Abstract
Background
The benefits of leaf stay-green for maintaining grain filling in sorghum under drought was largely demonstrated. However, its role in the stability of a dual production (grain, stem sugar) in tall sweet sorghum remains to be elucidated. This study aimed to analyze the impact of a post-anthesis drought on sugar accumulation along stem internodes in sweet and tall West-African sorghum with variable leaf stay-green and grain yield abilities.
Methods
Four accessions with similar phenology were studied in two consecutive years in the field at the National Agronomic Research Centre (CNRA) of Bambey (Senegal, West Africa) under two post-anthesis water treatments (irrigated, non-irrigated). Plant morphology, stem sugar related traits, grain production, and plant leaf area (PLA) variation were assessed. Carbohydrate contents (sucrose, hexoses, starch) were determined during grain filling in the whole stem juice and at three internode levels: bottom, median, top. Analysis of variance was performed to test post-anthesis water treatment, accession, organ, year effects and their interactions on the studied traits.
Results
Panicle dry weight (PDW) was not affected by drought, but strongly varied among years and accessions. The PDW/PLA ratio was negatively correlated with the variation of sucrose and hexoses at the three internodes levels. This carbohydrates reduction was mainly influenced by the PDW. The bigger the panicle the higher the carbohydrates remobilization from the stem to panicle for grain filling. This was mainly shown on accessions G3 and G11 which exhibited low stay green ability. However, G10 with low PDW/PLA ratio and showing higher stay green ability, exhibited a low reduction of total soluble sugars and sucrose and inversely higher increase of hexoses mainly at the median internode.
Conclusions
This ability to better maintain green leaf area and high hexoses in the stem under post-flowering drought could be an osmoregulation mechanism to adapt to drought. Therefore, stay-green is an important trait to consider for sweet sorghum breeding and particularly in the objective of developing dual purpose varieties in drought prone environments.
Similar content being viewed by others
Introduction
Sweet sorghum is able to accumulate remarkable amount of soluble sugars in the stem while ensuring grain filling and for this reason can be used as a dual purpose crop (Rutto et al. 2013). Sugar production by sweet sorghum depends on the quantity of sweet juice contained in the stem. Stem juiciness, juice sweetness and extractability are essential for such end-use (Burks et al. 2015; Zou et al. 2011). Stem non-structural carbohydrates are constituted of sucrose, glucose, fructose (Almodares et al. 2007; Qazi et al. 2012) and starch. Their concentration in the stem depends on the genotype, the environment and plant phenological stage (Gutjahr et al. 2013). Sucrose is the main type of non-structural carbohydrate present in the sorghum stem and starch is present in very small quantity (Massoud and Abd El-Razek 2011). Sucrose starts accumulating in a given internode when its expansion is completed and thus its concentration is initiated from the basis to the top of the plant. Accordingly, depending on crop cycle duration and internode rank, internode sucrose concentration can be almost completed at flowering or still increase until maturity. At this stage, sucrose concentration is almost constant along the stem, with a slight decrease in top internodes (Gutjahr et al. 2013).
Grain filling consists in the flux of carbohydrates from plant vegetative parts towards the panicle and is essential for grain yield elaboration. It depends firstly on plant carbohydrate availability, mainly ensured directly by leaf photosynthesis in sorghum (de Souza et al. 2015). Also the competition between grain filling and sugar accumulated in the stem is weak in sorghum, as suggested by Kouressy et al. (2008) on three grain sorghum varieties in optimal watering conditions. Tovignan et al. (2016), supported by Promkhambut et al. (2011) and Peloewetse (2012), even observed a significant increase of sugar content in stem juice between flowering and maturity in twelve tall, sweet and photoperiodic sorghum accessions. These authors suggested accordingly that under well-watered conditions, post-flowering photosynthesis can benefit to grain filling and stem sugar accumulation.
Although it is known to be drought tolerant (Sasaki and Baltazar 2009), grain sorghum yield can be dramatically affected by post-flowering drought, due to the reduction of green leaf area, photosynthetic capacity and thus carbohydrates availability (Beheshti and Behboodi 2010). This can be however partly compensated by the remobilization of nonstructural carbohydrates stored in the stem (Slewinski 2012; Fisher and Wilson 1971). This was already demonstrated in grain sorghum (Blum et al. 1997).
Several agronomic strategies were suggested to reduce post-anthesis drought effect on grain filling. Among them, some avoidance solutions were suggested as the reduction of pre-anthesis water use by reducing plant size (tillering) (van Oosterom et al. 2010) or the improvement of root system to enable the exploration of deeper soil layers (Kulathunga 2013). Regarding, physiological adaptations, leaf stay-green was shown to favor photosynthesis maintenance and carbohydrate availability for grain filling (Borrell et al. 2000). By comparing two water treatments (well-watered and post-flowering drought) on twelve sweet, tall grain sorghum accessions, Tovignan et al. (2016) pointed out that grain filling was not affected by a post-flowering drought, whereas stem sugar content was slightly reduced, to an extent positively correlated to the reduction rate of green leaf area between flowering and maturity. These results suggest that in sweet and tall sorghum types, drought impact can be buffered by the soluble sugars stored in the stem that, if associated to a stay-green aptitude, may ensure the maintenance of both grain and stem sugar productions, which might be of high agronomic value. As the production of soluble sugar by sweet sorghum depends on extractable juice quantity and sweetness (Burks et al. 2015), further insight must be provided to the way stay-green ability influences sugar partitioning in internodes along the stem depending on plant water status. To our knowledge this was not reported yet in the literature, although several studies provided evidence that drought affects plant C source-sink relationship and sugar partitioning on various cereals crops (Luquet et al. 2008; Rebolledo et al. 2013) on rice leaves; Gupta et al. (2011) on wheat internodes; Fu et al. (2010) on tall fescue leaves). Regarding, sweet sorghum, Qazi et al. (2014) recently reported a modification of sugar metabolism by drought (concentration of sucrose vs. hexoses and related enzyme activities) at the level of a single internode and peduncle. However, this study did not relate these results to whole plant production. This study was comforted by Ghate et al. (2016) but focusing on dwarf and short cycle sorghum isolines differing only for leaf stay-green QTL. Sweet sorghum is nevertheless classically tall with a long cycle, particularly in West-African ecotypes, which may have implications on the relationship between this type of results and their effect on plant production.
The present study aimed at better understanding the effect of post-flowering drought on stem sugar production by sweet and tall photoperiodic sorghum, depending on genotypic stay-green ability. For this purpose, four contrasted accessions studied by Tovignan et al. (2016) were chosen to evaluate carbohydrate partitioning variability depending on internode level along the stem. Results are discussed with respect to their implications for the improvement of sorghum cropping systems.
Materials and methods
Plant material
The four sorghum (Sorghum bicolor L. Moench) accessions (G3, G7, G10 and G11) studied are presented in Table 1 and were also previously described in Tovignan et al. (2016). They were tested in the field experimental station of CNRA of Bambey in Senegal (14° 42′ N, 16° 28′ W; 20 m above sea level), in two consecutive years (sowing on July17 and August 6 respectively in 2013 and 2014) and two post-anthesis water treatments (D treatments: NI, non-irrigated; IR, irrigated) with three replicates.
Experimental conditions
Weather conditions and field monitoring in both years, were previously described in Tovignan et al. (2016). As reminder, the rainfall, mean temperature, average relative humidity and global radiation distribution for the 2 years are again given in Fig. 1. Briefly, the trial was conducted in both years during the rainy seasons and was essentially rainfed before anthesis. However, when the dry spell occurred and lasted a week, the entire field was irrigated with 25 mm/week, using a sprinkler irrigation system. After anthesis, stressed plots were allowed to dry down and the controls were irrigated with 25 mm/week. Overall, the cumulative rainfall was 668 mm and 474 mm in 2013 and 2014 respectively. The supplied irrigation was 186 mm and 132 mm in 2013 and 2014 respectively. During the post-anthesis drought study, soil water content and predawn leaf water potential measured using the Diviner 2000 (Sentek Pty. Ltd., Adelaide, SA) and Scholander pressure chamber respectively, on two accessions used as reference in relation to their potential capacity to use water. These measurements (Fig. 2) were used to provide an indication of the variability of drought stress between water treatments, accessions and years.
Post-anthesis soil water content in each water treatment (NI: non irrigated, IR: irrigated) in 2013 and 2014 for the reference accessions SS6 (a) and SS8 (b) and predawn leaf water potential measured during post-anthesis drought in 2014 for SS6 (c) and SS8 (d) reported in Tovignan et al. (2016). Presented data are average value with standard error
In 2013, the soil in the field trial was sandy (86.3%) with low clay (10.2%), low loam (2.6%), low organic matter (0.49%) and low nitrogen (0.30‰) with a slightly alkaline pH (7.8). The experimental site was changed in 2014, but the soil characteristics of the 2 years were very similar. The soil in 2014 was sandy (88.1%) with low clay (7%), low loam (2.33%), low organic matter (0.61%) and low nitrogen (0.35‰) with a slightly alkaline pH (7.38). A split–split-plot design with three replicates was used to study three factors: 2 post-anthesis water regimes, 2 sowing dates and 12 accessions. However, only the first sowing date is taken into account in the present study for the four accessions represented.
Each unit plot comprised three rows 4.4 m in length each, 0.8 m apart, with a distance of 0.4 m between hills in the row. Around 15 days after emergence, the plots were thinned to three plants per hill. The sowing density applied was then 68,182 plants per ha. Fertilization consisted of an application of 150 kg ha−1 of NPK (15-10-10) after sowing. After thinning, 50 kg ha−1 of urea was applied and the same amount was provided during vegetative growth.
Post-anthesis plant leaf area, plant height, grain production and carbohydrates concentration measured at whole stem and internode level
Plant height (cm), panicle dry weight (g) and plant leaf area variation between anthesis and maturity (varPLA) were estimated on three plants per plot for each accession and water treatment as explained in Tovignan et al. (2016).
Stem sugar content was analyzed both years in both water treatments (NI, IR) at physiological maturity (MAT).
Sampling for carbohydrates analysis was performed at grain dough stage. For that, three plants per plot were selected and served to sample three levels of internodes on the main stem (INb: the second elongated internode from the base of the stem, INm: the median internode and INt: the internode below the peduncle), and extractable juice. All these samples were freeze dried in CERAAS (Thiès, Senegal) and carbohydrates content was analyzed using HPLC (CIRAD, Montpellier, France), as described in Gutjahr et al. (2013). Stem internode samples were ground using a mixer Mill MM 200 (Retsch, Germany) to obtain a powder (particle size < 100 µm) that was used for soluble sugar extraction. About 100 mg of powder was dried in the oven during 2 h at 65 °C. Analysis was performed on an aliquot of about 30 mg weighed precisely in a tube (2 mL). Sugars were extracted with 1 mL 80% ethanol for 30 min at 78 °C, then centrifuged (10,000 rpm, 10 min). The supernatant containing sugars was placed in 50 mL graduated flask filled previously with about 20 mL distilled water. The pellet was resuspended in 1 mL of 80% ethanol in same condition as previously, this procedure was carried out three times. At the last extraction the pellet which contains starch was added with 0.5 mL of 80-20 (v/v) ethanol–water medium and then cooled at − 30 °C. The flask was adjusted with distilled water. After homogenization and filtration with PES filter membrane 0.22 µm, the mono and di saccharide contents, total soluble sugars (SST), sucrose (SUC), glucose (GLU), fructose (FRU) were quantified using an HPAEC-PAD chromatography (Dionex, Salt Lake City, UT, USA). The separation was carried out by CarboPack PA1 Column at 30 °C with an isocratic elution of 150 mM sodium hydroxide.
The pellet containing starch was defrosted. A centrifugation (10,000 rpm/min) of tubes was carried out and the ethanol 80% was removed with pipette. Starch was gelatinized with 1 mL 0.02 N sodium hydroxide at 90 °C for 1.5 h and then hydrolysed with α-amyloglucosidase prepared in citrate buffer pH 4.5 at 50 °C for 1.5 h. Glucose produced was quantified as described by Boehringer (1984) using hexokinase and glucose-6-phosphate-dehydrogenase, followed by spectrophotometry of reduced nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm (spectrophotometer UV/VIS Jasco, V-530, Tokyo, Japan). Starch results (STA) were expressed in equivalent glucose.
Data analysis
Post-anthesis water treatment (D), accession (A), organ (O) and year effects as well as their interactions were tested through the variance analysis (ANOVA) and Tukey HSD test for mean comparison were performed using R software version 3.6.1 (R Core Team 2019).
Results
Stem carbohydrates, panicle production, plant height and post-anthesis plant leaf area variation
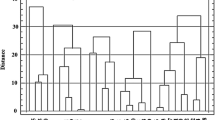
Figure 3 presents plant traits related to carbohydrates concentration in whole stem juice at dough grain stage, plant height, panicle dry weight measured at maturity and post-anthesis green leaf area variation. Table 2 shows the corresponding ANOVA. Sucrose was the most concentrated sugar (71%) in stem juice followed by glucose (19%) and fructose (10%). Among the four accessions, G10 and G7 observed in average the highest total soluble sugars and sucrose concentration in 2013. Total soluble sugars were not affected by drought in any year but sucrose was significantly affected in 2013 and slightly in 2014. By contrast, glucose and fructose were significantly affected by D and A. The interactions D × A were significant except for glucose in 2014. The highest concentrations of glucose and fructose were noted with G10. All traits observed a significant year effect (P < 0.001) except glucose and fructose concentration. Accession G3 had the biggest panicle and panicle dry weight was not affected by drought in any year. D × A was not significant (Fig. 3, Table 2). Post-anthesis plant leaf area variation was significantly affected by drought in both years and differed significantly among accessions only in 2014. D × A was significant in both years and Accession G10 systematically better maintained plant leaf area under drought in both years particularly in 2013 (Table 2, Fig. 3).
Carbohydrates concentration (a glucose, b fructose, c total soluble sugars and d sucrose) in stem juice (g L−1) measured at dough grain stage, e Juice weight at maturity (g), f Plant leaf area variation between anthesis and maturity (%) and g panicle dry weight (g) of the 4 studied accessions (G3, G7, G10, G11) under post-flowering drought (NI) and irrigated (IR) treatments in 2013 and 2014 field trials. * indicates difference between water treatments for a given accession at P < 0.05. Presented data are mean with standard error of three replications
Carbohydrates partitioning among stem internodes
Figure 4 presents the sugar content measured on the four accessions at three internode levels in 2013 and 2014 and Table 3 presents the corresponding ANOVA. Significant differences between water treatments and among internode ranks could be observed both years. Drought systematically decreased total soluble sugars, sucrose and starch and increased fructose and glucose in all internodes; and a decreasing gradient of content from the top to the bottom of the stem. The results showed that the strongest drought effect was observed on the top internode with the highest reduction of the total soluble sugars in both years. Sucrose reduction followed the similar trend as total soluble sugars. Whereas the highest reductions were noted in the top internode for total soluble sugars and sucrose, starch was strongly reduced in the bottom internode in both years. It can be mentioned that starch was in a very small proportion in stem: about 2% of all the stem carbohydrates. Contrary to the previous carbohydrates, the trend of the variation of hexoses was much more an accumulation than a reduction. The highest accumulation was shown in median internode in both years mainly in accession G10.
Carbohydrates (SST: total soluble sugars, SUC: sucrose, GLU: glucose, FRU: fructose and STA: starch) content (mg g−1) compared among stem internodes (INb: bottom, INm: median, INt: top) and water treatments (NI, IR) in 2013 and 2014. Data are average value with standard error of the 4 studied accessions (G3, G7, G10, G11)
Relationships between the sink source ratio and stem carbohydrates variation
The ratio between panicle dry weight and plant leaf area (PDW/PLA ratio), i.e. the amount of panicle dry matter produced per unit of green leaf area, was negatively correlated with the variation of the studied carbohydrates (SST, SUC, GLU and FRU) at the top (INt), median (INm) and bottom (INb) internodes (Fig. 5). The correlation coefficients computed for this relationship decreased gradually from INt to INm to INb for total soluble sugars and sucrose. The hexoses (GLU and FRU) content was similarly decreased at INt and INb internodes depending on PDW/PLA ratio. This decrease of hexoses was more pronounced at the median internodes. It was mainly influenced by panicle dry weight. The bigger the panicle the higher the carbohydrates remobilization from the stem to panicle filling. This way, accessions G3 and G11 with strong PDW/PLA ratio showed the highest reduction of SST, SUC and hexoses. However, G10 with low PDW/PLA ratio which moreover showed a low reduction of PLA between anthesis and maturity (Fig. 3), exhibited a low reduction of SST and SUC and inversely the highest increase of hexoses.
Linear regression between the variation of carbohydrates (between irrigated and non-irrigated treatments) at maturity (Y axis) and the PDW/PLA ratio (X axis) across the 4 studied sweet sorghum accessions and 2 years (2013 and 2014). Data are presented for total soluble sugars (a), sucrose (b), glucose (c) and fructose (d). Correlation coefficients (R) are presented for each internode level. The letters a and b following the accession number represent the 2013 and 2014 field trials respectively
Discussion
The present study aimed at providing further insight into the way post-flowering drought impacts stem sugar accumulation in sweet sorghum by assessing the nonstructural carbohydrates partitioning among internodes along the stem of the studied accessions.
At the whole stem level, quantification of soluble sugars content in the juice of the studied accessions showed sucrose as the most prominent sugars (71%) followed by glucose (19%) and fructose (10%). Similar proportions of soluble sugars (85%, 9% and 6% for sucrose, glucose and fructose, respectively) in sweet sorghum stem juice was reported by Gnansounou et al. (2005). Post-anthesis drought did not affect total soluble sugars in the stem juice but significantly decreased sucrose concentration and inversely increased glucose and fructose concentration. The highest concentrations of glucose and fructose were recorded in the accession G10. Similar trend of variations for sucrose and hexoses was observed for two sorghum varieties (ICSV213, ICSB338) subjected to a post-anthesis drought through a pot experiment (Njokweni et al. 2016).
At the level of the stem internodes, irrespective of water treatment, the present study showed that upper internodes contained less carbohydrates compared to bottom internodes along the stem, suggesting an increasing gradient from the top to the bottom of the stem. This is in line with Gutjahr et al. (2013) who reported similar trend at maturity across five levels of internode along the stem of sweet sorghum and suggested that by sampling internodes at three levels of tall sorghum should provide a good appraisal of this gradient. Post-anthesis drought resulted in a significant reduction of SUC and STA and an increase of hexoses (GLU and FRU) (Fig. 4). These results corroborate Qazi et al. (2014) on sweet sorghum and Iskandar et al. (2011) on sugarcane. The reduction in SUC and STA but the highest accumulation of GLU and FRU should reveal a conversion of the formers into hexoses to contribute to carbohydrates remobilization for panicle filling or to osmotic adjustment of the tissue in stay green genotype (Jagadish et al. 2015).
A negative correlation was observed between PDW/PLA ratio and the variation of the studied carbohydrates (sucrose, hexoses) with the correlation coefficients higher for upper internodes compared to the bottom internode. This suggests a higher reduction of these carbohydrates in the upper internodes. This reduction was mainly influenced by the panicle dry weight. The bigger the panicle the higher the amount of carbohydrates remobilized from the stem to panicle for grain filling. This was mainly shown on accessions G3 and G11 which exhibited low stay green ability. This finding substantiates that of Kouressy et al. (2008) who suggests, on a panel of West African accessions predominantly composed of grain sorghum, that grain yield is more sink- than source-limited.
By contrast, G10 with low PDW/PLA ratio and showing higher stay green ability, exhibited a low reduction of total soluble sugars and sucrose and inversely higher increase of hexoses mainly at the median internode. This ability to better maintain green leaf area and high hexoses in the stem under post-flowering drought could be an osmoregulation mechanism to adapt to drought. Many studies reported higher osmolytes accumulation in stay green sorghum genotypes in response to drought stress (de Souza et al. 2015; Anami et al. 2015; Saeidi and Abdoli 2015; Damame et al. 2016). Particularly, Ghate et al. (2016) comparing the performance of two sweet sorghum near-isogenic lines (NILs) differing from their parental line by stay green trait, showed higher increase in total sugars and reducing sugars at the fifth internode for the NILs compared to the parental line under post-anthesis drought. They also showed higher increase of total sugars in the peduncle only for the NILs and conclude on the stem carbohydrates contribution to panicle filling. Furthermore, these authors linked the carbohydrates remobilization from the stem to the higher accumulation of abscisic acid (ABA) in the leaves and internodes tissues of the stay green NILs (Ghate et al. 2019) and conclude that ABA accumulation induces sugars remobilization to the reproductive sinks under post-flowering drought.
Although the carbohydrates remobilization from the stem may seem inevitable, accumulating a large amount of sugars in the stem before anthesis can minimize losses during grain filling as it was the case in the present study with the genotype G10 (a sweet taller genotype) with the early sowing practiced. This genotype could serve as parent in a breeding program to design dual-purpose sorghum tolerant to drought stress.
Conclusion
This study showed that even if tall sweet photoperiod-sensitive sorghum of West-African origin could remarkably buffer post-flowering drought effect on sweet juice accumulation in the stem, drought affected sugar metabolism and partitioning differently in stem juice, and among internodes along the stem. Our results showed an increasing gradient of carbohydrates from the top to the bottom internodes along the stem. Under post-anthesis drought, the carbohydrates reduction from the stem was mainly influenced by the panicle size. Genotypes with strong panicle exhibiting low stay green ability, highly remobilized the carbohydrates from the stem to panicle for grain filling. The genotype with higher stay green ability showed a low reduction of total soluble sugars and sucrose but a higher increase of hexoses. This high hexoses accumulation suggests a contribution of stay green to osmoregulation processes. Future investigations could explore the genetic variability existing in these adaptive processes toward their genetic study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Almodares A, Taheri R, Adeli S. Inter-relationship between growth analysis and carbohydrate contents of sweet sorghum cultivars and lines. J Environ Biol. 2007;28(3):527–31.
Anami SE, Zhang L-M, Xia Y, Zhang Y-M, Liu Z-Q, Jing H-C. Sweet sorghum ideotypes: genetic improvement of stress tolerance. Food Energy Secur. 2015;4(1):3–24. https://doi.org/10.1002/fes3.54.
Beheshti AR, Behboodi Fard B. Dry matter accumulation and remobilization in grain sorghum genotypes (Sorghum bicolor L. Moench) under drought stress. Aust J Crop Sci. 2010;4(3):185–9.
Blum A, Golan G, Mayer J, Sinmena B. The effect of dwarfing genes on sorghum grain filling from remobilized stem reserves, under stress. F Crop Res. 1997;52:43–54.
Boehringer SA. Methods of enzymatic food analysis: using single reagents. Mannheim: Boehringer Mannheim GmbH; 1984. p. 79.
Borrell AK, Hammer GL, Douglas ACL. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Sci. 2000;40:1026–37.
Burks PS, Kaiser CM, Hawkins EM, Brown PJ. Genomewide association for sugar yield in sweet sorghum. Crop Sci. 2015;55(5):2138–48.
Damame SV, Naik RM, Chimote VP, Munjal SV. Molecular analysis of rabi sorghum genotypes differing in osmolytes accumulation under water stress. Int J Bioresour Stress Manag. 2016;7(5):1120–7.
de Souza AP, Cocuron J-C, Garcia AC, Alonso AP, Buckeridge MS. Changes in whole-plant metabolism during grain-filling stage in Sorghum bicolor L. (Moench) grown under elevated CO2 and drought. Plant Physiol. 2015;169(November):1755–65. https://doi.org/10.1104/pp.15.01054.
Fisher K, Wilson G. Studies of grain production in Sorghum bicolor (L. Moench). I. The contribution of pre-flowering photosynthesis to grain yield. Aust J Agric Res. 1971;22:33–7.
Fu J, Huang B, Fry J. Osmotic potential, sucrose level, and activity of sucrose metabolic enzymes in tall fescue in response to deficit irrigation. J Am Soc Hortic Sci. 2010;135(6):506–10.
Ghate T, Deshpande S, Bhargava S. Accumulation of stem sugar and its remobilisation in response to drought stress in a sweet sorghum genotype and its near-isogenic lines carrying different stay-green loci. Plant Biol. 2016. https://doi.org/10.1111/plb.12538.
Ghate T, Barvkar V, Deshpande S, Bhargava S. Role of ABA signaling in regulation of stem sugar metabolism and transport under post-flowering drought stress in sweet sorghum. Plant Mol Biol Rep. 2019;1–11.
Gnansounou E, Dauriat A, Wyman CE. Refining sweet sorghum to ethanol and sugar: economic trade-offs in the context of North China. Bioresour Technol. 2005;96:985–1002.
Gupta AK, Kaur K, Narinder K. Stem reserve mobilization and sink activity in wheat under drought conditions. Am J Plant Sci. 2011;2:70–7.
Gutjahr S, Clément-Vidal A, Soutiras A, Sonderegger N, Braconnier S, Dingkuhn M, et al. Grain, sugar and biomass accumulation in photoperiod-sensitive sorghums. II. Biochemical processes at internode level and interaction with phenology. Funct Plant Biol. 2013;40:355–68.
Iskandar HM, Casu RE, Fletcher AT, Schmidt S, Xu J, Maclean DJ, et al. Identification of drought-response genes and a study of their expression during sucrose accumulation and water deficit in sugarcane culms. BMC Plant Biol. 2011;11(1):1–14. https://doi.org/10.1186/1471-2229-11-12.
Jagadish KSV, Kavi Kishor PB, Bahuguna RN, von Wirén N, Sreenivasulu N. Staying alive or going to die during terminal senescence—an enigma surrounding yield stability. Front Plant Sci. 2015;6:1–14.
Kouressy M, Dingkuhn M, Vaksmann M, Clément-Vidal A, Chantereau J. Potential contribution of dwarf and leaf longevity traits to yield improvement in photoperiod sensitive sorghum. Eur J Agron. 2008;28(3):195–209.
Kulathunga MRDL. Traits associated for adaptation to water limited environment of cereal crops a review of literature. Int J Sci Technol Res. 2013;2(12):73–81.
Luquet D, Clément-Vidal A, This D, Fabre D, Sonderegger N, Dingkuhn M. Orchestration of transpiration, growth and carbohydrate dynamics in rice during a dry-down cycle. Funct Plant Biol. 2008;35:689–704.
Massoud MI, Abd El-Razek AM. Suitability of Sorghum bicolor L. stalks and grains for bioproduction of ethanol. Ann Agric Sci. 2011;56(2):83–7.
Njokweni A, Ibraheem O, Ndimba B. The role of sucrose metabolizing proteins in hyperosmotic stress tolerance in sweet sorghum (Sorghum bicolor L. Moench). Omi J. 2016;9(3):183–90.
Peloewetse E. Agro-industrial potential of sweet sorghum accessions grown under semi-arid conditions. Afr J Biotechnol. 2012;11(49):10970–5.
Promkhambut A, Polthanee A, Akkasaeng C, Younger A. Growth, yield and aerenchyma formation of sweet and multipurpose sorghum (Sorghum bicolor L. Moench) as affected by flooding at different growth stages. Aust J Crop Sci. 2011;5(8):954–65.
Qazi HA, Paranjpe S, Bhargava S. Stem sugar accumulation in sweet sorghum—activity and expression of sucrose metabolizing enzymes and sucrose transporters. J Plant Physiol. 2012;169(6):605–13.
Qazi HA, Srinivasa Rao P, Kashikar A, Suprasanna P, Bhargava S. Alterations in stem sugar content and metabolism in sorghum genotypes subjected to drought stress. Funct Plant Biol. 2014;41(9):954–62. https://doi.org/10.1071/FP13299.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing;. 2019.
Rebolledo MC, Luquet D, Courtois B, Henry A, Soulié JC, Rouan L, et al. Can early vigour occur in combination with drought tolerance and efficient water use in rice genotypes? Funct Plant Biol. 2013;40(6):582–94.
Rutto LK, Xu Y, Brandt M, Ren S, Kering MK. Juice, ethanol, and grain yield potential of five sweet sorghum (Sorghum bicolor [L.] Moench) cultivars. J Sustain Bioenergy Syst. 2013;3(2):113–8.
Saeidi M, Abdoli M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J Agric Sci Technol. 2015;17:885–98.
Sasaki T, Baltazar AA. Plant genomics: sorghum in sequence. Nature. 2009;457:547–8.
Slewinski TL. Non-structural carbohydrate partitioning in grass stems: a target to increase yield stability, stress tolerance, and biofuel production. J Exp Bot. 2012;25:1–24.
Tovignan TK, Fonceka D, Ndoye I, Cisse N, Luquet D. The sowing date and post-flowering water status affect the sugar and grain production of photoperiodic, sweet sorghum through the regulation of sink size and leaf area dynamics. F Crop Res. 2016;192:67–77.
van Oosterom EJ, Chapman SC, Borrell AK, Broad IJ, Hammer GL. Functional dynamics of the nitrogen balance of sorghum. II. Grain filling period. F Crop Res. 2010;115:29–38.
Zou G, Yan S, Zhai G, Zhang Z, Zou J, Tao Y. Genetic variability and correlation of stalk yield-related traits and sugar concentration of stalk juice in a sweet sorghum (Sorghum bicolor L. Moench) population. Aust J Crop Sci. 2011;5(10):1232–8.
Acknowledgements
The authors are also grateful to Mathieu AYENA for his critical review of the manuscript.
Funding
This study was funded by the West Africa Agricultural Productivity Program (WAAPP, Senegal). The authors are thankful to the Francophone University Agency (AUF) for supporting the short-term mobility of the first author for the biochemical analysis in Montpellier (France).
Author information
Authors and Affiliations
Contributions
TKT, NC, DL conceived and designed the study; TKT, CD, AC and AS performed the research; TKT and DL analyzed the data and wrote the manuscript; HA and CD contributed to the results interpretation and provided a critical review on the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tovignan, T.K., Adoukonou-Sagbadja, H., Diatta, C. et al. Terminal drought effect on sugar partitioning and metabolism is modulated by leaf stay-green and panicle size in the stem of sweet sorghum (Sorghum bicolor L. Moench). CABI Agric Biosci 1, 4 (2020). https://doi.org/10.1186/s43170-020-00003-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43170-020-00003-w