Abstract
Background
The predictive value of the new imaging sequences, especially T2 mapping in assessment of articular cartilage abnormalities of the medial knee compartments in patients with medial knee pain. The purpose of this study is to evaluate the additional value of T2 mapping over using a baseline standard knee MRI to detect cartilage lesions of the medial compartments in patients representing with medial knee pain.
Results
The study included 60 patients presented with medial knee pain, where divided into two groups ; control group (20 volunteers) with age range from 19 to 41 years old 26.80 ± 8.05 (mean ± SD) and patients (40 candidates) with age range from 13 to 57 years old with a mean age 33.00 ± 14.1 (mean ± SD).
Conclusion
On adding T2 mapping sequence to the routine MRI of the knee, the sensitivity for detecting knee cartilage lesions was increased, especially in the detection of early cartilage degeneration at the medial compartment.
Compositional MR imaging including T2 mapping plays an important role in the assessment of early and potentially reversible cartilage damage especially among the young population.
Similar content being viewed by others
Background
The articular cartilage is one of the most important structures involved in a degenerative joint disease like osteoarthritis (OA). Magnetic resonance imaging (MRI) is the imaging modality of choice for the diagnostic of the knee articular cartilage defect. Conventional MRI sequence can detect morphologic changes which normally occur in an advanced stage of OA whereas T2 and T2 mapping is sensitive to structural and biochemical changes in the extracellular cartilage matrix.
Both T2 and T2 can detect biochemical changes in the cartilage which occurs in the early stage of OA like the change of collagen orientation, the collagen content and tissue hydration [1].
Cartilage loss and clinical symptoms are preceded by collagen–proteoglycan matrix damage and elevated cartilage water content. Therefore, a sensitive technique for detecting these structural and functional changes during the early stages of osteoarthritis could be valuable for identifying the need for early treatment, monitoring response to treatment, and assessing efforts to prevent disease progression [2].
Magnetic resonance imaging (MRI) allows proper visualization of all tissues involved in the process including articular cartilage. In addition to qualitative or quantitative morphological assessment.
MRI-based techniques have been developed that allow characterization and quantification of the biochemical components of cartilage. These include relaxometry measurements (T2, T2 mapping, T1rho mapping, and T1), sodium imaging, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), glycosaminoglycan-specific chemical exchange saturation transfer (gagCEST), diffusion-weighted imaging (DWI), and diffusion tensor imaging (DTI). These compositional MRI techniques may have the potential to serve as quantitative, reproducible, non-invasive, and objective endpoints for arthritic changes, especially in early and pre-radiographic stages of the disease
Nowadays, non-surgical and surgical techniques exist with a goal to maintain and improve cartilage health and based on clinical evaluation of pain symptoms and subjective outcomes scoring. It appears that these preventative and repair strategies experience some success and goals [2].
Cartilaginous knee lesions are a frequent condition in young patients, can cause both pain and functional impairment, and consequently osteoarthritis.
The identification of early cartilage degeneration in symptomatic patients undergoing conventional standard magnetic resonance (MR) imaging of the knee joint is clinically important [3].
The macroscopic evaluation of cartilage repair according to the International Cartilage Repair Society (ICRS) classification (grade 0 normal, grade 1 nearly normal (soft indentation and/or superficial fissures and cracks, grade 2 abnormal (lesions extending down to < 50% of cartilage depth), grade 3 severely abnormal (cartilage defects > 50% of cartilage depth), grade 4 severely abnormal (through the subchondral bone) was compared to the MRI staging and showed accuracy for deep chondral defects (accuracy superior to 90% for the grades III and IV of the ICRS classification), but is less sensitive to the biochemical changes associated with early OA for ICRS grade I and II lesions. This means that morphological MRI shows chondral damage at a stage when cartilage is already irreversibly lost [4, 5].
In the early phase of osteoarthritis, elevated cartilage T2 values as well as cartilage damage have been shown to be associated with findings of pain.
It has also been hypothesized that a focal prolongation of the cartilage T2 value could be linked to focal cartilage damage, as foci of increased T2-weighted signal intensity are visible in such lesions on conventional T2 sequences [6, 7].
The conjunction of the anatomical and functional information may augment our ability to detect early cartilage degeneration and to differentiate between different stages of degeneration [6, 8].
The purpose of this study is to evaluate the additional value of T2 mapping over using a baseline standard knee MRI to detect cartilage lesions of the medial compartments in patients representing with medial knee pain.
Methods
The current study is a prospective analysis that was conducted during the period from June 2017 to June 2019, to rule out the preciseness of using T2 mapping when added to the conventional MRI protocol of the knee joint for patients complaining of medial knee pain.
The ethics commission of our institution approved this study and written informed consent for the present study from all patients prior to enrollment was obtained.
Patients
The study includes 60 persons: 24 females and 36 males with age range from 13 to 57 years old, divided into two groups:
Sample study group A
This includes 40 patients complaining of medial knee joint pain and not accurately diagnosed clinically to have established osteoarthritis during the period of the study.
Control group B
This includes 20 normal volunteers not complaining of knee joint pain.
Inclusion criteria
-
1.
Medial knee pain of at least one-month duration not clinically diagnosed to have established osteoarthritis.
-
2.
Normal radiographs for knee joint.
Exclusion criteria
-
1.
Patients with deformities, mal-alignment of the lower extremity, knee injury within the previous 3 months.
-
2.
History of knee surgery or arthroscopy was not included in the study.
-
3.
Patients contraindicated for MRI, e.g., cardiac pacemaker.
-
4.
Overweight, more than 150 kg (cannot fit in the MRI machine).
-
5.
Patients suffering from claustrophobia.
All patients were submitted to the following
-
1.
Demographic and clinical data collection including patient’s name, age, residence, phone number, complaint, duration of illness, and past history.
-
2.
Clinical provisional diagnosis.
-
3.
Imaging procedure.
As each patient had a single MRI examination including conventional MR routine protocol and added a single complementary T2 mapping, 60 MRI examinations and 60 corresponding complementary T2 mapping were analyzed.
MRI study
MRI imaging technique
MR examinations were performed on a 1.5 T whole body MR scanner (GE health care MAGNETOM Aera 1.5 T), using an eight-channel knee array coil. During the MR-scan, patients will be in supine position, feet first with full limb extension.
Patient preparation
Before the examination, patients were routinely questioned about any contraindication for MRI examination and instructed to remove any metal objects.
Patient positioning:
Technically, T2 mapping sequence is easy to acquire and does not require any knee re-positioning. The knee flexion angle was aligned 15°, and a dedicated holder was used to reduce motion artifacts at the time of imaging.
Protocol of MR imaging: Table 1
Preliminary scout localizers in axial, coronal, and sagittal planes were done. The axial images serve as a localizer for prescribing the coronal and sagittal sections.
The coronal sections are graphically prescribed on an axial image from the patella to the posterior surfaces of the femoral condyles. The planes is oriented parallel to the anterior/posterior surfaces of the femoral condyles.
The sagittal sections are graphically prescribed from the lateral to the medial collateral ligament and aligned parallel with the anterior cruciate ligament.
The coverage should include all the anterior, posterior, medial, and lateral supporting structures of the knee. Superiorly, the distal aspects of the quadriceps tendon should be included. The distal insertions of the patellar tendon should be included inferiorly.
The standard knee protocol (sagittal proton density-fat suppressed, coronal proton density-fat suppressed, axial T2-weighted image, sagittal T1-weighted image, and sagittal T2-weighted image) was done.
Complementary sagittal and axial T2 maps were displayed by using the available software tools provided by the MR scanner manufacturer.
Image interpretation and analysis
Firstly, the standard imaging planes of the knee were evaluated with assessment of articular cartilage as intact or thinned out. Thereafter, these standard planes were evaluated together with T2 maps, and articular cartilage was again assessed as of normal or elevated T2 signal in terms of milliseconds.
The medial knee joint cartilage was assessed according to the following regional compartments: 1—medial patellar, 2—medial trochlear, 3—central medial femoral, 4—peripheral medial femoral, 5—central medial tibial, 6—peripheral medial tibial.
An intact articular cartilage
-
○ On conventional MRI was the one with uniform thickness and bright hyper-intense signal on PDFS sequence (sagittal and axial) covering tibial, femoral, and patellar articular surfaces. Figure 1a–c shows bright hyper-intense signal of cartilage at medial (1a) and lateral (1b) tibio-femoral condyles and patellofemoral (1c) with uniform cartilage thickness (arrows).
-
○ On T2 maps was the one with normal T2 values not exceeding 40 ms delineated by color-coded map (red, yellow, or green) represented on a color coded scale with upper limit of 100 ms. Figure 1d–f shows normal color-coded values of cartilage (superficial and deep zones normal (red, yellow, or green)) at both medial and lateral tibiofemoral condyles and patellofemoral. ICRS grade 0–1 (arrows).
Bright hyper-intense signal of cartilage at medial (a) and lateral (b) tibio-femoral condyles and patellofemoral (c) with uniform cartilage thickness (arrows). d–f Normal color coded values of cartilage (superficial and deep zones normal (red, yellow, or green)) at both medial and lateral tibiofemoral condyles and patellofemoral. ICRS grade 0–1 (arrows)
Affected articular cartilage
-
○ On conventional MRI in early stages of osteoarthritis appears in PDFS sequence as an area with non-uniform thickness or of altered signal intensity.
-
○ On T2 maps in early osteoarthritis can be delineated as it takes a certain color (aqua or blue) corresponding to high T2 value on the color coded scale found in the scanner software.
-
○ The hyaline cartilage lesions (on T2 maps) were graded according to an ICRS-like score (inspired by the International Cartilage Research Society score) in each anatomical compartment (medial femoro-tibial and medial patello-femoral joints), the T2 value of the severest lesion was the one considered.
-
○ No degeneration: (grade 0–1): superficial and deep zones normal (red, yellow, or green). Figure 2 shows preserved bright hyper-intense signal of cartilage covering the lateral tibio-femoral condyle (arrow) (Fig. 2a) and normal color-coded values of cartilage (superficial and deep zones normal (red, yellow, or green)) covering the lateral tibiofemoral condyle ICRS grade 0–1 (arrow) (Fig. 2b).
-
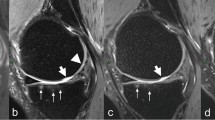
○ Grade 2 degeneration: superficial zone aqua and deep zone is normal (red, yellow or green). Figure 3 shows preserved bright hyperintense signal of cartilage covering the medial tibiofemoral condyle with average uniform cartilage thickness (arrow) (Fig. 3a), elevated color-coded values of cartilage (superficial zone (aqua), and deep zone is normal (red, yellow, or green)) covering the medial tibio-femoral condyle ICRS grade 2 (arrow) (Fig. 3b).
-
○ Grade 3 degeneration: superficial zone aqua or blue and deep zone aqua or blue with no associated bone degeneration. Figure 4 shows decreased bright hyperintense signal of cartilage covering the medial tibiofemoral condyle with decreased cartilage thickness (arrow) (Fig. 4a). Elevated color-coded values of cartilage (superficial zone aqua/blue and deep zone aqua/blue with no associated bone degeneration) covering the medial tibiofemoral condyle ICRS grade 3 (arrow) (Fig. 4b).
-
○ Grade 4 degeneration: superficial zone aqua or blue and deep zone aqua or blue with associated bone degeneration (Fig. 5). Decreased bright hyperintense signal of cartilage covering the medial tibiofemoral condyle with denudation and decreased cartilage thickness (star) (Fig. 5a), while on T2 mapping, elevated color-coded values of cartilage (superficial zone aqua/blue and deep zone aqua/blue with associated bone degeneration) covering the medial tibio-femoral condyle ICRS grade 4 (arrow) (Fig. 5b).
-
❖T2 mapping findings were compared to the initial standard sequences.
-
● Any associated meniscal, ligamentous, muscular, or bony abnormalities in the knee were reported.
Preserved bright hyperintense signal of cartilage covering the medial tibiofemoral condyle with average uniform cartilage thickness (arrow) (a). Elevated color-coded values of cartilage (superficial zone (aqua) and deep zone is normal (red, yellow, or green)) covering the medial tibio-femoral condyle ICRS grade 2(arrow) (b)
Decreased bright hyperintense signal of cartilage covering the medial tibiofemoral condyle with decreased cartilage thickness (arrow) (a). Elevated color-coded values of cartilage (superficial zone aqua/blue and deep zone aqua/blue with no associated bone degeneration) covering the medial tibiofemoral condyle ICRS grade 3 (arrow) (b)
Decreased bright hyperintense signal of cartilage covering the medial tibiofemoral condyle with denudation and decreased cartilage thickness (star) (a), while on T2 mapping, elevated color-coded values of cartilage (superficial zone aqua/blue and deep zone aqua/blue with associated bone degeneration) covering the medial tibio-femoral condyle ICRS grade 4 (arrow) (b)
Statistical analysis
Data was analyzed using SPSS win statistical package version 21 (SPSS Inc., Chicago, IL). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For quantitative data, a comparison between two groups was done using either Student’s t test or Mann-Whitney test (non-parametric t test) as appropriate. A p value of less than 0.05 was considered significant. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of T2 mapping for deepening and confirming on the diagnosis of articular cartilage early arthritic changes according to different sites of assessment were calculated.
Results
The included 60 patients 40 patients presenting with pain and 20 without pain as control. All 60 cases performed conventional MRI knee and complementary sagittal and axial T2 mapping.
the study population were divided:
According to the T2 mappings findings at different sites: medial patella (Table 2), medial trochlea (Table 3), medial (peripheral and central) femoral (Table 4), medial (peripheral and central) tibial (Table 5).
According to the p value, we declared that the significance was postulated and declared at the medial femoral showed distribution of central medial and peripheral femoral degeneration according to ICRS degeneration grades (Figs. 6 and 7) and tibial where distribution of central and peripheral medial tibial degeneration according to ICRS degeneration grades (Figs. 8 and 9) sites.
Discussion
Compositional MR imaging including T2 mapping play an important role in the assessment of early and potentially reversible cartilage damage [1, 9].
The main clinical application for T2 mapping involves the detection of early cartilage damage prior to the onset of symptoms and prior to detection using conventional screening techniques [1, 10].
Combining the anatomical and functional information may enhance our ability to detect early cartilage degeneration, and to distinguish between different stages of degeneration [8].
T2 mapping has been used to describe the composition of hyaline articular cartilage in the knee joint on the basis of collagen structure and hydration. Analyses of T2 relaxation times in the knee have been performed, usually at 1.5T or, more recently, 3.0 T, demonstrating the ability to depict abnormalities before there is evident morphologic change [1, 11].
The results of this study have the potential to deepen our information about the role of compositional imaging T2 mapping sequence in detection of stages of medial knee compartments articular cartilage degeneration beyond the usage of descriptive statistics.
Sensitivity and specificity of conventional techniques, and the absence of a defined threshold to classify OA, have been criticized in previous studies. However, our results show there may be more information.
Compared with other quantitative MRI techniques, T2 mapping has the advantage that it can be performed non-invasively without the injection of contrast agents; also, this sequence and its post-processing software are available in many commercial MRI scanner systems [11, 12].
In our study, the addition of a T2 mapping sequence to a routine MR imaging protocol significantly increased the sensitivity for detecting cartilage lesions within the knee joint, with the greatest improvement occurring in the identification of early cartilage degeneration. Our low sensitivity for detecting early cartilage degeneration by using the morphologic cartilage imaging sequences in the routine MR protocol is similar to the findings of previous studies, which have reported sensitivity values for detecting cartilage fibrillation and superficial partial-thickness cartilage defects
The study showed significant differences in T2 mapping ability to detect degree of cartilage degeneration between the two study groups according to ICRS degeneration grades in the weight-bearing regions of the knee joint (medial patella and trochlea, peripheral medial femoral and tibial, central and peripheral medial femoral and tibial regions).
This agreed with Zhang, et al. [13] for the patellar articular cartilage early changes as well as Apprich, et al. [14] for the medial femoral condyle, where T2 mapping appears to be sensitive to early cartilage changes, but also to the loading state of the joint. Even changes not yet visible in high-resolution MRI might be detectable with quantitative T2 mapping.
Furthermore Hesper et al. [15], who reported that the potential value of T2 mapping as a biomarker for early cartilage degeneration is highlighted by its inclusion in the MRI protocol for the early osteoarthritis.
Advanced quantitative MRI techniques such as T2 mapping are sensitive to subtle cartilage matrix alterations that occur early in the course of OA and therefore, these imaging modalities have the potential to provide biomarkers for disease onset and progression, which could be a meaningful addition in the diagnosis and follow-up of cartilage abnormalities [11, 15].
A study by Hesper et al. [15] concluded that “biochemically sensitive” MRI techniques as T2 mapping are able to add robust biomarkers for osteoarthritis onset and progression with varying degrees of sensitivity and specificity, and therefore could be meaningful assessment tools for the diagnosis and follow-up of cartilage abnormalities.
In our study, the age of presentation of the cases ranged from 13 to 57 years old. Out of the 20/30 (66%) cases presented by knee pain, 15% were anterior 35% medial, 15% lateral, and 35% global. All cases underwent conventional MRI with complementary T2 mapping sequence
T2 mapping despite all the enumerated advantages, and its contribution to enhancing cartilage status assessment, is still in its infancy, as its values are based on several factors, including the biochemical status of the cartilage, physics variables involved in obtaining the mapping, and the physiology of the individual patient. So, the current literature still lacks clinical correlation [4].
A study by Mars, et al. and Zhang, et al. [10, 13] reported that the potential clinical applications of quantitative mapping are vast, but, before the clinical community can take full advantage of this tool, it must be automated, standardized, validated, and have proven reproducibility prior to its implementation into the standard clinical care routine.
T2 mapping may provide the basis for diagnosis and follow-up evaluation of cartilage injury and response to cartilage treatment and repair. Once a set of standardized protocols are developed and proposed, subsequent studies are needed to determine specific values or distribution of values, which are associated with healthy and damaged cartilage [10, 13].
Pedoia et al. and Hesper et al. [12, 15] added that much remains to be understood and certain points have become apparent with the studies that are crucial to the further applications of T2 mapping technique, also added that further studies are needed to address protocol issues for this technique.
T2 mapping has proven to be a useful predictor of cartilage degeneration and repair tissue following surgical intervention in the knee [10].
Surgical approaches to repair focal cartilage damage were introduced several years ago and have shown promising results, but long-term observation of any of the repair techniques still have not been reported. The most commonly applied techniques are microfracture, osteochondral transfer, and autologous chondrocyte implantation [9].
Limitations
The limitation of this study is the lack of histological information or arthroscopic correlation. Further investigations in larger patient groups with histologic correlation would be beneficial in comparing T2 and T2 mapping values. Also, studies in larger number of patients with different age and activity levels are needed to quantify correctly these biochemical mapping methods.
Conclusion
On adding T2 mapping sequence to the routine MRI of the knee, the sensitivity for detecting knee cartilage lesions was increased, especially in the detection of early cartilage degeneration at the medial compartment.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- bSSFP:
-
Balanced steady-state free precession
- dGEMRIC:
-
Delayed gadolinium-enhanced MRI of cartilage
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- DEFT:
-
Driven equilibrium Fourier transform
- DESS:
-
Dual-echo steady state
- ECM:
-
Extracellular matrix
- FIESTA:
-
Fast imaging employing steady-state acquisition
- FLASH:
-
Fast low angle shot
- FSE:
-
Fast SE
- FS:
-
Fat saturation
- FOV:
-
Field of view
- FEMR:
-
Fluctuating equilibrium MRI
- GdDTPA:
-
Gadolinium diethylenetriaminepentaacetic acid
- GAGs:
-
Glycosaminoglycans
- GRE:
-
Gradient-recalled echo
- ICRS:
-
International cartilage repair society
- MR:
-
Magnetic resonance
- OA:
-
Osteoarthritis
- PGs:
-
Proteoglycans
- PD:
-
Proton density
- RF:
-
Radio frequency
- STIR:
-
Short tau inversion recovery
- SNR:
-
Signal to noise ratio
- SE:
-
Spin echo
- SPGR:
-
Spoiled gradient echo sequence
- SSFP:
-
Steady-state free precession
- STZ:
-
Superficial Tangential Zone
- 3D GRE:
-
Three dimension gradient-recalled echo
- 3D SE:
-
Three dimension spin echo
- True-FISP:
-
True fast imaging with SSFP
References
Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW (2015) Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr Cartil 23:1639–1653. https://doi.org/10.1016/j.joca.2015.05.026
Surowiec RK, Lucas EP, Ho CP (2014) Quantitative MRI in the evaluation of articular cartilage health: reproducibility and variability with a focus on T2 mapping. https://doi.org/10.1007/s00167-013-2714-6
Felson D (2006) Osteoarthritis of the knee. N Engl J Med 354(8):841–848
Dautry R, Bousson V, Manelfe J, Perozziello A, Boyer P, Loriaut P, Koch P, Silvestre A, Schouman-Claeys E, Laredo JD, Dallaudière B (2014) Correlation of MRI T2 mapping sequence with knee pain location in young patients with normal standard MRI. JBR–BTR 97(1):11–16
LaPrade RF, Cram TR, Mitchell JJ, Geeslin AG, Lockard CA, Fitzcharles EK, Dornan GJ (2018) Axial-oblique versus standard axial 3-t magnetic resonance imaging for the detection of trochlear cartilage lesions: a prospective study. Orthop J Sports Med 6(10):2325967118801009. https://doi.org/10.1177/2325967118801009
Colotti R, Omoumi P, Bonanno G, Ledoux J-B, van Heeswijk RB (2017) Isotropic three-dimensional t2 mapping of knee cartilage: development and validation. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25755
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage : Influence of aging and early symptomatic degeneration on the spatial variation of T2-preliminary findings at 3T. Radiology. 214:259–266 [PubMed]
Poh CL, Sheah K (2009) Visualization of articular cartilage using magnetic resonance imaging data. ICBME 2008, Proceedings, vol 23, pp 386–389
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F et al (2011) Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 31:37–61 [PubMed]
Mars M, Chelli M, Tbini Z, Ladeb F, Gharbi S (2018) MRI T2 mapping of the knee articular cartilage using different acquisition sequences and calculation methods at 1.5 Tesla. Med Princ Pract. https://doi.org/10.1159/000490796
Sveinsson B, Chaudhari AS, Gold GE, Hargreaves BA (2017) A simple analytic method for estimating T2 in the knee from DESS. Magn Reson Imaging 38:63–70. https://doi.org/10.1016/j.mri.2016.12.018
Pedoia y z V, Lee y J, Norman y a B, Link y TM, Majumdar S (2019) Diagnosing osteoarthritis from T2 maps using deep learning: an analysis of the entire Osteoarthritis Initiative baseline cohort. Osteoarthr Cartil 27:2002–2010. https://doi.org/10.1016/j.joca.2019.02.800
Zhang N, Lv Y, Liu Y, Yin G, Hu D, Runhui YP (2018) T2 mapping in the quantitative evaluation of articular cartilage changes in children with hemophilia: A pilot study. Wiley. https://doi.org/10.1002/ped4.12099
Apprich S, Mamisch TC, Welsch GH, Stelzeneder D, Albers C, Totzke U, Trattnig S 2011(2012) Quantitative T2 mapping of the patella at 3.0 T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 81:e438–e443. https://doi.org/10.1016/j.ejrad.2011.03.069
Hesper T, Hosalkar HS, Bittersohl D, Welsch GH, Krauspe R, Zilkens C, Bittersohl B (2014) T2* mapping for articular cartilage assessment: principles, current applications, and future prospects,# ISS 2014
Acknowledgements
The authors really appreciate the role of the Kasr El-Ainy Institute and Hospital as well as Nile Scan Radiology Center for providing the authors with the required type of patients and the help of reaching optimum diagnose for the proper management service.
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
NE gave idea and collected the patients’ data and analyze them. HK put study design and followed up the patients postoperatively. NE wrote the paper with revision. They all approved the final version of the manuscript. I did intend to submit the manuscript to the ‘Egyptian Journal of Radiology and Nuclear Medicine,’ and that it is not currently under consideration elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine at Cairo University in Egypt on 201572016 in compliance with Helsinki Declaration (DoH-oct20081)
All patients included in this study gave written informed consent to participate in this research. If the patient was less than 16 years old or unconscious at the time of the study, written informed consent for their participation was given by their parent or legal guardian.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was less than 16 years old, deceased, or unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their parent or legal guardian.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Liethy, N.E., Kamal, H.A. Advanced compositional imaging T2 mapping sequence in detection of stages of medial knee joint compartments articular cartilage degeneration. Egypt J Radiol Nucl Med 52, 46 (2021). https://doi.org/10.1186/s43055-020-00395-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00395-9