res. 2012;4(1):151–61.
Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–7.
Baran R, Coquard F. Combination of fluconazole and urea in a nail lacquer for treating onychomycosis. J Dermatol Treat. 2005;16(1):52–5.
Bohn M, Kraemer KT. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J Am Acad Dermatol. 2000;43(4):S57–69.
Kiran S, Chandrashekhar B, Vishnu P, Prasad M. Ungual drug delivery systems of ketoconazole nail lacquer. Int J Appl Pharm. 2010;4:17–9.
Tandel AA, Agrawal S, Wankhede SS. Transungual permeation of the voriconazole nail lacquer against Trichophyton rubrum. J Drug Deliv Ther. 2012;2(1).
Syed T, Qureshi Z, Ali S, Ahmad S, Ahmad S. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4(4):284–7.
Abrams BB, Hänel H, Hoehler T. Ciclopirox olamine: a hydroxypyridone antifungal agent. Clin Dermatol. 1991;9(4):471–7.
Rigopoulos D, Ioannides D, Prastitis N, Katsambas A. Nail psoriasis: a combined treatment using calcipotriol cream and clobetasol propionate cream. Acta Derm Venereol. 2002;82(2):140.
Balbás GM, Regaña MS, Millet PU. Tacalcitol ointment for the treatment of nail psoriasis. J Dermatol Treat. 2009;20(5):308–10.
Buselmeier T. Combination urea and salicyclic acid ointment nail avulsion in nondystrophic nails: a follow-up observation. Cutis. 1980;25(4):397. 405–397, 405.
Donnelly RF, McCarron PA, Lightowler JM, Woolfson AD. Bioadhesive patch-based delivery of 5-aminolevulinic acid to the nail for photodynamic therapy of onychomycosis. J Control Release. 2005;103(2):381–92.
Elewski B, Ghannoum M, Mayser P, Gupta A, Korting HC, Shouey R, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild‐to‐moderate toenail onychomycosis: results from three randomized studies using double‐blind vehicle‐controlled and open‐label active‐controlled designs. J Eur Acad Dermatol Venereol. 2013;27(3):287–94.
Ghannoum MA, Long L, Pfister WR. Determination of the efficacy of terbinafine hydrochloride nail solution in the topical treatment of dermatophytosis in a guinea pig model. Mycoses. 2009;52(1):35–43.
Parashar B, Kabra A, Chandel A. Formulation and evaluation of gel containing miconazole nitrate an antifungal agent. Int J Pharm Res Rev. 2013;2:8–28.
Helal DA, El-Rhman DA, Abdel-Halim SA, El-Nabarawi MA. Formulation and evaluation of fluconazole topical gel. Int J Pharm Pharm Sci. 2012;4(5):302–10.
Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, et al. Ungual and trans‐ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci. 2009;98(11):4130–40.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227–43.
Jain K, Kumar RS, Sood S, Gowthamarajan K. Enhanced oral bioavailability of atorvastatin via oil-in-water nanoemulsion using aqueous titration method. J Pharm Sci Res. 2013;5(1):18–25.
Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10(1):69–76.
Guideline IHT. Stability testing of new drug substances and products. Q1A (R2), Curr Step. 2003;4.
Shinde PB. Component screening of miconazole nitrate nanoemulsion. Asian J Biomed Pharm Sci. 2013;3(19):33–40.
Mutimer MN, Riffkin C, Hill JA, Glickman ME, Cyr GN. Modern ointment base technology II. Comparative evaluation of bases. J Am Pharm Assoc. 1956;45(4):212–8.
Coviello T, Trotta A, Marianecci C, Carafa M, Di Marzio L, Rinaldi F, et al. Gel-embedded niosomes: preparation, characterization and release studies of a new system for topical drug delivery. Colloids Surf B: Biointerfaces. 2015;125:291–9.
Rai VK, Yadav NP, Sinha P, Mishra N, Luqman S, Dwivedi H, et al. Development of cellulosic polymer based gel of novel ternary mixture of miconazole nitrate for buccal delivery. Carbohydr Polym. 2014;103:126–33.
Tan YT, Peh KK, Al-Hanbali O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech. 2000;1(3):69–78.
Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J. 2012;20(1):63–7.
Barot BS, Parejiya PB, Patel HK, Mehta DM, Shelat PK. Microemulsion-based antifungal gel delivery to nail for the treatment of onychomycosis: formulation, optimization, and efficacy studies. Drug Deliv Transl Res. 2012;2(6):463–76.
Kirkpatrick WR, Turner TM, Fothergill AW, McCarthy DI, Redding SW, Rinaldi MG, et al. Fluconazole disk diffusion susceptibility testing of Candida species. J Clin Microbiol. 1998;36(11):3429–32.
Zhu W, Guo C, Yu A, Gao Y, Cao F, Zhai G. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int J Pharm. 2009;378(1):152–8.
Barot BS, Parejiya PB, Patel HK, Gohel MC, Shelat PK. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. AAPS PharmSciTech. 2012;13(1):184–92.
Lala R, Awari N. Nanoemulsion-based gel formulations of COX-2 inhibitors for enhanced efficacy in inflammatory conditions. Appl Nanosci. 2014;4(2):143–51.
Shirsand S, Para M, Nagendrakumar D, Kanani K, Keerthy D. Formulation and evaluation of ketoconazole niosomal gel drug delivery system. Int J Pharm Investig. 2012;2(4):201.
Kute S, Saudagar R. Emulsified gel a novel approach for delivery of hydrophobic drugs: an overview. J Adv Pharm Educ Res. 2013;3(4).
López A, Llinares F, Cortell C, Herraez M. Comparative enhancer effects of Span® 20 with Tween® 20 and Azone® on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 2000;202(1):133–40.
Leser ME, Sagalowicz L, Michel M, Watzke HJ. Self-assembly of polar food lipids. Adv Colloid Interf Sci. 2006;123:125–36.
Smolinske SC. CRC handbook of food, drug, and cosmetic excipients. CRC press; 1992.
Delgado ÁV. Interfacial electrokinetics and electrophoresis. CRC Press; 2001.
Mustafa G, Khan ZI, Bansal T, Talegaonkar S. Preparation and characterization of oil in water nano-reservoir systems for improved oral delivery of atorvastatin. Curr Nanosci. 2009;5(4):428–40.
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma M. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3):102–10.
Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2(05):626.
Ramasamy T, Khandasami US, Ruttala H, Shanmugam S. Development of solid lipid nanoparticles enriched hydrogels for topical delivery of anti-fungal agent. Macromol Res. 2012;20(7):682–92.
Pande V, Patel S, Patil V, Sonawane R. Design expert assisted formulation of topical bioadhesive gel of sertaconazole nitrate. Adv Pharm Bull. 2014;4(2):121.
Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RFV. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75(2):186–93.
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):377–90.
Acknowledgments
This work was supported by the All India Council for Technical Education, Government of India. We sincerely acknowledge the kind help form Dr. Mohd. Samim, Department of Chemistry, Faculty of Science, Jamia Hamdard, New Delhi, for conducting microbiological studies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests and have received no payment for the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Mahtab, A., Anwar, M., Mallick, N. et al. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 17, 1477–1490 (2016). https://doi.org/10.1208/s12249-016-0488-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0488-0