Abstract
Background
This paper describes a blind randomized controlled trial (RCT) designed to evaluate the effect of gonadotropin-releasing hormone agonist (GnRH-a) administration on outcomes of intracytoplasmic sperm injection (ICSI) in subjects stimulated with the gonadotropin-releasing hormone (GnRH) antagonist protocol. A total of 268 women who underwent ICSI cycles with GnRH antagonist ovarian stimulation protocol were included in the study. Patients were randomly assigned to the intervention (GnRH-a) and control groups. The intervention group received a single dose injection of triptorelin (0.1 mg) subcutaneously 6 days after oocyte retrieval while the control group received placebo. The rates of chemical and clinical pregnancy were defined as the primary outcome values.
Results
Two hundred forty participants accomplished the study, and their data were analyzed. No significant difference was detected between the chemical pregnancy rates of the intervention and control groups. However, the clinical pregnancy rate was significantly higher in the GnRH-a group than in the placebo group.
Conclusions
The findings of the present study suggest that the GnRH-a support in the luteal phase can result in a significant improvement of pregnancy rates in ICSI cycles following the ovarian stimulation with GnRH antagonist protocol.
Similar content being viewed by others
Background
Stimulating the development of multiple follicles and, eventually, the formation of multiple embryos is achieved by controlled ovarian stimulation (COS) in assisted reproductive techniques (ARTs) [1]. Induction of multiple follicles in the COS procedure causes forming multiple corpora lutea which leads to abnormally increasing high estradiol production [1, 2]. The continuously high levels of estradiol inhibit the luteinizing hormone (LH) secretion by hypophysis. This effect shortens the luteal phase and causes premature luteolysis that can lead to decreased pregnancy rates [3, 4]. In this regard, the supporting medications including estradiol, progesterone, and hCG are prescribed as luteal phase support (LPS) in ART cycles [5]. However, trials investigating the efficacy of steroid combinations revealed contradictory results, and the human chorionic gonadotropin (hCG) carries the risk of ovarian hyperstimulation syndrome (OHSS) [6]. Therefore, researches are in progress for finding luteal phase support with fewer side effects.
Administration of gonadotropin-releasing hormone agonist (GnRH-a) has been introduced recently as a beneficial luteal phase support [7, 8]. Recent studies have shown the positive effect of a single-dose GnRH-a administration on the luteal phase in ICSI cycles [9,10,11,12,13]. GnRH-a administration in the mid-luteal phase has been detected to increase the efficacy of implantation and live birth rates in women undergoing ICSI [4, 14]. However, the effect of GnRH-a administration as luteal phase support on the pregnancy outcomes remains controversial [12]. Despite existing, several reports on the effectiveness of GnRH-a administration as a luteal phase support protocol, there are still several questions which need to be answered.
In this regard, the present randomized controlled trial (RCT) aimed to assess the effect of GnRH-a administration on ICSI outcomes in the luteal phase of cycles stimulated by the gonadotropin-releasing hormone (GnRH) antagonist protocol.
Methods
Study population
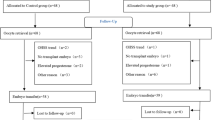
Between 2017 and 2019, 268 ICSI candidate patients were assessed for their eligibility to enter the study. Written informed consents were obtained from all patients. In total, 28 patients were excluded for different reasons, and 240 stayed included in the study (Fig. 1).
Age (between 20 and 38 years old), body mass index (BMI) below 30 kg/m2, and serum FSH level below 10 mIU/ml on the 2nd or 3rd days of the menstrual cycle were considered as inclusion criteria. Low response according to Bologna criteria, repeated implantation failure (RIF), polycystic ovary syndrome (PCOS), endometriosis, uterine abnormalities, hormonal disorders, OHSS, failed fertilization, less than two embryos available for transfer, male factor infertility, and non-adherence to the interventions were considered as exclusion criteria.
Study design
This was a single-center, double-blind, controlled trial with balanced randomization, which was conducted in the IVF Center of Taleghani Hospital, Tehran, Iran. Patients were randomly assigned to one of two groups including the GnRH agonist group or the placebo group. Patients, physicians, outcome assessors, and statisticians were kept blinded about randomization. Randomization was carried out using computer-generated simple random tables in a 1:1 ratio. The sample size was determined after consideration of type 1 statistical error < 5% and type 2 statistical error < 20%.
Treatment protocol
Patients were pretreated with 2 mg of oral estradiol valerate (Aburaihan Co., Tehran, Iran) twice daily from day 21 of the natural cycle continued until days 2–4 of the new cycle. Stimulation was started by daily injections of (150–225 IU) of recombinant FSH (Gonal F, Merck, Germany) from the second or third day of the cycle. The dose was adjusted according to the follicular growth that was monitored by transvaginal ultrasonography. GnRH antagonist (Cetrotide, Merck, Germany) administration was done when the leading follicles reached 13–14 mm in diameter and continued until the day of ovulation induction. When the diameter of three or more follicles reached 17 mm, 10,000 IU of hCG (Choriomon, IBSA, Switzerland) was administered as the ovulation induction. Thirty-six hours later, a transvaginal ultrasound-guided oocyte pick-up (OPU) was carried out.
Serum level of estradiol (E2), progesterone (P), and LH were measured by enzyme-linked immunosorbent assay (ELISA) kit, and endometrial thickness and pattern were assessed by transvaginal ultrasonography on the day of trigger (Table 1). Endometrial thickness was defined as the maximal distance in the plane between the echogenic interfaces of the endometrium and myometrium through the central longitudinal axis of the uterus. Endometrial pattern was classified as pattern A (a triple-line pattern consisting of a central hyperechoic line surrounded by two hypoechoic layers), pattern B (an intermediate isoechogenic pattern with the same reflectivity as the surrounding myometrium and a poorly defined central echogenic line), and pattern C (homogenous, hyperechogenic endometrium) [15].
Assisted reproduction techniques
After OPU, oocytes were washed in a handling medium (MHM™, Irvine Scientific, USA) and cultured in a culture medium (Sage, Cooper Surgical, USA) at 37 °C with 6% CO2 for 2 h prior to denudation. The oocytes were then denuded from granulosa cells by mechanical and enzymatic dissection using hyaluronidase (GM501 Hyaluronidase, Gynemed, Germany). ICSI was performed on all metaphase II oocytes 3–4 h after OPU. The injected oocytes were cultured in a cleavage medium (Sage, Cooper Surgical, USA) and evaluated by morphologic criteria on day 3. Embryos with at least six blastomeres and < 20% fragmentation were selected for embryo transfer (ET). The embryo transfers (ETs) were carried out 3 days after OPU with an embryo transfer catheter (Cook, USA) by an expert gynecologist under the trans-abdominal ultrasound guidance according to the American Society for Reproductive Medicine (ASRM) guidelines. According to the quality of the embryos and the condition of the females, 1–2 fresh embryos were transferred in each cycle (Table 1).
Luteal phase supplementation
After ovulation induction, the intervention stage started for patients in two groups. The intervention group received 0.1 mg of triptorelin (Decapeptyl, Ferring, Germany) by subcutaneous (SC) injection 6 days after oocyte retrieval. The control group received the placebo. All patients received 400 mg of vaginal progesterone (Cyclogest, Actavis, Barnstaple, UK) twice daily beginning from the day of oocyte retrieval until the 12th week of pregnancy.
Outcome assessment
Chemical and clinical pregnancies were detected by positive serum β-hCG 2 weeks after ET and the presence of a fetal heartbeat in transvaginal ultrasound 6 weeks after ET, respectively. Multiple pregnancies were defined as a gestation with more than one fetus in ultrasonography.
Statistical analysis
The results were provided as mean ± SD. Statistical analysis was conducted using the SPSS 21.0 statistical software package (SPSS Inc., Chicago, IL, USA). Student’s t test, chi-squared test, and exact test were used for comparing the study groups. The p < 0.05 was considered as statistically significant.
Results
Baseline characteristics
A total of 268 patients fulfilled the inclusion criteria and were enrolled in this study, from which 240 participants accomplished the study and their data were analyzed (Fig. 1). Table 2 provides a summary of the baseline characteristics of the two groups. No significant differences were detected in the baseline characteristics of the two groups.
Outcomes
No significant differences were detected in the chemical pregnancy rates between the two groups (40% vs. 41.7%, p value = 0.797). However, the clinical pregnancy rate was significantly higher in the intervention group compared to the placebo group (36.7% vs. 25.8%, p value = 0.0089). No significant difference was observed between the two groups in terms of multiple pregnancies (p value = 0.827) (Table 3).
Discussion
The present study was undertaken to evaluate the effect of a single dose administration of triptorelin as a LPS medication on pregnancy outcomes in ICSI candidate women. Our results showed no significant difference in the chemical pregnancy rates between the two groups. However, the clinical pregnancy rate was significantly higher in the group which received triptorelin. On the other hand, no significant difference was observed in multiple pregnancy rates between the two groups. These results can be interpreted as the positive effect of triptorelin treatment on the pregnancy outcome of ICSI-undergone women.
The positive effect of GnRH-a application as a LPS in ART cycles is collectively admitted, but the addressed evidences are in low quality as stated by some reviewers [1]. Some researchers showed that using GnRH-a could be considered feasible as the sole source of LPS instead of progesterone. For instance, the RCT conducted by Pirard et al. showed a better efficacy for GnRH-a administration in IVF/ICSI antagonist protocols than for progesterone administration as the classic LPS [16]. In another more recent and extended study by Hava et al., the efficacy of GnRH-a administration was compared to progesterone as LPS in classic antagonist protocols [17]. Despite the inherent biases in the later non-randomized study, a meaningful boost was observed in the rate of ongoing pregnancy in the GnRH-a receiving group [17]. The findings of Hava et al. were confirmed by other RCTs that suggested the GnRH-a administration as a practical LPS in antagonist protocol [18]. However, their results have been challenged by other studies addressing that the ICSI-assisted pregnancies supported by GnRH-a administration in the luteal phase in agonist protocols have not necessarily accomplished [19,20,21].
The observed inconsistency in the influence of GnRH-a administration between the antagonist and agonist protocols can be possibly due to the saturation and downregulation of the GnRH receptors in the reproductive organs due to the continuous administration of GnRH-a in agonist protocols [14, 20]. In support of this conclusion, Kung et al. reported that the luteal GnRH-a administration can increase the rates of the clinical pregnancy and live birth when the GnRH antagonist is prescribed in a co-treating protocol, whereas it does not make any difference in patients who are treated using a long GnRH-a downregulation protocol [22].
A variety of protocols have been studied to detect the reason underlying the inconsistent results in comparing the single-dose or continuous administration and SC or nasal administration of the GnRH-a. Nevertheless, lots of questions remain to be answered. There are some molecular studies which have shown interactions between GnRH-a and the endometrial function; however, there has been no significant clinical relation [23, 24].
The result obtained from our study is in line with the report presented by Zafardoust et al. that stated administration of GnRH-a in antagonist protocols in the luteal phase can improve pregnancy rate [25]. Zafardoust et al. showed that GnRH-a (Decapeptyl) administration (6 days after oocyte retrieval) could improve the implantation and pregnancy rates in women with a history of several IVF/ICSI failures [25]. However, Benmachiche et al. concluded that a single-dose administration of GnRH-a (6 days after oocyte retrieval) was inefficient to affect the reproductive outcomes [26]. They also observed a meaningful increase in the levels of mid-luteal endogenous gonadotropins and steroids [26].
Considering the broad heterogeneity between the trials, further studies including systematic reviews are required to provide enough evidence about the efficiency and safety of GnRH-a administration in the luteal phase.
Conclusion
Our study demonstrated that the administration of a single dose of triptorelin 6 days after oocyte retrieval in candidate women for ICSI cycles following the ovarian stimulation with GnRH antagonist protocol led to a significant improvement in pregnancy rate.
Availability of data and materials
The data that support the findings of this study are available from the IVF Center.
Abbreviations
- AFC:
-
Antral follicle count
- AMH:
-
Anti-Mullerian hormone
- ARTs:
-
Assisted reproductive techniques
- ASRM:
-
American Society for Reproductive Medicine
- BMI:
-
Body mass index
- COS:
-
Controlled ovarian stimulation
- ETs:
-
Embryo transfers
- FSH:
-
Follicle-stimulating hormone
- GnRH-a:
-
Gonadotropin-releasing hormone agonist
- ICSI:
-
Intracytoplasmic sperm injection
- NS:
-
Non-significant
- OPU:
-
Oocyte pick-up
- RCT:
-
Randomized controlled trial
- RIF:
-
Repeated implantation failure
- SC:
-
Subcutaneous
References
Martins WP, Ferriani RA, Navarro P, Nastri CO (2016) GnRH agonist during luteal phase in women undergoing assisted reproductive techniques: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 47(2):144–151
Griesinger G, Meldrum D (2018) Introduction: management of the luteal phase in assisted reproductive technology. Fertil Steril 109(5):747–748
Fatemi H (2009) The luteal phase after 3 decades of IVF: what do we know? Reprod BioMed Online 19:1–13
van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M (2015) Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 7
Gizzo S, Andrisani A, Esposito F, Noventa M, Di Gangi S, Angioni S, Litta P, Gangemi M, Nardelli GB (2014) Which luteal phase support is better for each IVF stimulation protocol to achieve the highest pregnancy rate? A superiority randomized clinical trial. Gynecol Endocrinol 30(12):902–908
Pritts E, Atwood A (2002) Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod 17(9):2287–2299
Fujii S, Sato S, Fukui A, Kimura H, Kasai G, Saito Y (2001) Continuous administration of gonadotrophin-releasing hormone agonist during the luteal phase in IVF. Hum Reprod 16(8):1671–1675
Tesarik J, Hazout A, Mendoza C (2004) Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Hum Reprod 19(5):1176–1180
Oliveira JBA, Baruffi R, Petersen CG, Mauri AL, Cavagna M, Franco JG (2010) Administration of single-dose GnRH agonist in the luteal phase in ICSI cycles: a meta-analysis. Reprod Biol Endocrinol 8(1):107
Tesarik J, Hazout A, Mendoza-Tesarik R, Mendoza N, Mendoza C (2006) Beneficial effect of luteal-phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist-and antagonist-treated ovarian stimulation cycles. Hum Reprod 21(10):2572–2579
Razieh DF, Maryam AR, Nasim T (2009) Beneficial effect of luteal-phase gonadotropin-releasing hormone agonist administration on implantation rate after intracytoplasmic sperm injection. Taiwanese Journal of Obstetrics and Gynecology 48(3):245–248
Kyrou D, Kolibianakis E, Fatemi HM, Tarlatzi T, Devroey P, Tarlatzis B (2011) Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: a systematic review and meta-analysis. Hum Reprod Update 17(6):734–740
Isik AZ, Caglar GS, Sozen E, Akarsu C, Tuncay G, Ozbıcer T, Vicdan K (2009) Single-dose GnRH agonist administration in the luteal phase of GnRH antagonist cycles: a prospective randomized study. Reprod BioMed Online 19(4):472–477
Aboulghar MA, Marie H, Amin YM, Aboulghar MM, Nasr A, Serour GI, Mansour RT (2015) GnRH agonist plus vaginal progesterone for luteal phase support in ICSI cycles: a randomized study. Reprod BioMed Online 30(1):52–56
Zhao J, Zhang Q, Wang Y, Li Y (2014) Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod BioMed Online 29(3):291–298
Pirard C, Loumaye E, Laurent P, Wyns C (2015, 2015) Contribution to more patient-friendly ART treatment: efficacy of continuous low-dose GnRH agonist as the only luteal support—results of a prospective, randomized, comparative study. Int J Endocrinol
Hava IB, Blueshtein M, Herman HG, Omer Y, David GB (2017) Gonadotropin-releasing hormone analogue as sole luteal support in antagonist-based assisted reproductive technology cycles. Fertil Steril 107(1):130–135. e131
de Ziegler D, Pirtea P, Andersen CY, Ayoubi JM (2018) Role of gonadotropin-releasing hormone agonists, human chorionic gonadotropin (hCG), progesterone, and estrogen in luteal phase support after hCG triggering, and when in pregnancy hormonal support can be stopped. Fertil Steril 109(5):749–755
Ata B, Yakin K, Balaban B, Urman B (2008) GnRH agonist protocol administration in the luteal phase in ICSI–ET cycles stimulated with the long GnRH agonist protocol: a randomized, controlled double-blind study. Hum Reprod 23(3):668–673
Inamdar DB, Majumdar A (2012) Evaluation of the impact of gonadotropin-releasing hormone agonist as an adjuvant in luteal-phase support on IVF outcome. Journal of Human Reproductive Sciences 5(3):279
Geber S, Sampaio M (2013) Effect of duration of the GnRH agonists in the luteal phase in the outcome of assisted reproduction cycles. Gynecol Endocrinol 29(6):608–610
Kung H-F, Chen M-J, Guua H-F, Chen Y-F, Yi Y-C, Ho JY-P, Chou M-M (2014) Luteal phase support with Decapeptyl improves pregnancy outcomes in intracytoplasmic sperm injection with higher basal follicle-stimulating hormone or lower mature oocytes. Journal of the Chinese Medical Association 77(10):524–530
Fauser BC, Devroey P (2003) Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab 14(5):236–242
Takeuchi S, Futamura N, Minoura H, Toyoda N (1998) Possible direct effect of gonadotropin releasing hormone on human endometrium and decidua. Life Sci 62(13):1187–1194
Zafardoust S, Jeddi-Tehrani M, Akhondi MM, Sadeghi MR, Kamali K, Mokhtar S, Badehnoosh B, Arjmand-Teymouri F, Fatemi F, Mohammadzadeh A (2015) Effect of administration of single dose GnRH agonist in luteal phase on outcome of ICSI-ET cycles in women with previous history of IVF/ICSI failure: a randomized controlled trial. Journal of Reproduction & Infertility 16(2):96
Benmachiche A, Benbouhedja S, Zoghmar A, Boularak A, Humaidan P (2017) Impact of mid-luteal phase GnRH agonist administration on reproductive outcomes in GnRH agonist-triggered cycles: a randomized controlled trial. Front Endocrinol 8:124
Acknowledgements
We acknowledge all staff who worked in the IVF Center, Taleghani Hospital.
Funding
The present paper was financially supported by the Researcher Department of the School of Medicine Shahid Beheshti University of Medical Sciences (Grant no 7205).
Author information
Authors and Affiliations
Contributions
LZ and NS were responsible for the conceptualization and methodology. LZ, NS, SS, SH, HH collected, analyzed, and interpreted the patients’ data. HH drafted the manuscript. LZ and HH revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval of this study was given by the ethics committee of Shahid Beheshti University of Medical Sciences (SBMU) and registered at the Iranian Registry of Clinical Trials, number IRCT2016101729027N2. Written informed consent was obtained from all individuals prior to participating in the study.
Consent for publication
Not applicable
Competing interests
The authors declare that there are no competing interests related to the subject matter or materials discussed in this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saharkhiz, N., Salehpour, S., Hosseini, S. et al. Effects of gonadotropin-releasing hormone agonist (GnRH-a) as luteal phase support in intracytoplasmic sperm injection (ICSI) cycles: a randomized controlled trial. Middle East Fertil Soc J 25, 20 (2020). https://doi.org/10.1186/s43043-020-00030-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-020-00030-7