Abstract
Background
Different inflammatory cells (i.e., CD4, CD8, CD20 and CD68) are involved in pathogenesis of DM muscle. In this context, the aim of this study was to assess and compare these inflammatory cell phenotyping in muscle samples of treatment naive juvenile and adult patients with dermatomyositis.
Methods
This is a cross-sectional study, in which 28 untreated juvenile and 28 adult untreated dermatomyositis patients were included. Immunohistochemical analysis was performed on serial frozen muscle sections. Inflammatory cell phenotyping was analyzed quantitatively in endomysium, perimysium, and perivascular (endomysium and perimysium) area.
Results
Mean age at disease onset was 7.3 and 42.0 years in juvenile and adult dermatomyositis, respectively. Both groups had comparable time duration from symptom’s onset to biopsy performance. CD4 and CD8 positive cells distributions were similar in both groups in all analyzed area, except for more predominance of CD4 in perimysium at juvenile muscle biopsies. The CD20 and CD68 positive cells were predominantly observed in adult muscle biopsy sections, when compared to juvenile samples, except for similar distribution of CD20 in perivascular endomysium, and CD68 in perimysium.
Conclusions
These data show that the differences between juvenile and adult dermatomyositis may be restricted not only to patients’ age, but also to different inflammatory cell distribution, particularly, in new-onset disease. Further studies are necessary to confirm the present study data and to analyze meaning of the different inflammatory cell phenotyping distribution finding in these both diseases.
Similar content being viewed by others
Introduction
Dermatomyositis (DM) is a rare systemic autoimmune myositis with characteristic cutaneous manifestations, such as heliotrope rash and Gottron’s papules [1,2,3,4,5,6,7]. The annual incidence of DM is 5–10 cases per million, with the adult DM primarily affecting patients between 45 and 55 years old, whereas the juvenile DM affects individuals between 5 and 10 years of age [3, 4].
Muscle biopsy is one of the important diagnostic procedures in DM. The classical findings of muscle biopsies for DM evident are presence of mononuclear, inflammatory cell exudate arranged in a perivascular and perifascicular distribution with degenerating and regenerating muscle fibers and perifascicular atrophy [3,4,5,6,7,8]. Furthermore, the major histocompatibility complex expression and inflammatory cell phenotyping have been extensively described in both juvenile and adult DM muscle biopsies [3,4,5,6,7,8,9]. Until recently, these two parameters have not been simultaneously assessed and compared in both juvenile and adult DM. In that point, a recent study has shown at the first time that there is different major histocompatibility complex expression in simultaneously analysed juvenile and adult myositis [9]. However, the inflammatory cell phenotyping had not yet been studied.
Therefore, the aim of the present study was to compare the inflammatory cell phenotyping in the naive juvenile and adult DM muscle samples.
Subject and methods
Twenty-eight juvenile and 28 adult DM consecutive patients fulfilling Bohan and Peter’s criteria [1, 2] were included in this cross-sectional study. The study was approved by the local Research Ethics Committee (Number 0335/11).
The patients were followed between 1990 and 2010 in the Pediatric Rheumatology Unit and the Inflammatory Myopathies Unit of our tertiary center.
All demographic, clinical, laboratory parameters were based on the previous study [9]. Demographics and clinical manifestations at disease onset (cutaneous involvement: heliotrope rash and Gottron’s papules; articular involvement: arthralgia and/or arthritis; pulmonary involvement: pulmonary alterations in computer tomography and with symptoms like dyspnea; muscular strength of the limbs (degree 0: absence of muscle contraction, degree I: signs of mild contractility, degree II: normal amplitude movements but that do not overcome the action of gravity, degree III: normal amplitude movements against the action of gravity; degree IV: integral mobility against the action of gravity and some degree of resistance, degree V: complete mobility against severe resistance and against the action of gravity) [10]; and laboratory data were obtained through a systematic review of patients records.
The laboratory data corresponds to information collected at disease onset. Creatine phosphokinase (normal range: 24–173 U/L) and aldolase (normal range: 1.0–7.5 U/L) were determined by the automated kinetic method.
Sequential frozen 5 μm-thickness sections were stained for haematoxylin-eosine (HE), and then by immunohistochemistry. Monoclonal antibodies (CD4 and CD8: EnVision-AP technique, CD20 and CD68: LSB+ system) were used in immunohistochemical analysis. Frozen sections were fixed for 10 min in acetone at 4 °C. Endogenous peroxidase was blocked with H2O2 1% in absolute methanol three times for 10 min. After a rinse in phosphate-buffered-saline (PBS 0.01 M, pH 7.4) for 5 min, the specimens were incubated in fetal serum in a wet chamber for 1 h at 37 °C. Primary antibodies diluted in PBS and bovine serum albumin 1% were applied in a wet chamber at 37 °C, overnight. Slides were then washed in PBS, the prepared secondary mouse biotinylated (StreptABComplex/HRP) was applied for 30 min at 37 °C, and rinsed in PBS. Subsequently, the prepared StreptABComplex/HRP complex was applied and incubated for 30 min at 37 °C. After rinsing in PBS, reactions were visualized after incubation with a chromogenic substrate (3.3′-diaminobenzidine tetrahydrochloride) solution for peroxidase. After a final rinse, haematoxylin counterstaining was performed. Slides were mounted and cover-slipped with an aqueous based mounting medium. The preparations of all muscle specimens were done at the same time as a batch. Human tonsil was used as a positive control. Inflammatory cell phenotyping (CD4, CD8, CD20, CD68) was analyzed quantitatively in endomysium, perimysium, pericapillar (endomysium and perimysium) areas in 10 distinct fields (200x magnification). Each muscle biopsy specimen was coded and analyzed by two independent investigators (SKS and AMES), blinded to diagnosis and clinical status. When any discrepancy was noted, the case was reviewed concomitantly.
Statistical analysis. The Kolmogorov-Smirnov test was used to evaluate the distribution of each parameter. The data were expressed as mean ± standard deviation or median (25th - 75th interquartile range). Comparisons between juvenile and adult DM patient parameters were made using Student’s t-test or the Mann-Whitney test. All of the analyses were performed using the SPSS 15.0 statistics software (Chicago, Illinois, EUA). P < 0.05 was considered to indicate statistical significance.
Results
The demographic, clinical, laboratory parameters of adult and juvenile DM are shown in the Table 1. Mean age at onset of disease was 7.3 ± 3.4 and 42.0 ± 15.9 years-old in juvenile and adult DM, respectively, whereas median disease time within muscle biopsy was similar in both groups: 4.0 (2.0–16.5) vs. 7.0 (3.3–12.0) months, P = 0.274). There was no difference between both groups in relation to clinical and laboratory data, except for higher hampered muscle strength (upper limbs) in juvenile DM group.
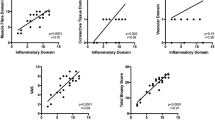
Concerning immunohistochemical analysis, CD4 and CD8 positive inflammatory cells distributions in muscle biopsies were comparable between juvenile and adult DM, except for a higher number of CD4 positive cells in perimysium area in juvenile DM, when compared to adult DM (Fig. 1). Cells expressing CD20 were predominantly present in adult DM muscle biopsies (endomysium, perimysium, pericapillar perimysium) in relation to juvenile DM, whereas the distribution of CD20 positive cells was similar in pericapillar endomysium in both groups. Additionally, inflammatory cells which express CD68 were also predominant in adult DM muscle biopsies (endomysium, pericapillar endomysium and perimysium), except in perimysium, where CD68 positive cells distribution was similar in both groups.
Absolute number of inflammatory cell infiltrations in 10 different areas of muscle biopsies from untreated adult and juvenile dermatomyositis patients at 200x magnification. Bars represent the lower and higher values and the mean of the group. ADM: adult dermatomyositis; JDM: juvenile dermatomyositis; muscle areas: a, endomysium; b, perimysium; c, pericapillar endomysium; d, pericapillar perimysium *P < 0.05; **P = 0.001; ***P < 0.001
Moreover, all inflammatory cells distributions (CD4, CD8, CD20 and CD68) did not correlate to any demographics, to clinical data and to laboratory features (P > 0.05).
Discussion
Similarly to major histocompatibility complex expression in juvenile and adult DM [9], the present study showed that there is different inflammatory cell phenotyping distribution in juvenile and adult DM muscle biopsy samples.
DM is an autoimmune systemic myopathy characterized by the presence of cellular infiltrates in muscle biopsies, autoantibodies in the peripheral blood and association with major histocompatibility complex overexpression [3, 5, 11, 12]. In this context, the presence and activation of CD4+ and CD8+ T cells as well as B cells in muscle tissues promote the humoral mediated pathogenesis in DM [11, 12]. However, corroborating with literature data, there were few inflammatory cell infiltrations in muscle biopsies, because an early histological and primarily feature in DM is a complement-mediated microangiopathy leading to capillary drop-out, necrosis of muscle fibers [11], even in the absence of inflammation [6, 13].
In the present study, even with few inflammatory cell infiltrations in muscle biopsies, a different inflammatory cell phenotype aspect was observed. There was a higher CD4+ T-cell distribution on perimysium area in juvenile DM biopsy, in contrast to a more evident CD20+ and CD68+ cells in muscle tissue areas of adult DM.
The predominance of CD4+ T lymphocyte infiltrations had also observed in juvenile DM muscle biopsies [14]. These cells are important to active CD20+ and autoantibody production in DM. On the other hand, CD20+ and CD68+ cells were more predominant in adult DM muscle biopsies. The relevance of CD20+ in DM is supported by the favorable clinical response to rituximab, a B cell blocking immunobiologic [15,16,17].
Additionally, in a recent report, it was demonstrated that Th2 T cells are increased in juvenile DM compared to adult DM muscle, indicating different immune cells regulation [18]. CD4 T-cells, and also B lymphocytes and macrophage cells may also play a major role [19, 20] in a immunological-mediated mechanism underlying an important pathologic aspect of this disease.
Overall, all these different immunohistochemical aspects can partially explain why patients with DM show different disease prognosis, clinical manifestations and/or response to conventional treatment.
Further multicentric and international studies will be necessary to confirm the present study data and to analyse the meaning of the different inflammatory cell phenotyping distribution finding in both diseases.
Conclusions
Our data show that the differences between juvenile and adult DM could be restricted not only to age of onset, but also possibly to histological muscle biopsies with different inflammatory cell distribution, particularly, in new-onset disease.
Abbreviations
- DM:
-
Dermatomyositis
- HE:
-
Haematoxylin-eosine
- PBS:
-
Phosphate-buffered-saline
References
Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344–7.
Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403–7.
Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;373:393–4.
Drake LA, Dinehart SM, Farmer ER, Goltz RW, Graham GF, Hordinsky MK, et al. Guidelines of care for dermatomyositis. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:824–9.
Dalakas MC, Sivakumar K. The immunopathologic and inflammatory differences between dermatomyositis, polymyositis and sporadic inclusion body myositis. Curr Opin Neurol. 1996;9:235–9.
Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990;27:343–56.
Engel AG, Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986;17:704–21.
Lorenzoni PJ, Scola RH, Kay CS, Prevedello PG, Espindola G, Weneck LC. Idiopathic inflammatory myopathies in childhood: a brief review of 27 cases. Pediatr Neurol. 2011;45:17–22.
Shinjo SK, Sallum AME, Silva CA, Marie SKN. Skeletal muscle major histocompatibility complex class I and II expression differences in adult and juvenile dermatomyositis. Clinics. 2012;67:885–90.
Medical Research Council. Aids to the examination of the peripheral nervous system. War memorandum (revised 2nd edition). London: HMSO, 1943.
Dalakas MC. An update on inflammatory and autoimnmune myopathies. Neuropathol Appl Neurobiol. 2011;37:226–42.
Haq SA, Tournadre A. Idiopathic inflammatory myopathies: from immunopathogenesis to new therapeutic targets. Int J Rheum Dis. 2015;18:818–25.
Engel AG, Hohlfeld R, Banker BQ. The polymyositis and dermatomyositis syndrome. In: Engel AG, Franzini-Armstrong C, editors. Myology. New York: McGraw-Hill; 2006. p. 1135.83.
Papa V, Romanin B, Bergamaschi R, Cordelli DM, Costa R, De Giorgi LB, et al. Juvenile dermatomyositis: a report of three cases. Ultrastruct Pathol. 2016;40:83–5.
Krystufkova O, Vallerskog T, Helmers SB, Mann H, Putová I, Belácek J, et al. Increased serum levels of B cell activating factor (BAFF) in subsets of patients with idiopathic inflammatory myopathies. Ann Rheum Dis. 2009;68(6):836–43.
Oddis CV, Reed AM, Aggarwa R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximabe in the treatment of refractory adult and juvenile dermatomyositis an adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314–24.
Couderc M, Gottenberg JE, Mariette X, Hachulla E, Sibilia J, Fain O, et al. Efficacy and safety of rituximab in the treatment of refractory inflammatory myopathies in adults: results from the AIR registry. Rheumatology. 2011;50:2283–9.
López De Padilla CM, Crowson CS, Hein MS, Pendegraft RS, Strausbauch MA, Niewold TB, et al. Gene expression profiling in blood and affected muscle tissues reveals differential activation pathways in patients with new-onset juvenile and adult dermatomyositis. J Rheumatol. 2017;44:117–24.
Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies: I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208.
Engel AK. Monoclonal antibody analysis of mononuclear cells in myopathies. III: Immunoelectron microscopy aspects of cell-mediated muscle fiber injury. Ann Neurol. 1986;19:119–25.
Acknowledgments
Not applicable.
Funding
Federico Foundation, Fundação Faculdade de Medicina, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) #2014/09079–1 to SKS.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to write and review the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local research ethics committee (Number 0335/11) and the patient consent for participation was waived by this ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shinjo, S.K., Sallum, A.M.E., Oba-Shinjo, S.M. et al. Comparison between treatment naive juvenile and adult dermatomyositis muscle biopsies: difference of inflammatory cells phenotyping. Adv Rheumatol 58, 37 (2018). https://doi.org/10.1186/s42358-018-0037-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-018-0037-5