Abstract
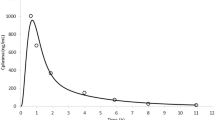
A physiologically based pharmacokinetic (PBPK) human model for alpelisib, an oral α-specific class I phosphatidylinositol-3-kinase (PI3K) inhibitor, was established to simulate oral absorption and plasma pharmacokinetics of healthy subjects to allow model-informed drug development. The GastroPlus™ model consisted of an advanced absorption gut model, which was linked to a 2-compartmental model. Systemic clearance and volume of distribution were estimated using population pharmacokinetics (popPK). Various food effect and pH-mediated absorption drug–drug interaction (DDI) scenarios were modeled. In fasted healthy subjects, simulated absorption was lower (ca. 70% for a 300-mg dose) due to pH and bile acid concentration-dependent solubility. Ranitidine showed a significant pH-mediated DDI effect only in the fasted but not fed state. The PBPK model identified that more drug is absorbed in the fed state, and alpelisib intestinal permeability is rate limiting to systemic exposure. Simulations for healthy subject showed a positive food effect with ca. 2-fold increase in plasma Cmax and 1.5-fold increase in AUC0-inf with a meal compared with fasted conditions. The PBPK model was verified using clinical food effect data with pivotal clinical formulation (PCF) and then applied to predict the performance of a commercial formulation (CF) in healthy volunteers. The model successfully predicted the outcome of a clinical bioequivalence study for PCF and CF with included in vitro dissolution data, both fasted and fed state. Estimated predictive errors (based on plasma Cmax, AUC0-t) were equal or below 30%. The alpelisib model for healthy subjects enables future bioequivalence formulation assessments, in fasted, fed, or altered pH conditions.

Graphical Abstract






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the plasma concentration-time curve
- Cmax:
-
Maximum plasma concentration
- PBPK:
-
Physiologically based pharmacokinetics
- PK:
-
Pharmacokinetics
References
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–40.
James A, Blumenstein L, Glaenzel U, Jin Y, Demailly A, Jakab A, et al. Absorption, distribution, metabolism, and excretion of [14C]BYL719 (alpelisib) in healthy male volunteers. Cancer Chemother Pharmacol. 2015;76:751–60.
Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, et al. Phosphatidylinositol 3-kinase a–selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J Clin Oncol. 2018.
Marbury T, El-Hashimy M, Blumenstein L, Letellier F, Sengupta T, Lorenzo S, et al. Pharmacokinetics and safety of alpelisib in subjects with hepatic impairment: a phase 1, open-label, multicenter, parallel-group study. J Cancer. 2019;(In press).
Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, et al. In vitro models for the prediction of in vivo performance of oral dosage forms: recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm. 2019;136:70–83.
Parrott NJ, Yu LJ, Takano R, Nakamura M, Morcos PN. Physiologically based absorption modeling to explore the impact of food and gastric pH changes on the pharmacokinetics of alectinib. AAPS J. 2016;18:1464–74.
Tistaert C, Heimbach T, Xia B, Parrott N, Samant TS, Kesisoglou F. Food effect projections via physiologically based pharmacokinetic modeling: predictive case studies. J Pharm Sci. 2019;108:592–602.
Mitra A, Parrott N, Miller N, Lloyd R, Tistaert C, Heimbach T, et al. Prediction of pH-dependent drug-drug interactions for basic drugs using physiologically based biopharmaceutics modeling: industry case studies. J Pharm Sci. 2019.
Heimbach T, Suarez-Sharp S, Kakhi M, Holmstock N, Olivares-Morales A, Pepin X, et al. Dissolution and translational modeling strategies toward establishing an in vitro-in vivo link—a workshop summary report. AAPS J. 2019;21:29.
Markopoulos C, Andreas CJ, Vertzoni M, Dressman J, Reppas C. In-vitro simulation of luminal conditions for evaluation of performance of oral drug products: choosing the appropriate test media. Eur J Pharm Biopharm. 2015;93:173–82.
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res. 2006;23:1144–56.
Oncology Clinical Protocol CBYL719C2301 / NCT02437318 [Internet]. SOLAR-1: a phase III randomized double-blind, placebo controlled study of alpelisib in combination with fulvestrant for men and postmenopausal women with hormone receptor positive, HER2-negative advanced breast cancer which progressed on or after aromatas. 2017 [cited 2020 Apr 3]. p. 1–210. Available from: https://clinicaltrials.gov/ProvidedDocs/18/NCT02437318/Prot_000.pdf.
Kletzl H, Giraudon M, Ducray PS, Abt M, Hamilton M, Lum BL. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anti-Cancer Drugs. 2014.
Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH-dependent drug-drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther. 2014;96:266–77.
Boxenbaum H, Ronfeld R. Interspecies pharmacokinetic scaling and the Dedrick plots. Am J Physiol Regul Integr Comp Physiol. 1983;245:R768–75.
Vuppugalla R, Marathe P, He H, Jones RDO, Yates JWT, Jones HM, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 4: prediction of plasma concentration-time profiles in human from in vivo preclinical data by using the Wajima approach. J Pharm Sci. 2011;100:4111–26.
Sutton SC, Nause R, Gandelman K. The impact of gastric pH, volume, and emptying on the food effect of ziprasidone oral absorption. AAPS J. 2017;19:1084–90.
Kakuda TN, Falcon RW. Effect of food and ranitidine on saquinavir pharmacokinetics and gastric pH in healthy volunteers. Pharmacotherapy. 2006.
Fischer M, Fadda HM. The effect of sex and age on small intestinal transit times in humans. J Pharm Sci. 2016;105:682–6.
Urgesi R, Cianci R, Bizzotto A, Costamagna G, Riccioni ME. Evaluation of gastric and small bowel transit times in coeliac disease with the small bowel PillCam®: a single centre study in a non gluten-free diet adult italian population with coeliac disease. Eur Rev Med Pharmacol Sci. 2013.
Ring BJ, Chien JY, Adkison KK, Jones HM, Rowland M, Jones R Do, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 3: comparative assessement of prediction methods of human clearance. J Pharm Sci. 2011.
FDA- Approved Drugs [Internet]. New Drug Application (NDA): 212526. [cited 2020 Apr 27]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=212526.
Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62.
NDA/BLA multi-disciplinary review and evaluation [Internet]. Application number 212526Orig1s000. 2016 [cited 2020 Jun 4]. p. 1–225. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212526Orig1s000MultidisciplineR.pdf.
Framework for assessing pH-dependent drug-drug interactions. Dly J United States Gov [Internet]. 2018;No. 2018–1:23688–9. Available from: https://www.federalregister.gov/documents/2018/05/22/2018-10927/framework-for-assessing-ph-dependent-drug-drug-interactions-establishment-of-a-public-docket-request.
Bethesda M. No title. Natl Inst Diabetes Dig Kidney Dis. 2012.
Litou C, Vertzoni M, Goumas C, Vasdekis V, Xu W, Kesisoglou F, et al. Characteristics of the human upper gastrointestinal contents in the fasted state under hypo- and A-chlorhydric gastric conditions under conditions of typical drug – drug interaction studies. Pharm Res. 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The Novartis authors as indicated by their affiliation are Novartis employees and own Novartis stocks. A Sinn and M Velinova have nothing to disclose.
Additional information
Guest Editor: Filippos Kesisoglou
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 174 kb)
Rights and permissions
About this article
Cite this article
Gajewska, M., Blumenstein, L., Kourentas, A. et al. Physiologically Based Pharmacokinetic Modeling of Oral Absorption, pH, and Food Effect in Healthy Volunteers to Drive Alpelisib Formulation Selection. AAPS J 22, 134 (2020). https://doi.org/10.1208/s12248-020-00511-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00511-7