Abstract
Background
In eusocial animal colonies, individuals from different castes often play divergent behavioral roles and confront distinct cognitive demands. Neuroecology theory predicts variation in cognitive demands will correspond to differences in brain investment because brain tissue is energetically expensive. We hypothesized colony-level selection for efficient energy allocation will favor reduced brain investment for castes with reduced cognitive demands. Neotropical army ants (genus Eciton) have morphologically-based worker castes; the specialized soldiers have reduced behavioral repertoires. We asked whether Eciton worker brain size and brain architecture varied with body size, and predicted the specialized soldier caste would have reduced investment in brain tissue.
Results
Eciton brain size generally increased with body size, but relative brain size (brain size/body size ratio) decreased sharply with body size. Soldiers were the largest-bodied workers, but their total brain volume overlapped extensively with other workers and was small relative to body size. Furthermore, soldier chemosensory antennal lobes and central-processing mushroom bodies were smaller relative to their brain size.
Conclusions
These patterns suggest colony-level selection on brain investment efficiency has led to adaptive adjustments in brain tissue allocation among Eciton worker castes, with reduced brain investment in the behaviorally specialized soldiers.
Similar content being viewed by others
Background
Increases in animal cognitive abilities are often associated with greater investment in neural tissue [1, 2]. Because neural tissue is among the most expensive to produce and maintain [3,4,5], brain tissue investment is constrained and should evolve to match the cognitive demands a species confronts [6]. Species’ brain sizes (relative to body size) are often positively correlated with differences in cognitive capacity [6,7,8]. Furthermore, brains often show structural specialization. Anatomically-distinct brain regions typically perform distinct cognitive functions [9, 10], and brain regions can evolve in size and complexity at different rates (mosaic brain evolution: [11, 12]). Superimposed on the evolution of total brain size, the size ratios of brain regions correspond to the particular cognitive demands imposed by a species’ ecological conditions [13,14,15].
Eusocial animals present an opportunity to explore brain evolution under super-organismal (colony-level) selection. In eusocial animals, specialized sub-groups of individuals (castes) perform distinct functions for their colonies [16]. Most members of eusocial colonies are workers, which rarely or never reproduce. The fitness costs and benefits of variation in individual cognitive ability, and of brain tissue investment, must therefore accrue at the group or colony level. When castes differ in the cognitive challenges they face, colony-level selection should favor adaptive variation in brain investment among castes.
We tested this idea by comparing brain structure among the behaviorally distinct worker castes of army ants. Neotropical army ants (Dorylinae: Ecitonini) possess a complex caste system with some of the strongest within-colony variation in worker morphology among ants [17,18,19]. Army ant worker behavior and task specialization are related to body size and shape [20,21,22]. In the army ant genus Eciton, most species possess several morphologically distinct worker castes including large-bodied soldiers. Soldiers perform few or no tasks other than colony defense, primarily against vertebrates. Other workers size-classes (henceforth, “workers”) perform a wide array of tasks including temporary nest (bivouac) assembly, queen and brood care, and the capture, dismembering, and transport of prey [23]. Solders are distinguished by their sharply-pointed, sickle-shaped mandibles that are effective at piercing and gripping vertebrate flesh in colony defense (Fig. 1). Soldiers’ mandibles cannot be used to capture prey, nor to carry prey or brood; soldiers are even fed by other workers [21, 24, 25]. Because of their reduced behavioral complexity, we predicted investment in total brain volume, and in key brain regions associated with sensory perception and complex cognitive processing, would be reduced in the Eciton soldier caste relative to their nest-mate workers.
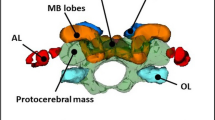
a Foraging worker (gray arrow) and soldier (white arrow) of Eciton burchellii cupiens, Tiputini Biodiveristy Station, Ecuador. b Same-scale 3-D reconstructions of head capsules and brains of army ant (Eciton burchellii parvispinum) worker (A) and soldier (B). Scale bar represent 0.5 mm. Brain structures are the colored bodies in the center of each head capsule. Green: protocerebral mass; dark blue: mushroom body calyces; red: optic lobes; light blue: antennal lobes
We first quantified the allometry of total brain size with body size variation and between castes. There is substantial overlap in body-size range among Eciton species, allowing tests of species differences in brain size for size-matched workers [26]. Within many animal clades, the brain size/body size ratio decreases with increasing body size (Haller’s rule: [27, 28]). We tested for Haller’s rule by comparing total brain size against Eciton worker and soldier body size. We then assessed whether the soldier brain size/body size allometric relationship differed from that of workers (Fig. 1). We predicted soldiers would have smaller relative brain sizes, because investment in soldier brains would be minimized.
We also asked whether brain architecture differed between workers and soldiers, independent of brain size variation. First, we analyzed caste differences in the relative sizes of peripheral sensory processing brain lobes. Army ant brains include anatomically-distinct antennal lobes (henceforth, AL) that receive olfactory information, and optic lobes (henceforth, OL) that process visual inputs [29,30,31]. The relative sizes of these peripheral brain regions correspond to ants’ species-typical environments during behavioral activity, such as ambient light intensity [31,32,33,34]. We tested for caste differences in AL and OL investment by measuring the volumes of these structures relative to total brain volume. Second, we analyzed caste differences in the relative sizes of the mushroom body calyces. The mushroom bodies (henceforth, MB) are brain neuropils that receive inputs from the peripheral lobes and are involved in learning, memory and sensory integration [35,36,37]. MB calyx size is positively associated with task performance by social insect workers [37,38,39].
Methods
Subject animal collections and caste identification
Ants were collected in the field directly into either 96% ethanol or buffered aldehyde-based fixative; ants collected into ethanol were later transferred into buffered aldehyde-based fixative. Ants were stored refrigerated at 4 °C in fixative until histological processing. We chose subjects from each species to span the range of body sizes among the workers we collected. We identified soldiers by their elongated, curved mandibles that ended in sharp points. We collected brain and head size data from n = 109 army ant workers and n = 39 soldiers. For each subject species the total sample sizes of workers and soldiers used as brain anatomy subjects, number of colonies sampled, and dates and geographic coordinates of field collections were: E. burchellii foreli - workers n = 13, soldiers n = 4, n = 2 colonies, Mar 2010 and Jul 2014, La Selva Biological Station, Costa Rica (84°1’W, 10°26’N); E. burchellii cupiens - workers n = 13, soldiers n = 4, n = 1 colony, Jun 2007, Tiputini Biodiversity Station, Ecuador (76°9’W, 0°38’S); E. burchellii parvispinum - workers n = 13, soldiers n = 9, n = 6 colonies, Jul 2009, Jun 2010, Jul 2014, Monteverde, Costa Rica (84°48’W, 10°18’N); E. dulcium - workers n = 15, soldiers n = 6, n = 2 colonies, Mar and Apr 2013, La Selva, Costa Rica; E. hamatum - workers n = 11, soldiers n = 7, n = 3 colonies, Jun 2007 and Jul 2014 Tiputini, Ecuador and La Selva, Costa Rica; E. lucanoides, workers n = 13, soldiers n = 3, n = 2 colonies, Feb 2013 and Mar 2014, La Selva, Costa Rica; E. mexicanum - workers n = 15, soldiers n = 3, n = 3 colonies, Apr and Jul 2014, Santa Elena, Costa Rica (84°48’W, 10°21’N) and La Selva, Costa Rica; E. vagans, workers n = 16, soldiers n = 3, n = 2 colonies, Apr 2014 La Selva, Costa Rica.
Morphological (head capsule) measurements
We estimated head capsule volume for each brain anatomy subject. We dissected the ant’s head capsules from the body at the foramen (the narrow attachment-point to the mesosoma), and photographed each head using a digital camera mounted on a dissecting scope. We used the ruler tool in ImageJ version 1.46 software and photographs of a stage micrometer to convert pixels to mm. Heads were photographed face-on in frontal view with the foramen area facing away from the camera resting against a horizontal glass surface. We measured head width at the level of the antennal sockets, head height from the center of the clypeus to the vertex, and we used ½ head width as an approximation of head depth. We then estimated head capsule volume for each individual using the formula for an ellipsoid:
Histology and neuroanatomical measurements
After photographing, we dehydrated the head capsules through an ethanol series, acetone, then increasing concentrations of plastic resin [40]. Individual ant heads were incubated in 0.2 ml resin in pyramid-shaped flexible molds at 60 °C for 72 h. The solid resin pyramids were mounted on acrylic posts. Each head was sectioned into 12–14 μm thick slices using a rotary microtome with disposable steel histology blades. We placed the sections on gelatin-coated microscope slides and stained the tissue with Toluidine blue. We cleared in an ethanol series and Histochoice clearing agent (Sigma-Aldrich), then cover slipped under transparent mounting medium. We used a digital camera mounted on a compound light microscope using a 5X or 10X objective to photograph the tissue sections, with a digital image resolution of 2048 × 1536 pixel. For each subject, we photographed every section starting at the section where brain tissue first became visible. ImageJ version 1.46 software was used to quantify the volumes of brain structures. To quantify brain regions on each section we outlined the target brain regions and used ImageJ to count the number of image pixels in the structure. We converted the pixel counts to area using a photograph of a stage micrometer taken at the same magnification with the same microscope and camera as a size reference, then multiplied the areas by section thickness to yield a volume estimate. Only brain neuropils were measured; we did not measure adjacent cell body regions. We measured and analyzed the volumes of the following brain sub-regions: OL, AL:, and MB calyx. As in some other ants, army ant MB calyces are simplified in structure and the visual and olfactory sensory sub-regions are not anatomically differentiated [31, 41]. We measured and analyzed the volume of the entire MB calyx as a unit. Volumes of other brain regions were pooled as an index of brain size: MB peduncle and lobes, central complex, and the remainder of the protocerebrum.
Statistical analyses
Standard parametric analyses were performed with SPSS v.24 software (IMB corporation, 2016). We included species identity as a random-factor predictor variable in all regression analyses.
We used paired t-tests (with species as the pairing factor) to compare the mean relative sizes of targeted brain regions (MB calyces, AL and OL) for workers and soldiers. We used a paired t-test with phylogenetic correction to assess whether the caste differences in relative total MB and MB calyx sizes were significant after accounting for the effects of phylogeny using Phytools v. 0.5–38 software in R [42]. The species-level phylogeny for Eciton from [43] was our source of hypothesized species relationships.
Results
Caste differences in brain allometry with body size
Brain size relative to body size was strongly allometric, with size-relative brain volume increasing sharply as the smallest worker body sizes were approached (in support of Haller’s rule, Fig. 1). Soldiers had larger head capsules than workers (Figs. 1 and 2; F1,139 = 516.5, p < 0.001). Total brain size increased with body size (head capsule volume), but at a decelerating rate (Fig. 2). Despite the greater size of soldiers, soldiers did not differ significantly from workers in total brain volume (Fig. 2; F1,13 = 1.56, p = 0.21, NS). After accounting for head size effects, soldier total brain volume was significantly smaller (F1,138 = 7.45, p = 0.007).
a The ratio of total brain volume to body size (head capsule volume) plotted against head capsule volume for workers (gray symbols) and soldiers (white symbols) of eight species of Eciton army ants. The symbol shapes used to plot the data points for each species are indicated in the legend. b Total brain volume (normalized, relative to species maximum value) plotted against head capsule volume (normalized, relative to species maximum value) for workers (gray symbols) and soldiers (white symbols) of eight species of Eciton army ants. Species symbols for data points as in (a)
Caste differences in brain architecture: Relative sizes of sensory lobes and MB calyces
Army ant AL are exceptionally large [31, 32]; in our sample, AL volume ranged from 9.7 to 18.1% of total brain volume, while the MB calyces comprised 13.4 to 24.5% of total brain volume. Relative to workers, soldiers had significantly lower AL:rest of brain ratios (paired t-test, t = 7.01, df = 7, p < 0.001) and significantly lower MB calyx:rest of brain ratios (Fig. 3; paired t-test, t = 7.68, df = 7, p < 0.001). These significant caste differences were robust to corrections for effects of phylogeny (phylogenetic paired t-tests; for MB calyx: t = 6.17, p = 0.002; for AL: t = 3.8, p = 0.01). The OL comprised a relatively small fraction of total brain volume (0.2 to 1.6%); soldiers of some species had higher OL:rest of brain ratios than workers (Fig. 2), but this difference was not significant (paired t-test, t = − 1.43, df = 7, p = 0.20, NS).
Mean relative volumes of functionally distinct brain regions for workers (gray bars) and soldiers (white bars) of eight species of Eciton army ants. In each graph, species are arranged in descending order of the mean relative structure volume for workers. Top: Calyx neuropil of the mushroom bodies (MB calyx). Middle: Antennal lobes (AL). Bottom: optic lobes (OL)
Discussion
Our data suggest that, as predicted, high investment constraints on neural tissue [3,4,5] affect the allometry of brain structure among social insect workers. Colony-level selection for efficiency of resource allocation is thought to explain investments in different castes [16, 44, 45]. When a caste has consistently reduced cognitive demands, colony-level selection should favor reduced brain tissue investment in that caste.
Eciton army ants provide a particularly strong system for testing adaptive caste differences in brain structure. Eciton army ants present some of the most extreme examples of developmentally-based specialization within social insect worker forces. Nest-mate Eciton workers cluster into multiple allometrically distinct size classes [21, 22]. The morphologically specialized Eciton soldiers are a unique worker caste with highly modified, pincer-like mandibles. Other than colony defense, Eciton soldiers have a reduced task load [18, 24]. We assumed the limited task repertoire of Eciton soldiers would reduce the range of cognitive challenges that soldiers confront, thereby reducing demands for individual investment in brain tissue [31, 34]. We predicted soldiers would have lower total brain size relative to body size, and reduced brain size relative investment in both sensory-processing peripheral brain regions (AL: and OL), and in the central-processing MB calyces.
In support of our predictions, both total brain size and the relative sizes of key brain regions (MB calyces and AL) were reduced in soldiers. Although soldiers were larger, their absolute brain volume was similar to that of workers. This pattern suggests a relatively low overall investment in soldier brain tissue. Furthermore, the soldiers’ brains had relatively small AL and MB calyces. MB calyces increase in volume and neuronal complexity when social insect workers perform complex tasks such as foraging [37, 46, 47]. These volume differences result in part from increases in the length and branching complexity of the dendritic fields of MB neurons in the calyx, suggesting plastic neuron development in the MB calyx is necessary for complex task performance by workers [37, 48]. Eciton soldier head capsules house relatively large muscles that attach to the base of the mandibles [49], suggesting the interesting possibility that brain investment may trade off against muscle development among castes. Morphological worker castes (majors and minors) in several species of Pheidole ants also showed a positive correlation of caste task plasticity with MB size [38]. The pattern we documented may be widespread among social insects with behaviorally specialized castes.
The reduced size of the AL in Eciton soldiers further suggests their chemosensory abilities may be relatively weak. In some ant taxa, species differences in AL size and structure correspond to species differences in ecology [11, 50]. In an interspecific comparison, the two most above-ground active Neotropical army ant genera (Eciton and Neviamyrmex) had relatively larger average AL than the more subterranean genera [31]. Like army ants, leaf cutter ants’ (genus Atta) worker forces comprise workers of varying sizes and distinct behavioral repertoires. In A. vollenweideri, worker AL structure and sensitivity to trail pheromones covaries with worker size [30].
However, contrary to our predictions, relative OL size was higher for soldiers than workers in most species, although this pattern was not significant overall. Army ants have reduced external eyes, a pattern seen in many subterranean ants [51]. The external eyes of Eciton are reduced to single external facets; although their visual capacity is poorly understood, their eyes probably cannot form images. Army ant species vary widely in their degree of above- versus below-ground activity when nesting, foraging and emigrating, but Eciton is the most above-ground active Neotropical genus [31]. Greater relative soldier OL size in some species could be related to visual detection of vertebrate predators or cleptoparasites [52], but whether and how Eciton workers and soldiers rely on vision remains to be tested.
Conclusion
Like Eciton soldiers, Polyergus slavemaking ant workers have modified piercing mandibles and a reduced task repertoire. Polyergus colonies rely on captured and enslaved Formica ant workers to perform most foraging, nest maintenance, and brood care tasks. Like Eciton soldiers, Polyergus slavemaking ant workers have reduced MB investment relative to their enslaved Formica host workers [39]. However, slave-maker/host brain structure differences represent species-level adaptations. Our data suggest the reduced brain region investment in Eciton soldiers is adaptive at the colony level, given their reduced task repertoire.
Abbreviations
- AL:
-
Antennal lobes
- MB:
-
Mushroom bodies
- OL:
-
Optic lobes
References
Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–49.
Herculano-Houzel S. Numbers of neurons as biological correlates of cognitive capability. Curr Opin Behav Sci. 2017;16:1–7.
Laughlin SB. Energy as a constraint on the coding and processing of sensory information. Curr Opin Neurobiol. 2001;11:475–80.
Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–804.
Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480:91–3.
Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cog Sci. 2005;9:250–7.
Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr Biol. 2013;23:168–71.
Benson-Amram S, Dantzer B, Stricker G, Swanson EM, Holekamp KE. Brain size predicts problem-solving ability in mammalian carnivores. Proc Nat Acad Sci. 2016;113:2532–7.
Street SE, Navarrete AF, Reader SM, Laland KN. Coevolution of cultural intelligence, extended life history, sociality, and brain size in primates. Proc Nat Acad Sci. 2017;114:7908–14.
Farris SM. Evolutionary convergence of higher order brain centers spanning the protostome deuterostome boundary. Brain Behav Evol. 2008;72:106–22.
Ilies I, Muscedere ML, Traniello JFA. Neuroanatomical and morphological trait clusters in the ant genus Pheidole: evidence for modularity and integration in brain structure. Brain Behav Evol. 2015;85:63–76.
Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–8.
Smaers JB, Soligo C. Brain reorganization, not relative brain size, primarily characterizes anthropoid brain evolution. Proc Biol Sci. 2013;280:20130269.
Allman JM. Evolving brains. New York: Scientific American Library; 2000.
Catania KC. Evolution of sensory specializations in insectivores. Anatom Rec A. 2005;287:1038–50.
Hölldobler B, Wilson EO. The superorganism: the beauty, elegance, and strangeness of insect societies. In: WW Norton & Company; 2009.
Schneirla TC. Army ants: A study in social Organization Oxford. England: W. H. Freeman; 1971.
Gotwald WH Jr Army ants: the biology of social predation. Cornell University Press (1995).
Brady SG. Evolution of the army ant syndrome: the origin and long-term evolutionary stasis of a complex of behavioral and reproductive adaptations. Proc Nat Acad Sci. 2003;100:6575–9.
Franks NR, Sendova-Franks AB, Anderson C. Division of labour within teams of new world and old world army ants. Anim Behav. 2001;62:635–42.
Powell S, Franks NR. Ecology and the evolution of worker morphological diversity: a comparative analysis with Eciton army ants. Funct Ecol. 2006;20:1105–14.
Powell S, Franks NR. How a few help all: living pothole plugs speed prey delivery in the army ant Eciton burchellii. Anim Behav. 2007;73:1067–76.
Powell S, Franks NR. Caste evolution and ecology: a special worker for novel prey. Proc R Soc B. 2005;272:2173–80.
Rettenmeyer CW. Behavioral studies of army ants. Univ Kans Sci Bull. 1963;44:281–465.
Franks NR. Reproduction, foraging efficiency and worker polymorphism in army ants. In: Experimental Behavioral Ecology and Sociobiology: in Memoriam Karl Von Frisch,1886–1982 (eds B. Holldobler & M. Lindauer) 31:91–107. Sunderland: Sinauer Associates; 1985.
Baudier KM, Mudd AE, Erickson SC, O'Donnell S. Microhabitat and body size effects on heat tolerance: implications for responses to climate change (army ants: Formicidae, Ecitoninae). J Anim Ecol. 2015;84:1322–30.
Seid MA, Castillo A, Wcislo WT. The allometry of brain miniaturization in ants. Brain Behav Evol. 2011;77:5–13.
Eberhard WG, Wcislo WT. Grade changes in brain–body allometry: morphological and behavioural correlates of brain size in miniature spiders, insects and other invertebrates. Adv Ins Phys. 2011;40:155–214.
Strausfeld NJ. The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arth Struct Devel. 2005;34:235–56.
Kubler LS, Kelber C, Kleineidam CJ. Distinct antennal lobe phenotypes in the leaf-cutting ant (Atta vollenweideri). J Comp Neurol. 2010;518:352–65.
Bulova S, Purce K, Khodak P, Sulger E, O'Donnell S. Into the black, and back: the ecology of brain investment in Neotropical army ants (Formicidae: Dorylinae). Sci Nat. 2016;103:1–11.
Gronenberg W, Liebig J. Smaller brains and optic lobes in reproductive workers of the ant Harpegnathos. Naturwissenschaften. 1999;86:343–5.
O'Donnell S, Clifford MR, DeLeon S, Papa C, Zahedi N, Bulova SJ. Brain size and visual environment predict species differences in paperwasp sensory processing brain regions (Hymenoptera: Vespidae, Polistinae). Brain Behav Evol. 2013;82:177–84.
O'Donnell S, Bulova SJ, DeLeon S, Khodak P, Miller S, Sulger E. Distributed cognition and social brains: reductions in mushroom body investment accompanied the origins of sociality in wasps (Hymenoptera: Vespidae). Proc R Soc B. 2015;282:20150791.
Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem. 1998;5:11–37.
Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annu Rev Entomol. 2006;51:209–32.
Farris SM, Robinson GE, Fahrbach SE. Experience and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J Neurosci. 2001;21:6395–404.
Muscedere ML, Traniello JFA. Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct sub-caste- and age-related patterns of worker brain organization. PLoS One. 2012;7:e31618.
Sulger E, Mcaloon N, Bulova SJ, Sapp J, O'Donnell S. Evidence for adaptive brain tissue reduction in obligate social parasites (Polyergus mexicanus) relative to their hosts (Formica fusca). Biol J Linn Soc. 2014;113:415–22.
O’Donnell S, Bulova SJ, DeLeon S, Barrett M, Fiocca K. Caste differences in the mushroom bodies of swarm-founding paper wasps: implications for brain plasticity and brain evolution (Vespidae, Epiponini). Behav Ecology Sociobiol. 2017;71:116.
Gronenberg W. Modality-specific segregation of input to ant mushroom bodies. Brain Behav Evol. 1999;54:85–95.
Revell LJ. Phytools: An R package for phylogenetic comparative biology (and other.. things). Methods Ecol Evol. 2012;3:217–23.
Winston ME, Kronauer DJ, Moreau CS. Early and dynamic colonization of central America drives speciation in Neotropical army ants. Molec Ecol. 2017;26:859–70.
Wilson EO. The sociogenesis of insect colonies. Science. 1985;228:1489–95.
Sutcliffe GH, Plowright RC. The effects of food supply on adult size in the bumble bee Bombus terricola Kirby (Hymenoptera: Apidae). Canad Entomol. 1988;120:1051–8.
Gronenberg W, Heeren S, Hölldobler B. Age dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J Exp Biol. 1996;199:2011–9.
Rehan SM, Bulova SJ, O'Donnell S. Cumulative effects of foraging behavior and social dominance on brain development in a facultatively social bee (Ceratina australensis). Brain Behav Evol. 2015;85:117–24.
Jones TA, Donlan NA, O’Donnell S. Growth and pruning of mushroom body Kenyon cell dendrites during worker behavioral development in the paper wasp, Polybia aequatorialis (Hymenoptera: Vespidae). Neurobiol Learn Mem. 2009;92:485–95.
Whelden RM. Anatomy of adult queen and workers of army ants Eciton burchelli Westw. And E. Hamatum Fabr. (Hymenoptera: Formicidae). J N Y Ent Soc. 1963;1:14–30.
Stieb SM, Kelber C, Wehner R, Rössler W. Antennal-lobe organization in desert ants of the genus Cataglyphis. Brain Behav Evol. 2011;77:136–46.
Weiser MD, Kaspari M. Ecological morphospace of new world ants. Ecol Entomol. 2006;31:131–42.
Wrege PH, Wikelski M, Mandel JT, Rassweiler T, Couzin ID. Antbirds parasitize foraging army ants. Ecology. 2005;86:555–9.
Acknowledgements
Katherine Fiocca, Vishakha Hariawala, Annette Kang, and Sumaiya Zahid assisted with neuroanatomy data collection. Phil Ward and Sean Brady shared insights into the evolutionary history of doryline army ants. Yamile Molina, Bill Wood, Karina O’Donnell, Siobhan O’Donnell, Tom Soare, Sean Tully, Marie Clifford, and Kaitlin Baudier assisted with collections and field research. Ants were collected in Costa Rica and Ecuador under research permits from those countries.
Funding
Research was funded by NSF grant IOS 1209072 and Drexel University startup funds to S. O’D., and by funds from the German Science Foundation (DFG: BE 5177/1–1 and BE 5177/4–1) to CvB.
Availability of data and materials
All data generated and analysed during this study are included in this published article as Additional file 1: Table S1.
Author information
Authors and Affiliations
Contributions
SOD and CvB collected subject ants. SB and MB performed histology and collected neuroanatomical data. SOD performed the data analyses and wrote the paper; all authors helped to edit the manuscript. All authors read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Brain region volume and head capsule size data for all subjects used in the analysis of caste differences in army ant brain investment. Species and caste for each subject are indicated. (XLSX 40 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
O’Donnell, S., Bulova, S., Barrett, M. et al. Brain investment under colony-level selection: soldier specialization in Eciton army ants (Formicidae: Dorylinae). BMC Zool 3, 3 (2018). https://doi.org/10.1186/s40850-018-0028-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-018-0028-3