Abstract
Background
China is one of 22 countries with a high tuberculosis (TB) burden in the world. Healthcare workers (HCWs) have a high risk of contracting Mycobacterium tuberculosis infection due to insufficient infection control practices. We conducted a cross-sectional study to explore the prevalence of TB and its associated risk factors among HCWs in Chinese TB facilities.
Methods
Two hundred and forty-one TB facilities employing a total of 9663 HCWs were selected from 12 provinces in China to represent healthcare settings at the provincial, prefectural, and county levels. Structured questionnaires were used to collect information on TB infection control practices and HCWs in those facilities. Data was double entered into EpiData 3.1; TB prevalence and associated risk factors were analyzed using SPSS 21.0 with bivariate and multivariate regression models.
Results
The results showed that 71 HCWs had been diagnosed with TB, accounting for a prevalence of 760/100 000. The multivariate analysis showed that associated risk factors included belonging to the age group of 51 years and above (aOR: 6.17, 95% CI: 1.35–28.28), being a nurse (aOR = 3.09, 95% CI: 1.15–8.32), implementation of 0–9 items of management measures (aOR = 2.57, 95% CI: 1.37–4.80), and implementation of 0–1 items of ventilation measures (aOR = 2.42, 95% CI: 1.31–4.47).
Conclusion
This was the first national large sampling survey on TB prevalence among HCWs in China. It was found that the implementation of TB infection control practices in some facilities was poor. The TB prevalence in HCWs was higher than that in the general population. Therefore, TB infection control practices in Chinese medical facilities should be strengthened.
Similar content being viewed by others
Multilingual abstract
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
Tuberculosis (TB) is caused by the bacillus Mycobacterium tuberculosis (MTB) and remains one of the major problems threatening public health worldwide [1]. There were 10.4 million new cases diagnosed in 2015, accounting for a global estimated incidence of 142/100 000 [1].
China is one of 22 high TB burden countries for both TB and multidrug resistant TB cases [1,2,3,4]. The fifth national TB epidemiological survey conducted in 2010 showed that national prevalence rates of active TB, sputum smear-positive, and sputum culture-positive cases were 459/100 000, 66/100 000, and 119/100 000, respectively [5]. Hence, the epidemic of TB is one of the most important public health problems that China currently faces.
Tuberculosis is primarily a respiratory infectious disease; infection is airborne and transmitted via aerosols. Healthcare workers (HCWs) that are exposed to MTB have a 10- to 20-fold risk of contracting TB than the general population [6,7,8]. The paucity of occupational health programs tailored to HCWs resulting in inadequate information and equipment to prevent or minimize exposure to TB, in addition to structurally obsolete healthcare facilities with inadequate ventilation, are likely key drivers of such increased risks. Moreover, as people with latent TB infections (LTBIs) usually go undiagnosed, the implementation of TB infection control policies in healthcare facilities, which includes the diagnosis of LTBIs, is imperative to decrease TB transmission, incidence, and prevalence among HCWs [9]. Systematic application of TB infection risk assessments, formulation and implementation of infection control strategies, early diagnosis, and prompt treatment are vital strategies that should be incorporated into TB infection control programs to effectively reduce nosocomial TB infection risk [7].
Despite the World Health Organization establishing an international policy that aims to protect HCWs from acquiring TB [8], infection control practices have been insufficiently implemented in China, where LTBI screening policy, personal protective strategies, and risk assessments that should be embedded in routine workflows [9, 10] are not consistently undertaken. It is therefore imperative to understand the current status of TB infection control in Chinese TB facilities, in which HCWs are at a higher risk of contracting TB infection.
Study conducted in 22 TB facilities in three Chinese provinces provided a general understanding on the implementation of TB infection control policies and associated risk factors for TB infection in HCWs [11, 12]. This study, however, aims to assess the nationwide status of TB infection control in China: we conducted a cross-sectional study involving TB healthcare facilities of different levels of complexity in 12 Chinese provinces. We aimed to determine the prevalence of TB among HCWs, identify the key components driving TB infection risk among HCWs, and lay out the evidence for policy development in TB infection control.
Methods
Study design and sampling
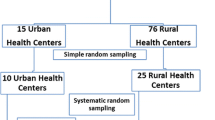
Based on the composition of HCWs at the different facilities, stage of the implementation of TB infection control measures, and willingness to be involved in this study, healthcare facilities from four eastern provinces (Jiangsu, Zhejiang, Shandong, and Liaoning); four central provinces (Anhui, Jiangxi, Henan, and Shanxi); and four western provinces (Shaanxi, Yunnan, Gansu, and Sichuan) were enrolled in the study. In each province, facilities from three prefectures were included and from each prefecture, facilities from four counties were included.
In order to reflect the organizational and structural heterogeneities of healthcare facilities in China, the TB facilities were selected as follows: (i) one TB dispensary and one TB specialized hospital from each provincial level; (ii) one TB dispensary or one infectious disease hospital from each prefectural level; (iii) one specialized hospital or general hospital with a TB ward from each prefectural level and; (iv) one TB dispensary with an outpatient department or designated hospital with an outpatient department, or general hospital with a TB ward from each county level. In total, 241 facilities were enrolled in this study.
All the HCWs in these facilities including administrative staff, doctors, nurses, and medical technicians were enrolled. Both long-term and temporary employees were included. In total, 9663 HCWs participated in this study.
Data collection
Data were collected via structured questionnaires between February and October 2013.
Provincial-level staff members were trained on the principles and practices of TB infection control by researchers from the Department of Science and Technology at the Chinese Centre for Disease Control and Prevention (China CDC). Those working in provincial facilities were responsible for collecting the data in all facilities (“investigators”) following training and assessment by the China CDC. The structured questionnaires were designed in accordance with the Chinese standard practice procedures for TB infection control [13] and included TB infection control questions around healthcare facilities and HCWs.
The questionnaire for healthcare facilities contained 11 sections (see Table 1) and was completed by the investigators. It elicited general background information on the facility, its management structure, administrative implementation, and the implementation of infection control strategies in outpatient departments, laboratories, or inpatient wards. Observations were made about administrative measures, design of the premises, environmental measures, and individual protection of HCWs in outpatient departments, wards, and laboratories.
The questionnaire for the HCWs elicited information on demographics, duration of employment at TB facility, professional category and length of service in current position, history of training to conduct TB infection control, smoking history, previous and/or current history of TB infection, and, if infected, details of laboratory results and treatment. The investigators collated this information and completed the questionnaires by reviewing professional and medical records, and by interviewing the relevant staff members in the event clarification was required.
Quality control
Data were collected by investigators who were deemed eligible after being examined by China CDC researchers. All healthcare facilities that were approached by our team agreed to participate in the study. Senior researchers from the China CDC were available to provide technical support and supervision as required. All questionnaires were double-checked for completeness by investigators. Healthcare facilities in Shandong, Henan, and Shanxi provinces in the eastern, central, and western provinces, respectively, were randomly selected for audit of filled questionnaires by senior researchers.
Data were double entered into EpiData 3.1(manufacture: The EpiData Association, location: Denmark) and checked for coincidence rate, abnormal values, and logical errors by trained data entry clerks.
Data analysis
Data were analyzed using SPSS 21.0 (manufacture: Chinese SPSS company, serial number: 20131105-LSZU, location: Shanghai in China). The associations between the prevalence of TB and its risk factors were assessed by bivariate regression analysis. In the bivariate analysis, variables (P ≤ 0.2) were retained in the multivariate regression analysis. Then, forward selection of variables was used in the multivariate regression model, and odds ratios (ORs) and their 95% confidence intervals (CIs) were determined.
Results
Distribution of survey sites enrolled in the study
Of the 241 facilities, 29 were excluded from the study as they did not have outpatient departments or wards, or lacked information on managerial strategies and environmental control to tackle nosocomial TB infections (e.g. floor plans, ventilation systems, ultraviolet germicidal lamps, etc.). Therefore, the final analysis was undertaken using data provided by 212 facilities and 9336 HCWs.
Of the 212 survey sites, 30.7% were in eastern China, 34.0% were in central China, and 35.3% were in western China. To break it down further, 71.7, 22.2, and 6.1% of the sites were at the county, prefectural, and provincial levels, respectively. Of all the sites, 78.8% were TB dispensaries and only 3.3% were TB specialized hospitals; 46.2% had wards, outpatient departments, and laboratories; and 92.0% had TB prevention departments (see Table 2).
Of the 9336 HCWs, 49.0% were from the eastern, 23.3% from the central, and 27.7% from the western provinces, respectively. Of all the HCWs, 54.5% worked in prefectural facilities. In terms of sex, 35.2% were male and 64.8% were female. The average age of HCWs was 38 years old (range 18–81) and 31.8% were below 30 years of age. In terms of education, 39.9% had a bachelor’s degree, 31.1% had a junior college degree, and only 4.7% had a master’s degree. The average duration of employment in all facilities was 16.7 years (range 1–64 years); 25.4% worked in TB prevention facilities for less than 5 years and 26.1% worked for more than 26 years. In terms of qualifications, 33.4% had a primary professional qualification and 11.4% had a senior professional qualification. Of all the HCWs, 35.1% were doctors and 28.4% were nurses (see Table 3).
Regarding infection with TB, 71 HCWs had been diagnosed with TB during the study period. This comprised: 30 nurses (42.3%), 28 doctors (39.4%), eight administrative staff members (11.3%), and five medical technicians (7.0%). Of the 71 TB cases, 28 (39.4%) were male and 43 (60.6%) were female; 28 (39.4%) were 30 years old or less. Five (7.0%) of the 71 TB cases were classified as sputum smear-positive and two (2.8%) were sputum-culture positive. In contrast, three (4.2%) had no bacterial evidence. In terms of outcome, 50 (81.7%) had been cured, while 13 (18.3%) were still under treatment upon the survey’s completion (see Table 4).
Of all the facilities under study, 85.4% (181/212) had carried out suspected symptom screening for healthcare visitors. Moreover, 92.3% (12/13) of provincial facilities successfully implemented this practice. Of all the facilities, 99.1% (210/212) had implemented TB infection control evaluation, but only one measure was evaluated in 66.0% (140/212) of the facilities. Of the facilities, 39.6% (84/212) conducted administrative implementation, with 17.0% (36/212) conducting 1–9 administrative measures. Almost all facilities (97.2%; 206/212) had appropriate ventilation systems and 94.3% (200/212) implemented 2–4 measures. In addition, 36.3% (77/212) conducted implementation of ultraviolet germicidal lamps installation with proper maintenance (see Table 5).
Prevalence of and risk factors for TB
The overall prevalence of TB among HCWs was 760/100 000 (71/9336 × 100 000). The TB prevalence of males was 851/100 000 and of females it was 711/100 000. The TB prevalence of non-smokers was 756/100 000 and of smokers it was 973/100 000. No statistical significance (P > 0.05) was found between educational status (master’s degree: 913/100 000; bachelor’s degree: 724/100 000; junior college: 931/100 000; and junior school or below: 572/100 000).
Based on the result of the bivariate analysis, variables such as inpatient wards, screening of patients, department and staff setting, staff training, and evaluation measures showed no association with TB prevalence. Tuberculosis prevalence was associated with variables related to the healthcare setting, such as different level of healthcare facility, geographical area, local implementation of policies and use of allocated budgets, availability of personal protective equipment, implementation of administrative measures, promotion and education, floor plan design, ventilation, ultraviolet lamp installation and maintenance, and variables related to the individual HCWs, such as age group, occupational category, professional qualification, and duration of employment. All these variables were found to have a P-value of ≤0.2 and were entered in the multivariate regression model (see Table 6).
The multivariate regression analysis showed that HCWs aged 51 years and above were more susceptible to TB (aOR = 6.17, 95% CI: 1.35–28.28) and nurses were considered a very high-risk group (aOR = 3.09, 95% CI: 1.15–8.32). Implementation of 0–9 items of administrative measures (aOR = 2.57, 95% CI: 1.37–4.80) and of 0–1 items of ventilation measures (aOR = 2.42, 95% CI: 1.31–4.47) were shown to be risk factors for TB (see Table 6).
Discussion
This study found that HCWs have a high risk of contracting TB infection due to frequent contact with TB patients and poor observance of protection measures [12, 14, 15]. Although previous reports have described TB prevalence among HCWs in local areas of China [16], this is the first national large sampling survey on TB prevalence in HCWs and associated risk factors, involving both demographics of HCWs and TB infection control policies [16, 17].
The TB prevalence among HCWs in this study was 760/100 000, which is much higher than the TB prevalence in both the general population of China in 2010 (459/100 000) [5] and TB prevalence among HCWs in other countries in 2010 and 2005 respectively [12, 18]. For example, TB prevalence in a survey of 22 healthcare institutions in Beijing, Inner Mongolia, and Shanghai was shown to be 665/100 000 [12]; another survey conducted among HCWs in Mexico showed a prevalence of 439/100 000 [18].
The prevalence of TB among HCWs in eastern China (612/100 000) was lower than that in the west (889/100 000) and central China (919/100 000); both of the latter are considered developing areas when compared to the east. The prevalence in the three areas was also higher than that in the general population (east area: 291/100 000; central area: 463/100 000; west area: 695/100 000) [5]. Other studies showed that the prevalence of LTBIs among HCWs in high-income countries was generally low [19, 20], and a high prevalence of TB was associated with living in poor economic areas and having a low-level education. We did not analyze the association between TB prevalence and economy due to a lack of economic information. However, we did confirm that the prevalence of TB among HCWs in provincial facilities (505/100 000) was lower than that in prefectural (825/100 000) and county-level (836/100 000) facilities. The reason for this might be that provincial facilities had the best practices of TB infection control compared with the prefectural and county-level facilities, which resulted from sufficient manpower and material resources. In addition, HCWs with higher education who had a better understanding of TB infection control practices might pay more attention to TB infection control at higher complexity level facilities.
Nurses were likely to acquire TB in our study, corroborating existing literature (e.g. a study conducted in the Limpopo province of South Africa) [21]. Several previous studies showed a higher TB prevalence of male HCWs than female ones [12, 14, 15, 22], which was contrary to our finding. Our study did not find that HCWs who had worked longer periods in TB facilities were more susceptible to be infected with TB [6, 18, 23]. However, results indicated that HCWs aged 51 years or above were at higher risk of contracting TB, in line with the available literature [24]. This might be due to impaired immunity in older people [25] and higher exposure to TB patients [16]. In terms of occupation, TB prevalence was highest in nurses, followed by doctors. This is probably due to this group’s close contact with patients, particularly the most infectious ones who are subjected to high-risk investigations, such as bronchoscopy examinations [26].
Tuberculosis prevalence among HCWs was associated with administrative control implementation of TB infection control measures, environmental control measures, personal protection [27]. China published a manual for TB infection prevention and control in 2012, which included guidelines primarily on organizational management, plus three other overarching groups of measures: (i) administrative control; (ii) environmental control; and (iii) individual protection [13]. However, these measures were not being implemented well in healthcare facilities [11, 28]. A study in Brazil showed that TB prevalence among HCWs decreased from 580/100 000 to 370/100 000 person-months after implementation of effective administrative control measures [29]. Another study in Northern Ireland showed that TB prevalence among HCWs working in facilities with good implementation of infection control measures and individual protection was not higher than in the general population [30]. Regular screening of HCWs has also been shown to be effective for TB prevention [31].
In this study, the implementation of administrative measures and appropriate ventilation was associated with TB prevalence among HCWs. Lack of good practice in terms of implementation of administrative measures (e.g. independent waiting areas, early diagnosis, cohorts of TB and non-TB patients in wards and outpatient departments) and the absence of appropriate ventilation systems (e.g. removal of mechanical ventilation equipment in outpatient departments or wards) increased the risk of TB.
Our study had limitations that may have impacted on the general validity of the findings. Firstly, facilities were not randomly selected—purposive and typical sampling can cause selection bias. Secondly, data on HCWs were collected by provincial staff members using either personal files or interviews, and false or outdated information may have been collected. Thirdly, HCWs could have been infected either in TB facilities or in the community, and the fact this distinction was not established may have overestimated the prevalence of infection as a result of occupational exposure. Fourthly, not all TB-infected HCWs had microbiological confirmation and this could have also led to overestimation of prevalence. Finally, the cross-sectional design of our study was not ideal to infer causality.
Conclusions
This was the first large national survey conducted on TB prevalence among HCWs in China. We confirmed that TB prevalence among HCWs is higher than in the general population and is associated with age, professional category, and implementation of managerial infection control measures and ventilation systems in TB facilities. Our study indicates a pressing need to strengthen TB infection control practices in China.
Abbreviations
- China CDC:
-
Chinese Center for Disease Control and Prevention
- CI :
-
Confidence interval
- HCW:
-
Healthcare worker
- LTBI:
-
Latent tuberculosis infection
- MTB:
-
Mycobacterium tuberculosis
- OR :
-
Odds ratio
- TB:
-
Tuberculosis
References
World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization Press; 2016.
World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization Press; 2015.
World Health Organization. Global Tuberculosis Report 2012. Geneva: World Health Organization Press; 2012.
World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB):2010 global report on surveillance and response. Geneva: World Health Organization Press; 2010.
Wang Y. Report on the fifth national epidemiological sampling survey of tuberculosis. Beijing: Press of Military Medical Sciences; 2011. (in Chinese)
Dimitrova B, Hutchings A, Atun R, Drobniewski F, Marchenko G, Zakharova S, Fedorin I, Coker RJ. Increased risk of tuberculosis among health care workers in Samara oblast, Russia: analysis of notification data. Int J Tuberc Lung Dis. 2005;9(1):43–8.
Granich R, Binkin NJ, Jarvis WR. Guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. Geneva: World Health Organization; 1999.
World Health Organization. Jonit WHO/ILO policy guidelines on improving health workers access to prvention, treatment and care services for HIV and TB. Geneva: World Health organization Press; 2010.
Wang FT, Deng YF, Li Y, Zhang YN, Zheng JL, Liu ZM. Study on risks of tuberculosis infections among health care workers in tuberculosis special hospitals. Chin J Nosocomiology. 2012;22(8):1674–6. (in Chinese)
Zhao F, Cheng J, Cheng SM, Zhang H, Zhao YL, Zhang CY, Hu DM, Fan HY, Huang F, Qu Y, He GX, Wang LX. The current status and challenges regarding tuberculosis infection control in health care facilities in China. Biomed Environ Sci. 2015;28(11):848–54.
Xiong YC, He GX, Zhao JZ, Hou YY, Hong F, He XX, Zhang WM, Zhang ZS, Cui ZL, Ren YL, Ren LP, Guo H, Zhao F, Li M. Status of tuberculosis infection control in different levels of health care facilities. Chin J Infect Control. 2012;11(4):247–51. (in Chinese)
Hou YY, Tan JB, He GX, Gao TJ, Xiong YC, Hong F, He XX, Zhang WM, Cui ZL, Ren YL, Ren LP, Guo H, Zhao F, Li M. Analysis of the prevalence of tuberculosis disease among health care workers in three regions and its associated factors. Chin J Antituberculosis. 2012;34(6):341–5. (in Chinese)
Wang LX, Cheng SM, He GX. Chinese standard practice procedure for tuberculosis infection control. Beijing: People’s Medical Publishing House; 2012. (in Chinese)
Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007;11(6):593–605.
He GX, Wang LX, Chai SJ, Klena JD, Cheng SM, Ren YL, Ren LP, Gao F, Li YY, He GM, et al. Risk factors associated with tuberculosis infection among health care workers in Inner Mongolia. China Int J Tuberc Lung Dis. 2012;16(11):1485–91.
He GX, van DenHof S, van der Werf MJ, Wang GJ, Ma SW, Zhao DY, Hu YL, Yu SC, Borgdorff MW. Infection control and the burden of tuberculosis infection and disease in health care workers in China: a cross-sectional study. BMC Infect Dis. 2010;10:313.
He GX, Li Y, Zhao F, Wang LX, Cheng SM, Guo H, Klena JD, Fan HY, Gao FF, Gao F, et al. The prevalence and incidence of latent tuberculosis infection and its associated factors among village doctors in China. PLoS One. 2015;10(5):e124097.
Laniado-Laborín RCN. Tuberculosis in healthcare workers at a general hospital in Mexico. Infect Control Hosp Epidemiol. 2006;27(5):449–52.
Seidler A, Nienhaus A, Diel R. Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration. 2005;72(4):431–46.
Blumberg HM, Watkins DL, Berschling JD, Antle A, Moore P, White N, Hunter M, Green B, Ray SM, McGowan JE Jr. Preventing the nosocomial transmission of tuberculosis. Ann Intern Med. 1995;122(9):658–63.
Malangu N, Legothoane A. Analysis of occupational infections among health Care Workers in Limpopo Province of South Africa. Glob J Health Sci. 2012;5(1):44–51.
Kayanja HK, Debanne S, King C, Whalen CC. Tuberculosis infection among health care workers in Kampala, Uganda. Int J Tuberc Lung Dis. 2005;9(6):686–8.
Zhang X, Jia H, Liu F, Pan L, Xing A, Gu S, Du B, Sun Q, Wei R, Zhang Z. Prevalence and risk factors for latent tuberculosis infection among health care workers in China: a cross-sectional study. PLoS One. 2013;8(6):e66412.
Zhou F, Zhang L, Gao L. Latent tuberculosis infection and occupational protection among health care workers in two types of public hospitals in China. PLoS One. 2014;9(8):e104673.
Xia H. Comparative study of CT and MRI clinical symptoms of elderly spinal tuberculosis. J Med Imaging. 2012;22(2):321–2.
Harries AD, Maher D, Nunn P. Practical and affordable measures for the protection of health care workers from tuberculosis in low-income countries. Bull World Health Organ. 1997;75(5):477–89.
Hou YY, Xiong YC, He GX, Guo H, Zhao F, Zhang WM, Li M. Analysis of the prevalence of tuberculosis disease among health care workers and its associated factors. Chin J Nosocomiology. 2012;22(19):4428–30. (in Chinese)
Zhang WM, Hong F, Wang HD, He GX, Guo H, Zhang XM. The implementation of “handbook of Chinese control and prevention of TB infection” in four health-care facilities. Chin J Antituberculosis. 2011;33(8):475–9. (in Chinese)
Da Costa PA, Trajman A, Mello FC. Administrative measures for preventing Mycobacterium tuberculosis infection among healthcare workers in a teaching hospital in Rio de Janeiro. Brazil J Hosp Infect. 2009;72(1):57–64.
Riley M, Loughrey CM, Wilkinson P, Patterson CC, Varghese G. Tuberculosis in health service employees in Northern Ireland. Respir Med. 1997;91(9):546–50.
Tudor C, Van der Walt M, Hill MN, Farley JE. Occupational health policies and practices related to tuberculosis in health care workers in KwaZulu-Natal, South Africa. Public Health Action. 2013;3(2):141–5.
Acknowledgments
We thank all healthcare workers and investigators for their collaboration. We also acknowledge Dong-Mei Hu, Meng Li, Jun Cheng, and Shi-Cheng Yu for their help in this study.
Funding
Funding was obtained from the Global Fund Project (TB12-0010) and the Research Project and Achievement Management of Department of Science and Technology of the China CDC. The contents of this publication are the sole responsibility of the authors.
Availability of data and materials
According to the consent for participants, the original data can be used only by our researchers and cannot be provided to others.
Author information
Authors and Affiliations
Contributions
X-NW implemented the study, managed and analyzed the data, interpreted the data and wrote the manuscript. G-XH and FZ convinced the idea and designed the research, supervised the study, revised the manuscript. T-LH, M-JG and Y-DS managed and analyzed the data, drafted the manuscript. J-CW, ML, SJH, and ALGC interpreted the data and revised the manuscript. Y-LZ and YP provided support in study design and revised the manuscript. All the authors reviewed and approved the final version of the manuscript submitted to the journal.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our study was approved by the Chinese Tuberculosis Research Institutional Review Board for TB Operational Research (approval notice: NO.002). Official representatives of healthcare facilities were briefed about the study. All participants were adult HCWs and provided written informed consent, after being explained the purpose of the research, collection of information, and use of data for publishing. All information obtained served exclusively for the purposes of this analysis. Data were kept confidentially and questionnaires were disposed of appropriately after the research.
Consent for publication
Written informed consent was obtained from the participants for the publication of this paper.
Competing interests
The authors declare that they have no competing interests.
Additional file
Additional file 1:
Multilingual abstract in the five official working languages of the United Nations. (PDF 433 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, XN., He, TL., Geng, MJ. et al. Prevalence of and risk factors for tuberculosis among healthcare workers in Chinese tuberculosis facilities. Infect Dis Poverty 7, 26 (2018). https://doi.org/10.1186/s40249-018-0407-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-018-0407-6