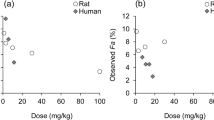
Abstract
Quantifying the multiple processes which control and modulate the extent of oral bioavailability for drug candidates is critical to accurate projection of human pharmacokinetics (PK). Understanding how gut wall metabolism and hepatic elimination factor into first-pass clearance of drugs has improved enormously. Typically, the cytochrome P450s, uridine 5′-diphosphate-glucuronosyltransferases and sulfotransferases, are the main enzyme classes responsible for drug metabolism. Knowledge of the isoforms functionally expressed within organs of first-pass clearance, their anatomical topology (e.g. zonal distribution), protein homology and relative abundances and how these differ across species is important for building models of human metabolic extraction. The focus of this manuscript is to explore the parameters influencing bioavailability and to consider how well these are predicted in human from animal models or from in vitro to in vivo extrapolation. A unique retrospective analysis of three AstraZeneca molecules progressed to first in human PK studies is used to highlight the impact that species differences in gut wall metabolism can have on predicted human PK. Compared to the liver, pharmaceutical research has further to go in terms of adopting a common approach for characterisation and quantitative prediction of intestinal metabolism. A broad strategy is needed to integrate assessment of intestinal metabolism in the context of typical DMPK activities ongoing within drug discovery programmes up until candidate drug nomination.




Similar content being viewed by others
References
Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–5.
Lesko LJ, Rowland M, Peck CC, Blaschke TF. Optimizing the science of drug development: opportunities for better candidate selection and accelerated evaluation in humans. Pharm Res. 2000;17(11):1335–44.
Hellriegel ET, Bjornsson TD, Hauck WW. Interpatient variability in bioavailability is related to the extent of absorption: implications for bioavailability and bioequivalence studies. Clin Pharmacol Ther. 1996;60(6):601–7.
Hurst S, Loi C-M, Brodfuehrer J, El-Kattan A. Impact of physiological, physicochemical and biopharmaceutical factors in absorption and metabolism mechanisms on the drug oral bioavailability of rats and humans. Expert Opin Drug Metab Toxicol. 2007;3(4):469–89.
Ballard P, Brassil P, Bui KH, Dolgos H, Petersson C, Tunek A, et al. The right compound in the right assay at the right time: an integrated discovery DMPK strategy. Drug Metab Rev. 2012;44(3):224–52.
Chen Y, Jin JY, Mukadam S, Malhi V, Kenny JR. Application of IVIVE and PBPK modeling in prospective prediction of clinical pharmacokinetics: strategy and approach during the drug discovery phase with four case studies. Biopharm Drug Dispos. 2012;33(2):85–98.
Jones HM, Parrott N, Jorga K, Lavé T. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45(5):511–42.
Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46–58.
Van den Bergh A, Sinha V, Gilissen R, Straetemans R, Wuyts K, Morrison D, et al. Prediction of human oral plasma concentration-time profiles using preclinical data. Clin Pharmacokinet. 2011;50(8):505–17.
Zou P, Yu Y, Zheng N, Yang Y, Paholak HJ, Lawrence XY, et al. Applications of human pharmacokinetic prediction in first-in-human dose estimation. AAPS J. 2012;14(2):262–81.
Poulin P, Jones R, Jones HM, Gibson CR, Rowland M, Chien JY, et al. PHRMA CPCDC initiative on predictive models of human pharmacokinetics, part 5: prediction of plasma concentration-time profiles in human by using the physiologically‐based pharmacokinetic modeling approach. J Pharm Sci. 2011;100(10):4127–57.
De Buck SS, Sinha VK, Fenu LA, Nijsen MJ, Mackie CE, Gilissen RA. Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos. 2007;35(10):1766–80.
Zhang T, Heimbach T, Lin W, Zhang J, He H. Prospective predictions of human pharmacokinetics for eighteen compounds. J Pharm Sci. 2015;104(9):2795–806.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Akabane T, Tabata K, Kadono K, Sakuda S, Terashita S, Teramura T. A comparison of pharmacokinetics between humans and monkeys. Drug Metab Dispos. 2010;38(2):308–16.
Cao X, Gibbs ST, Fang L, Miller HA, Landowski CP, Shin H-C, et al. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharm Res. 2006;23(8):1675–86.
Grass GM, Sinko PJ. Physiologically-based pharmacokinetic simulation modelling. Adv Drug Deliv Rev. 2002;54(3):433–51.
Musther H, Olivares-Morales A, Hatley OJ, Liu B, Hodjegan AR. Animal versus human oral drug bioavailability: do they correlate? Eur J Pharm Sci. 2014;57:280–91.
Lin JH, Chiba M, Baillie TA. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol Rev. 1999;51(2):135–58.
Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34(5):880–6.
Yang J, Tucker GT, Rostami‐Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clin Pharmacol Ther. 2004;76(4):391.
Gertz M, Houston JB, Galetin A. Physiologically based pharmacokinetic modeling of intestinal first-pass metabolism of CYP3A substrates with high intestinal extraction. Drug Metab Dispos. 2011;39(9):1633–42.
Kadono K, Akabane T, Tabata K, Gato K, Terashita S, Teramura T. Quantitative prediction of intestinal metabolism in humans from a simplified intestinal availability model and empirical scaling factor. Drug Metab Dispos. 2010;38(7):1230–7.
Kaminsky LS, Fasco MJ. Small intestinal cytochromes P450. Crit Rev Toxicol. 1992;21(6):407–22.
Varma MV, Obach RS, Rotter C, Miller HR, Chang G, Steyn SJ, et al. Physicochemical space for optimum oral bioavailability: contribution of human intestinal absorption and first-pass elimination. J Med Chem. 2010;53(3):1098–108.
Fan J, Chen S, Chow EC, Pang SK. PBPK modeling of intestinal and liver enzymes and transporters in drug absorption and sequential metabolism. Curr Drug Metab. 2010;11(9):743–61.
Furukawa T, Yamano K, Naritomi Y, Tanaka K, Terashita S, Teramura T. Method for predicting human intestinal first-pass metabolism of UGT substrate compounds. Xenobiotica. 2012;42(10):980–8.
Pang SK. Modeling of intestinal drug absorption: roles of transporters and metabolic enzymes (for the Gillette Review Series). Drug Metab Dispos. 2003;31(12):1507–19.
Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007;8(7):676–84.
Galetin A, Gertz M, Houston JB. Potential role of intestinal first-pass metabolism in the prediction of drug-drug interactions. Expert Opin Drug Metab Toxicol. 2008;4(7):909–22.
Tang C, Prueksaritanont T. Use of in vivo animal models to assess pharmacokinetic drug-drug interactions. Pharm Res. 2010;27(9):1772–87.
Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42(6):620–43.
Chiou W, Ma C, Chung S, Wu T, Jeong H. Similarity in the linear and non-linear oral absorption of drugs between human and rat. Int J Clin Pharmacol Ther. 2000;38(11):532–9.
Chiou WL, Buehler PW. Comparison of oral absorption and bioavailability of drugs between monkey and human. Pharm Res. 2002;19(6):868–74.
Lennernäs H. Human intestinal permeability. J Pharm Sci. 1998;87(4):403–10.
Sjöberg Å, Lutz M, Tannergren C, Wingolf C, Borde A, Ungell A-L. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur J Pharm Sci. 2013;48(1):166–80.
Ungell AL, Artursson P. An overview of Caco‐2 and alternatives for prediction of intestinal drug transport and absorption. In: Van de Waterbeemd H, Testa B, editors. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability. 40. 2nd ed. KGaA, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co; 2009. p. 133–59.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–21.
Parrott N, Lave T. Applications of physiologically based absorption models in drug discovery and development. Mol Pharm. 2008;5(5):760–75.
Sjögren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernäs H, et al. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: application of the mechanistic absorption model GI-Sim. Eur J Pharm Sci. 2013;49(4):679–98.
Van de Waterbeemd H. In silico models to predict oral absorption. In: Taylor JB, Triggle DJ, editors. Comprehensive medicinal chemistry II. 5. Oxford: Elsevier Science Ltd; 2007. p. 669–97.
Chiou WL, Barve A. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharm Res. 1998;15(11):1792–5.
DeSesso JM, Williams AL. Contrasting the gastrointestinal tracts of mammals: factors that influence absorption. Annu Rep Med Chem. 2008;43:353–71.
Chiou WL, Jeong HY, Chung SM, Wu TC. Evaluation of using dog as an animal model to study the fraction of oral dose absorbed of 43 drugs in humans. Pharm Res. 2000;17(2):135–40.
Dressman JB. Comparison of canine and human gastrointestinal physiology. Pharm Res. 1986;3(3):123–31.
Becker D, Zhang J, Heimbach T, Penland RC, Wanke C, Shimizu J, et al. Novel orally swallowable IntelliCap® device to quantify regional drug absorption in human GI tract using diltiazem as model drug. AAPS PharmSciTech. 2014;15(6):1490–7.
He YL, Murby S, Warhurst G, Gifford L, Walker D, Ayrton J, et al. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly (ethylene glycol) and D‐peptides. J Pharm Sci. 1998;87(5):626–33.
Grime KH, Barton P, McGinnity DF. Application of in silico, in vitro and preclinical pharmacokinetic data for the effective and efficient prediction of human pharmacokinetics. Mol Pharm. 2013;10(4):1191–206.
McGinnity DF, Collington J, Austin RP, Riley RJ. Evaluation of human pharmacokinetics, therapeutic dose and exposure predictions using marketed oral drugs. Curr Drug Metab. 2007;8(5):463–79.
Thomas VH, Bhattachar S, Hitchingham L, Zocharski P, Naath M, Surendran N, et al. The road map to oral bioavailability: an industrial perspective. Expert Opin Drug Metab Toxicol. 2006;2(4):591–608.
Sohlenius-Sternbeck A-K, Afzelius L, Prusis P, Neelissen J, Hoogstraate J, Johansson J, et al. Evaluation of the human prediction of clearance from hepatocyte and microsome intrinsic clearance for 52 drug compounds. Xenobiotica. 2010;40(9):637–49.
Sohlenius-Sternbeck A-K, Jones C, Ferguson D, Middleton BJ, Projean D, Floby E, et al. Practical use of the regression offset approach for the prediction of in vivo intrinsic clearance from hepatocytes. Xenobiotica. 2012;42(9):841–53.
Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human micro-somal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8(1):33–45.
Grime K, Riley R. The impact of in vitro binding on in vitro-in vivo extrapolations, projections of metabolic clearance and clinical drug-drug interactions. Curr Drug Metab. 2006;7(3):251–64.
Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6(2):140–8.
Hewitt NJ, Gómez Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39(1):159–234.
Riley RJ, McGinnity DF, Austin RP. A unified model for predicting human hepatic, metabolic clearance from in vitro intrinsic clearance data in hepatocytes and microsomes. Drug Metab Dispos. 2005;33(9):1304–11.
Ito K, Houston JB. Prediction of human drug clearance from in vitro and preclinical data using physiologically based and empirical approaches. Pharm Res. 2005;22(1):103–12.
Soars MG, Webborn PJ, Riley RJ. Impact of hepatic uptake transporters on pharmacokinetics and drug-drug interactions: use of assays and models for decision making in the pharmaceutical industry. Mol Pharm. 2009;6(6):1662–77.
FDA Approved Drug Products (2012) 2012. Retrieved 27th September 2012 from]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
De Waziers I, Cugnenc P, Yang C, Leroux J, Beaune P. Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990;253(1):387–94.
Nishimuta H, Nakagawa T, Nomura N, Yabuki M. Significance of reductive metabolism in human intestine and quantitative prediction of intestinal first-pass metabolism by cytosolic reductive enzymes. Drug Metab Dispos. 2013;41(5):1104–11.
Ritter JK. Intestinal UGTs as potential modifiers of pharmacokinetics and biological responses to drugs and xenobiotics. Expert Opin Drug Metab Toxicol. 2007;3(1):93–107.
Thummel KE, Kunze KL, Shen DD. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev. 1997;27(2):99–127.
Kolars JC, Watkins P, Merion RM, Awni W. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338(8781):1488–90.
Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, et al. First‐pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60(1):14–24.
Floren LC, Bekersky I, Benet LZ, Mekki Q, Dressler D, Lee JW, et al. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther. 1997;62(1):41–9.
Glaeser H, Drescher S, Hofmann U, Heinkele G, Somogyi AA, Eichelbaum M, et al. Impact of concentration and rate of intraluminal drug delivery on absorption and gut wall metabolism of verapamil in humans. Clin Pharmacol Ther. 2004;76(3):230–8.
Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Investig. 1997;99(10):2545.
Mistry M, Houston JB. Quantitation of extrahepatic metabolism. Pulmonary and intestinal conjugation of naphthol. Drug Metab Dispos. 1985;13(6):740–5.
van de Kerkhof EG, de Graaf IA, Groothuis GM. In vitro methods to study intestinal drug metabolism. Curr Drug Metab. 2007;8(7):658–75.
Cubitt HE, Houston JB, Galetin A. Relative importance of intestinal and hepatic glucuronidation—impact on the prediction of drug clearance. Pharm Res. 2009;26(5):1073–83.
Galetin A, Gertz M, Houston JB. Contribution of intestinal cytochrome P450-mediated metabolism to drug-drug inhibition and induction interactions. Drug Metab Pharmacokinet. 2010;25(1):28–47.
Beddies G, Fox PR, Papich MD, Kanikanti V-R, Krebber R, Keene BW. Comparison of the pharmacokinetic properties of bisoprolol and carvedilol in healthy dogs. Am J Vet Res. 2008;69(12):1659–63.
Bueters T, Juric S, Sohlenius-Sternbeck A-K, Hu Y, Bylund J. Rat poorly predicts the combined non-absorbed and presystemically metabolized fractions in the human. Xenobiotica. 2012;43(7):607–16.
Furukawa H, Imventarza O, Venkataramanan R, Suzuki M, Zhu Y, Warty VS, et al. The effect of bile duct ligation and bile diversion on FK506 pharmacokinetics in dogs. Transplantation. 1992;53(4):722.
Heykants J, Knaeps A, Meuldermans W, Michiels M. On the pharmacokinetics of domperidone in animals and man I. Plasma levels of domperidone in rats and dogs. Age related absorption and passage through the blood brain barrier in rats. Eur J Drug Metab Pharmacokinet. 1981;6(1):27–36.
Higuchi S, Shiobara Y. Comparative pharmacokinetics of nicardipine hydrochloride, a new vasodilator, in various species. Xenobiotica. 1980;10(6):447–54.
Komura H, Iwaki M. In vitro and in vivo small intestinal metabolism of CYP3A and UGT substrates in preclinical animals species and humans: species differences. Drug Metab Rev. 2011;43(4):476–98.
Le Traon G, Burgaud S, Horspool L. Pharmacokinetics of cimetidine in dogs after oral administration of cimetidine tablets. J Vet Pharmacol Ther. 2009;32(3):213–8.
Walker D. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29(3):297–310.
Prueksaritanont T, Gorham LM, Hochman JH, Tran LO, Vyas KP. Comparative studies of drug-metabolizing enzymes in dog, monkey, and human small intestines, and in Caco-2 cells. Drug Metab Dispos. 1996;24(6):634–42.
Takahashi M, Washio T, Suzuki N, Igeta K, Yamashita S. The species differences of intestinal drug absorption and first‐pass metabolism between cynomolgus monkeys and humans. J Pharm Sci. 2009;98(11):4343–53.
Nishimura T, Amano N, Kubo Y, Ono M, Kato Y, Fujita H, et al. Asymmetric intestinal first-pass metabolism causes minimal oral bioavailability of midazolam in cynomolgus monkey. Drug Metab Dispos. 2007;35(8):1275–84.
Darwich A, Neuhoff S, Jamei M, Rostami-Hodjegan A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr Drug Metab. 2010;11(9):716–29.
Jeong EJ, Liu Y, Lin H, Hu M. Species-and disposition model-dependent metabolism of raloxifene in gut and liver: role of UGT1A10. Drug Metab Dispos. 2005;33(6):785–94.
Furukawa T, Naritomi Y, Tetsuka K, Nakamori F, Moriguchi H, Yamano K, et al. Species differences in intestinal glucuronidation activities between humans, rats, dogs and monkeys. Xenobiotica. 2013;44(3):205–16.
Fisher MB, Labissiere G. The role of the intestine in drug metabolism and pharmacokinetics: an industry perspective. Curr Drug Metab. 2007;8(7):694–9.
Wilson Z, Rostami‐Hodjegan A, Burn J, Tooley A, Boyle J, Ellis S, et al. Inter‐individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br J Clin Pharmacol. 2003;56(4):433–40.
Hatley OJD. Mechanistic prediction of intestinal first-pass metabolism using in vitro data in preclinical species and in man [PhD thesis]. Manchester: The University of Manchester; 2014.
Cubitt HE, Houston JB, Galetin A. Prediction of human drug clearance by multiple metabolic pathways: integration of hepatic and intestinal microsomal and cytosolic data. Drug Metab Dispos. 2011;39(5):864–73.
Orzechowski A, Schrenk D, Bock-Hennig BS, Bock KW. Glucuronidation of carcinogenic arylamines and their N-hydroxy derivatives by rat and human phenol UDP-glucuronosyltransferases of the UGT1 gene complex. Carcinogenesis. 1994;15(8):1549–53.
Ebner T, Burchell B. Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metab Dispos. 1993;21(1):50–5.
Kilford PJ, Stringer R, Sohal B, Houston JB, Galetin A. Prediction of drug clearance by glucuronidation from in vitro data: use of combined cytochrome P450 and UDP-glucuronosyltransferase cofactors in alamethicin-activated human liver microsomes. Drug Metab Dispos. 2009;37(1):82–9.
Bach P, Antonsson T, Bylund R, Björkman J-A. Österlund, Giordanetto F, et al. Lead optimization of ethyl 6-aminonicotinate acyl sulfonamides as antagonists of the P2Y12 receptor. Separation of the antithrombotic effect and bleeding for candidate drug AZD1283. J Med Chem. 2013;56(17):7015–24.
Nishimuta H, Houston JB, Galetin A. Hepatic, intestinal, renal, and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog, and rat: implications for in vitro-in vivo extrapolation of clearance of prodrugs. Drug Metab Dispos. 2014;42(9):1522–31.
Crow JA, Borazjani A, Potter PM, Ross MK. Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol Appl Pharmacol. 2007;221(1):1–12.
Rudakova E, Boltneva N, Makhaeva G. Comparative analysis of esterase activities of human, mouse, and rat blood. Bull Exp Biol Med. 2011;152(1):73–5.
Peters SA. Evaluation of a generic physiologically based pharmacokinetic model for lineshape analysis. Clin Pharmacokinet. 2008;47(4):261–75.
Peters SA. Identification of intestinal loss of a drug through physiologically based pharmacokinetic simulation of plasma concentration-time profiles. Clin Pharmacokinet. 2008;47(4):245–59.
Acknowledgments
This work was contributed to the OrBiTo project (http://www.imi.europa.eu/content/orbito) as side ground.
O.J.D.Hatley was funded by a PhD grant awarded through the CASE award scheme, receiving support from both the MRC and AstraZeneca.
All in vivo work conducted within AstraZeneca was subject to internal ethical review and conducted in accordance with Home Office requirements under the Animals Scientific Procedures Act (1986).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Structure features of AZ12470164. (GIF 3 kb)
Figure S2
Structure of AZD1283. (GIF 4 kb)
Figure S3
Structure of AZD7009. (GIF 3 kb)
Figure S4
Physiologically based PK simulations of PK profiles of AZD7009 in rats. Simulations of (a) IV and (b) oral profiles against the observed data from different animals, reprinted with permission (100). (GIF 18 kb)
Figure S5
Physiologically based PK simulations of PK profiles of AZD7009 in humans. (a) Simulation of the intravenous profile without enterohepatic recirculation. (b) Simulation of the intravenous profile with inclusion of enterohepatic recirculation. (c) Simulated oral profile against the observed when intestinal loss was not considered; the permeability used was 5.8 cm/s. (d) Simulated oral profile against the observed when intestinal loss was not considered; the permeability used was 1.9 cm/s. (e) Simulation of the oral profile with intestinal loss rate constants introduced and refinement with enterohepatic recirculation. (f) Simulation of the oral profile excluding enterohepatic recirculation and including intestinal loss. (g) Simulation of the oral profile including enterohepatic recirculation and excluding intestinal loss. Reprinted with permission (100). (GIF 35 kb)
Rights and permissions
About this article
Cite this article
Jones, C.R., Hatley, O.J.D., Ungell, AL. et al. Gut Wall Metabolism. Application of Pre-Clinical Models for the Prediction of Human Drug Absorption and First-Pass Elimination. AAPS J 18, 589–604 (2016). https://doi.org/10.1208/s12248-016-9889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9889-y