Abstract
Background
Tuta absoluta (Meyrick), a major invasive pest of Solanaceous plants, was recently detected in Botswana. Abiotic and biotic factors, together with a suite of population demographic traits are likely key for species propensity and invasion success. First, we determined the movement of T. absoluta from its core detection centre to new invasion areas using pheromone baiting and established likely biotic dispersal drivers. Second, we measured thermal tolerance vis critical thermal limits and lower and upper lethal limits to determine how these traits shape population establishment.
Results
We detected T. absoluta in all 67 pristine sites across nine districts of Botswana. Within-district trap catches varied between cultivated and wild hosts but were generally not statistically significant (P > 0.001). We report three major wild host plants for T. absoluta as biotic dispersal drivers: Solanum coccineum (Jacq.), Solanum supinum (Dunal) and Solanum aculeatissimum (Jacq.). Solanum coccineum and S. supinum were omnipresent, while S. aculeatissimum distribution was sporadic. Thermal tolerance assays showed larvae were more heat tolerant, with a higher critical thermal maxima (CTmax) than adults (P < 0.001), whereas the adults were more tolerant to cold with a significantly lower (P < 0.001) critical thermal minima (CTmin) compared to larvae. The upper lethal temperatures ranged from 37–43 °C, whereas the lower lethal temperatures ranged from − 1 to − 12 °C for 0–100% mortality, respectively. In the light of prevailing environmental (habitat) temperatures (Thab), warming temperature (7.29 °C) and thermal safety margin (22.39 °C) were relatively high.
Conclusion
Tuta absoluta may not be under abiotic physiological or biotic constraint that could limit its geographical range extension within Botswana. The ubiquity of wild Solanaceous plants with the bridgehead of year-round intensive monocultures of Solanaceous crops within a favourable climatic framework may mean that environmental suitability aided the rapid spread of T. absoluta.
Similar content being viewed by others
Background
Invasive species are a major threat to agroecosystems and global change [1, 2] and increased global connectivity [3] has drastically increased the diversity and magnitude of such invasions especially in the hot-dry Afrotropical region. Tomato leaf miner, (Tuta absoluta) (Meyrick) (Lepidoptera: Gelechiidae), is one of the most destructive insect pests of tomatoes globally [2, 4]. It is of South American origin and was first detected in Spain in 2006 [5, 6] before rapidly spreading and establishing in novel environments in the Mediterranean Basin, Europe, Middle East, South Asia (India), north, east and west Africa [5,6,7,8,9] and recently Southern Africa [10,11,12]. Because of its high reproductive potential, multivoltinism and potential to acclimatize to different climatic conditions [1], T. absoluta is currently considered a key limiting phytosanitary factor affecting the global Solanaceous crops value chain [13].
The larvae of T. absoluta feeds on all aerial parts of the plants including the fruits, resulting in significant yield losses and cosmetic damages as well as secondary infection [14, 15]. Characteristic larval mesophyll mining also compromises photosynthetic capacity of crops significantly reducing yields [14]. In the absence of control, yield losses ranging 80–100% have been reported in open and protected tomato fields [5]. A cost–benefit analysis has shown a significant increase in cost of production through high use of insecticides [2, 16], increased tomato market prices as farmers try to recover the high production cost, spatial prohibition of tomato seedlings and fruits trade [17] culminating into overall increased food and nutrition insecurity [18].
In tropical sub-Saharan Africa, irrigated tomatoes are an essential component of horticulture, a major pillar of sustainable development, with a significant contribution to food and nutritional security as well as household source of income especially for resource-poor farmers [18]. However, a major constraint to growing field horticultural crops in Southern Africa is the reduction in yield and quality caused by insect pests [19]. The potential invasion of Southern Africa by T. absoluta has already been described [1, 3, 6] with models based on its invasion history and global warming [20]. However, there are no reports based on field data on its thermal fitness and how this correlates with availability and distribution biotic resources, e.g. wild host plant species. Although T. absoluta survival on wild Solanaceae, Amaranthaceae, Fabaceae, Chenopodiaceae and Asteraceae plant families was reported, no report has so far combined this knowledge with its on-going invasive movement in the light of prevailing climate data.
For an invasive species to be established, it first has to overcome several environmental barriers [21] including transport, introduction, population establishment and spread [22]. Upon introduction into a novel environment, high propagule pressure [23], species genetic and demographic characteristics [24] and physiological tolerance allow the establishment and habitat permeability [3]. Climate synchrony should exist between introduced areas’ and species’ environmental stress tolerance to allow successful spread during transience and niche occupation post-invasion [3, 25]. As such, physiologists often use species’ thermal tolerance assays as proxy for determining potential for establishment of invasive species. Similarly, it has also been clear from modelling studies, that even when propagule material is high, environmental suitability remains an overriding factor for invasive species successful establishment [3, 23, 26]. Indeed, physiological assays have found use in niche modelling and invasive species risk assessments to determine critical risk invasion areas [27, 28]. Tuta absoluta is known to respond naturally to rapidly changing environments [29]. This is characteristic of successful invaders, which should inherently possess high basal and plastic physiological tolerance, including rapid genetic adaptive shifts [30]. Nevertheless [3], also show that native environmental heterogeneity may contribute to species invasive success. This means, species coming from a more heterogeneous environment may likely cope with a changing novel environment through phenotypic adjustment, compared to those coming from a more stable environment.
Temperature is the most important abiotic factor exerting direct and indirect effects on T. absoluta population dynamics [1] and consequently invasion success [31, 32]. Therefore, temperature forms a first abiotic ‘ecological filter’ [33] for successful invasion in a new environment [34], and failure to mount any compensatory mechanisms against it may result in the species failing to establish [35, 36]. The proximity of the environmental temperatures to species thermal physiological limits can therefore indicate species vulnerability and dispersal fitness [31]. Species introduced into habitats close to their thermal tolerance limits are more affected by environmental temperature [37] than those introduced into habitats far from their thermal tolerance limits.
Insects have been reported to experience multiple overlapping abiotic and biotic stressors such as temperature, starvation and desiccation in the wild [3, 38, 39]. Hence, an understanding of bioecology of invasive species is of paramount importance in enlightening mechanisms underlying the successful spread and establishment of invasive alien species [1, 40]. This will also involve determining how the invasive species may respond to native wild host plants. The availability and distribution of alternative wild (non-cultivated) host plants play a significant inoculum sink–source role across the novel landscapes [6]. Since its detection in Zambia [41], South Africa [10, 42] and Botswana [12], no work has documented T. absoluta spread and establishment across the biotic and abiotic frontiers. Here, we ought to establish whether T. absoluta was indeed spreading and elucidate the major environmental drivers to successful establishment in Botswana. We measured its thermal tolerance vis limits to activity (critical thermal minima [CTmin] and critical thermal maxima [CTmax]) and lethal limits (lower and upper lethal limits [LLT] and [ULT], respectively) and compared this with prevailing ambient climatic environment. Second, we investigated wild Solanaceous host diversity and linked this to T. absoluta invasion. To date, data on T. absoluta invasion potential in tropical climates have only been derived from modelling [1, 2]. No studies have looked at T. absoluta physiological thermal tolerance limits with field climate data to test the possible role of climate on its range expansion and spread. Similarly, no study has coupled physiological tolerance and its interaction with host availability on T. absoluta invasion pathway. The objective of this study was therefore to investigate whether T absoluta has spread and established from its core detection site across other pristine districts of Botswana, since its first detection [12]. Such information is important for pest risk assessments, niche modelling and may aid in developing phytosanitary regulations for effective invasive pest management.
Methods
Insect trapping and sites
Following the detection of T. absoluta at Genesis farm (S21.14776; E27.64744), Matshelagabedi village in the North East District of Botswana December 2016 [12], a follow-up surveillance trapping was conducted across 9 of the 10 districts of Botswana (Fig. 1). Traps were not set in Kgalagadi as it is largely part of the Kalahari Desert with very minimal vegetation, human settlement and agricultural activity. A total of 201 (67 sites with 3 traps per site) yellow delta traps (Chempac-Progressive-Agricare®) (Suider Paarl, South Africa) equipped with sticky pads were placed ~ 1 m above ground in tomato fields (cultivated host) and open forests (wild hosts) in each of the study districts during the hot-rainy summer season when the wild hosts were flourishing. High temperature, high relative humidity [1] and presence of the host [2] were reported to possibly enhance its propensity to spread. The major male-attracting synthetic sex pheromone (3E,8Z, 11Z)-3,8,11-tetradecatrienyl acetate (TDTA) loaded on grey rubber dispensers at a dosage of 110 µg per lure, (Tuta absoluta-optima PH-937-OPTI Russel IPM, Flintshire, UK) was used. Trap catch data were collected after ~ 30 days, and trapped moths were counted using dyed pointers (chopsticks dipped in insect dye) and mechanical (tally) counters following gross morphological identification [43]. Global Positioning System (GPS) points were recorded for each trapping site using a Garmin® (GPSMAP 62 model, Olathe, USA). Climate data for the sampling areas were obtained from the Meteorological Department, Ministry of Environment, Wildlife and Tourism (MEWT), Republic of Botswana.
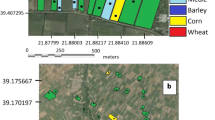
a New detection sites for T. absoluta (Meyrick) from traps set in the wild (empty rhombus) and on cultivated host plants (Solanum lycopersicum (L.)) (black rhombus) as well as the core detection District (red) [11] in Botswana, and b occurrence and distribution of wild alternative wild host plants S. aculeatissimum, S. coccineum and S. supinum in Botswana for T. absoluta. (Based on data courtesy of National Botanical Gardens, Botswana National Museums, Gaborone, Botswana)
Basal thermal tolerance experiments
Insect culture
Larvae were collected on damaged tomato fruits into insect cages (BugDorm®, MegaView Science Co., Ltd. Taiwan) from Noka farm (North East District) (S21.12860; E27.48830), with a general temperature range of 3.4–35.5 °C; mean, mean minimum and monthly temperature range of 20.5–22.6 °C, 11.9–13.3 and 29.1–30.4 °C, respectively [44]. These were allowed to pupate in the laboratory in climate chambers Memmert® climate chambers (HPP 260, Memmert GmbH + Co.KG, Germany) set at 25 ± 1 °C, 65 ± 5% relative humidity (RH) and 12L–12D photoperiod. This laboratory rearing temperature closely approximated mean annual temperature from the environment from which the specimens were collected. Eclosed T. absoluta adults were placed in 25-cm3 clean cages, where they fed on 10% sucrose solution ad libitum using the cotton dental wick source method (a feeding apparatus for liquid-feeding insects; insects suck the liquid from a wet cotton wick that draws the solution through capillarity) and provided with organically produced tomato-fruiting plants to lay eggs. Experiments were conducted using fourth instar F1 generation larvae and freshly emerged unsexed adults (± 2 days old). Sex was not considered a factor in our experiments since it has been reported not to affect thermal tolerance traits in some related species (e.g. [45,46,47]).
Lethal temperature assays
Lethal temperatures were determined using established methods as outlined in [48]. Upper and lower lethal temperatures (ULTs and LLTs) were determined using direct plunge protocol at 2-h duration at temperatures that elicited 0–100% mortality. Ten insects were placed in 60-ml polypropylene vials with gauzed lids and placed in a 33 × 22 cm ziplock bag, replicated three times. This was then plunged into a Merck® water bath (Modderfontein, South Africa) filled with 99.9% circulating ethanol. For ULT, tiny wet filter paper was suspended in each vial to maintain benign humidity and prevent desiccation-related mortality. Following treatment (ULT and LLT), test insects were placed at 25 ± 1 °C and 65 ± 5% RH in Memmert® climate chambers for 24 h before scoring survival. All insects had access to food and water ad libitum during the 24-h recovery period. Survival was defined as the ability to coordinate muscle response to stimuli such as gentle prodding, or normal behaviours such as feeding, flying or mating [48, 49].
Critical thermal limits (CTLs)
CTLs were assayed using a programmable waterbath (LAUDA Ecogold® RE 2025, Lauda-Königshofen, Germany) connected to a transparent double-jacketed chamber as outlined by [45]. A thermocouple (type K 36SWG) connected to a digital thermometer (Fluke 54 series IIB) was inserted into the central organ pipe (control chamber) to record chamber temperature. A total of ten test insects replicated three times to yield 30 replications per treatment were used in these experiments. Test insects were individually placed into the organ pipes of the double-jacketed chamber connected to a programmable water bath filled with 1:1 water: propylene glycol to allow for subzero temperatures [50]. Both CTmax and (CTmin experiments started from an ambient set point temperature of 25 °C from which temperature was ramped up (CTmax) or down (CTmin) at 0.25 °C/min until CTLs were recorded. Although it is likely faster than natural diurnal heating or cooling rates in the wild [45], this ramping rate was chosen as a compromise between ecological relevance and maximum throughput (see also discussions in [45, 51]). In this study, we defined CTLs as the temperature at which each individual insect lost coordinated muscle function and the ability to respond to mild stimuli (e.g. prodding with a thermally inert object).
Data analyses
New detection sites and the distribution of wild Solanaceous host plants were presented on maps (ArcGIS, ArcMap 10.2.2). Trap catch and thermal tolerance data analyses were carried out in STATISTICA, version 13.2 (Statsoft Inc., Tulsa, Oklahoma) and R version 3.3.0 [52]. CTLs met the linear model assumptions of constant variance and normal errors; therefore, they were analysed using one-way ANOVA in STATISTICA. LLT and ULT assays results did not meet the assumptions of ANOVA, and thus, they were analysed using generalized linear models (GLM) assuming a binomial distribution and a logit link function in R. Tukey–Kramer’s post hoc tests were used to separate statistically heterogeneous means.
Warming tolerance (WT) and the thermal safety margin (TSM) of T. absoluta under Botswana conditions were calculated as outlined by [53]:
where CTmax = critical thermal maximum for T. absoluta adult (the migratory stage), Thab = habitat temperature—Botswana mean annual temperature for 2015/16. Topt = optimum temperature for T. absoluta.
Results
The spread of T. absoluta in Botswana
Apart from North East District, the area of T. absoluta first detection [12], the species was recorded in eight other districts (Fig. 1a). Moths were detected both in the wild (forests, grazing lands and national parks distant from agroecosystems) and on cultivated solanaceous crops; mainly tomato Solanum lycopersicum (L.). We detected T. absoluta in areas such as Moremi Island (Okavango Delta) more than 200 km from the nearest human settlements and agricultural activities and bordered by Moremi and Chobe Game Reserves) (Fig. 1a). Surveillance results support our hypotheses that T. absoluta spread and successfully established across Botswana (Fig. 1a).
Tuta absoluta wild host plants belonging to the Solanaceae family showed a cosmopolitan distribution (Fig. 1b). Wild host species diversity showed three dominant species; Solanum aculeatissimum (Jacq.), Solanum coccineum (Jacq.) and Solanum supinum (Dunal). Solanum supinum was the most widely distributed, occurring in all districts except only in Chobe, North-East and South-East and was found on the Moremi Island of the Okavango Delta (Fig. 1b) giving credence to the occurrence of T. absoluta in such a remote area. Solanum coccineum had more sporadic distribution, occurring in Chobe, Ngamiland, Ghanzi, Kgalagadi, Kweneng districts and the surrounding areas of the Okavango Delta. However, S. aculeatissimum was only found in Kgatleng district (Fig. 1b).
Moths abundance in wild and cultivated hosts
Large numbers of T. absoluta moths were captured in all districts, in both cultivated and wild hosts. The cultivated host, S. lycopersicum hosted significantly higher numbers (P < 0.001) (Table 1) than the wild host plants within districts, especially in Kweneng and Central districts (Fig. 2). Inter-district populations were also generally not significantly different within the same host type (Fig. 2). Overall, in the wild host plants, we recorded a grand mean of 411.1 ± 13.38 moths/trap/month from the cultivated S. lycopersicum which was significantly higher (P < 0.001) (Table 1) than 187.4 ± 12.21 moths/trap/month recorded from the wild hosts. High numbers were recorded on S. lycopersicum in Central, South-East, Chobe, Kgatleng and Southern districts and in tunnels compared to open fields in other districts. There were no significant interaction effects between the host plant and the district (P > 0.05) (Table 1) signifying that in each district host type was not a significant factor affecting abundance (trap catches).
Basal thermal tolerance
Both life stages of T. absoluta showed relatively high temperature tolerance although the larvae had significantly higher (47.9 ± 1.25 °C) CTmax than the adult (44.1 ± 0.43 °C) (Fig. 3A). The highest temperature where T. absoluta could not survive (ULT0) was 43.0 °C, while the highest temperature for 100% survival (ULT100) was 37 °C (for a 2-h stressful high-temperature exposure). There were significant differences (χ2 = 107.29, df = 4, P < 0.001) in survival between test temperatures, again signifying the role of temperature severity and duration in its survival (Fig. 3B). However, on low temperature tolerance, the adult had a significantly lower CTmin (− 5.2 ± 0.23 °C) than the larvae (3.5 ± 0.07 °C) (Fig. 3C), and the LLTs ranged from − 12.0 to − 1.0 °C for LLT0 and LLT100, respectively, based on a 2-h duration at stressful low temperature (Fig. 3D). There were significant differences (χ2 = 163.73, df = 6, P < 0.001) in survival between the low test temperatures, implying that survival was determined by both temperature severity and duration of exposure.
High temperature; A Critical thermal maxima (CTmax) and B upper lethal temperature (ULT), and low temperature; C Critical thermal minima (CTmin), and D lower lethal temperature (ULT) for field collected Tuta absoluta F1 applied for a 2-hour duration for 0–100% survival. CTmin and CTmax were conducted on both larvae and adult while lethal temperature assays were only conducted on adults (the migratory stage)
Climate data and basal thermal tolerance
Field temperature data from eight districts of Botswana in 2015/2016 seasons (period post-first detection prior to and during establishment and spread of T. absoluta) are shown in Fig. 4a and b. The mean monthly maximum temperatures ranged from a low of 22.3 °C (Kweneng district) to a high of 37.4 °C. (South-East district) (Fig. 4a). Highest maximum field temperatures were below T. absoluta CTmax by about 6 °C (adults) and above 10 °C (larvae). Relating ULTs to the field maximum temperature data showed that the T. absoluta ULT0 of 43 °C (Fig. 4a) was well above the highest maximum temperatures recorded in nature (37.4 °C; Fig. 4a), implying that T. absoluta was not under high-temperature-related physiological stress that could limit its spread and establishment.
The mean monthly minimum temperatures ranged from a low of 1.1 °C (Kweneng district) to a high of 21.3 °C in December 2015 (Ngamiland district) (Fig. 4b). The lowest minimum field temperatures were above T. absoluta adult CTmin by about 6 °C (adult) and below that of the larvae by about 2.4 °C (see Fig. 4b). This implied that the minimum field temperatures were not physiologically constraining survival of T. absoluta adult but the larvae. Adult T. absoluta LLT0 was − 12.0 °C, while LLT100 was − 1.0 °C for a 2-h stressful low-temperature exposure (see Fig. 3D). Both temperatures fell well below the most extreme low temperatures recorded in the environment (see Fig. 4b), implying that adult T. absoluta may not be under diurnal low-temperature physiological stress.
Warming tolerance (WT) and thermal safety margin (TSM)
The annual mean temperature for Botswana in 2015/2016 was 22.71 °C considered in this study as the habitat temperature (Thab), and the optimum temperature for T. absoluta performance and population growth is 30 °C [54] considered as Topt. The adult CTmax was recorded as 44.1 °C (see “Basal thermal tolerance” section). Based on these data, the warming tolerance (WT) and the thermal safety margin (TSM) of T. absoluta under Botswana conditions were calculated according to [53]:
and similarly,
Discussion
Following its first invasion in Botswana in December 2016 [12], our results confirm that T. absoluta has spread and successfully established in almost all districts of Botswana, thus potentially eliciting widespread economic damage to Solanaceous crops. Although T. absoluta was first recorded in North east district of Botswana, evidence from this work suggest its rapid and wide extension of its distribution horizons with new records reported in various districts in the country within an 8-month period (January to August 2017) (Fig. 1). Indeed, this trend is not unusual for the species [2]. The species has been reported to spread at a rate of ~ 800 km/year aided through wind currents and plant material belonging to families Amaranthaceae, Convolvuceae, Fabaceae, Malvaceae and Solanaceae identified through volatile cues by female moths for egg laying [2]. Therefore, the observed rapid spread and successful niche establishment may be directly linked to the reported availability of host plants in the wild [3], climate suitability and physiological thermal tolerances [26]. These characteristics are consistent with other globally invasive economic insect pest species, e.g. Chilo partellus (Swinhoe) [55], Bactrocera dorsalis (Hendel) [56], Ceratitis capitata (Wiedemann) [57] and Drosophila suzuki (Matsumura) [58]. Our results associated T. absoluta with a wide range of cultivated and wild host plants [as in example 2, 59], consistent with polyphagic characteristic of many invasive species [22]. Tuta absoluta thermal activity physiological thresholds examined here also suggest that there is a conducive climate niche across the country and that species activity and hence invasion may not be constrained by temperature. Our survey showed Botswana hosts wild solanaceous plants: S. aculeatissimum, S. coccineum and S. supinum, which are all suitable hosts to T. absoluta [see 2, 15]. Amongst these wild host plants, S. supinum was the most widely distributed, occurring in all districts of the country, while S. coccineum and S. aculeatissimum distribution was sporadic. Therefore, it is highly likely that these wild host plants provide biotic resources, (food and shelter) supporting the invasion pathway of T. absoluta in Botswana. Although tomato is the preferred host for T. absoluta, the species can switch hosts from cultivated to wild as a survival strategy, a notion supported by [5] and [60]. Such availability of biotic resources and suitable environmental conditions are also known to impair diapausing in T. absoluta larvae [1] resulting in increased breeding propagule pressure even under less favourable climate conditions, with implications on niche invasion success.
Short-distance dispersal (adjacent field to field or field to tunnels) of T. absoluta is known to be facilitated by wind especially soon after introduction [13] with moths capable of active flights of up to 100 km [59], a characteristic that may aid the species’ dispersal [2, 32, 61]. Pressure distribution from the Indian Ocean was reported to traditionally create strong east-westerly air masses in the Southern African region [62]. This supports the possible movement of T. absoluta through wind currents from the north-east district (core detection district) to the central, southern and western parts of the country. On the other hand, long-distance dispersal may occur through open tomato trade, markets and other related activities [32]. These attributes together may to a larger extent have promoted the spread and establishment of T. absoluta propagule moths which could easily locate either cultivated or wild hosts during dispersal. However, the detection of T. absoluta in Moremi Island (Fig. 1a); (~ 200 km from human settlements and agroecosystems) suggests that wind and wild host plants might have played a more significant role in its invasion success.
High populations of T. absoluta were recorded on S. lycopersicum in Central, South-East, Chobe, Kgatleng and Southern districts (Fig. 2) where production of tomatoes is done in tunnels. The reason may be that the moths were contained within tunnels and hence highly concentrated resulting in the observed high trap catches. Amongst these districts, South-East recorded the highest moth catches. The district is a horticultural hotspot with high concentration and prolonged availability of the cultivated host plants (tomato, green and red pepper) which hosts T. absoluta. Since production of tomatoes is carried out throughout the year in this district, the tunnels also act as inoculum reservoirs that form bridgeheads for further introduction and reinfestation of outdoor cultivated and wild host plants [1,2,3]. Similarly, relatively high T. absoluta moths were recorded in the wild (Fig. 2) signifying its ability to survive outside the cultivated host plant ranges (agroecosystems). This therefore nullifies the possibility of controlled production of Solanaceous crops as a management measure against T. absoluta, as has been the case, e.g. Pectinophora gossypiella (Saunders) in cotton [63].
Native environmental heterogeneity may also contribute to invasion success [3]. Propagules from a more heterogeneously stressful environment are more adaptable to multiple stressful conditions [64] and, together with other factors, may work synergistically towards the succession of ecological filters [reviewed in 3]. Climate matching between the native and novel environment is known to aid invasion success of invasive alien species [30, 65, 66]. Interestingly, African biotic and climatic conditions are closely related to T. absoluta’s native region [1]. Insect species have specific optimum temperatures at which they optimally perform and develop [31, 37]. In addition, lower and upper developmental thresholds mark the temperatures beyond which they cannot perform and develop [31, 37, 67]. As such, basal environmental stress tolerance, phenotypic plasticity and rapid genetic adaptive shifts are key to invasive species establishment [30]. Tuta absoluta tolerance to temperature and relative humidity versus typical Botswana climate [68] may form the primary characteristics defining its range expansion [1, 37, 68]. Prior predictions using climatic suitability indices defined the eco-climatic index (EI) of Botswana to fall within 20–50, classified as high risk of establishment for T. absoluta [1]. Our results are thus in agreement with this prediction. Climatic conditions (chiefly temperature) are known to significantly influence generation numbers of multivoltine insects, with higher temperatures facilitating faster degree day accumulation and shorter generation times [69]. At an optimum temperature of 30 °C, the life cycle of T. absoluta ranges approximately 26 days [53] accounting to ~ 12 generations per year [2]. Global warming comes with increased mean temperatures and variability thereof and is reported to increase insect metabolism [70]. With African temperatures projected to increase, future populations of this pest may likely increase in tropical relative to temperate regions [1]. Botswana is arid to semi-arid with mean monthly maximum temperatures recorded in 2015/2016 season ranging 23.6 to 35.1 °C (Fig. 4a). Given that the optimum temperature for T. absoluta is 30 °C [54], a TSM of 7.29 °C was relatively high [37, 53]. This signifies that T. absoluta can tolerate an increase in atmospheric temperature of 7.29 °C from current Botswana ambient environmental temperatures of 22.71 °C (Thab) before its population growth and general performance can drop to critical levels. This is a considerably high TSM compared to most tropical species whose TSM is ~ 0 °C [53]. This, coupled with a wider WT (22.39 °C), further supports that Botswana environmental temperatures were conducive for the performance, rapid spread and establishment of T. absoluta. The lower and upper developmental threshold for T. absoluta is ~ 14 and 34.6 °C, respectively [54], translating to a wide thermal window (~ 20.6 °C) which is known to optimize key insect activity and life-sustaining behaviours such as development, mating and dispersal [3, 55], and may potentially facilitate the invasion pathway of T. absoluta. Thus, conducive climatic conditions might have chiefly facilitated the rapid accumulation of degree days hence culminating into shorter generation time. This high reproductive capacity may also have contributed to its increased invasion success in novel environments in the country [as in 1].
Improved environmental tolerance and thermal plasticity are the key contributing factors towards invasion success of invasive alien species into a novel environment [30, 39, 71]. Lower and upper lethal temperatures (LLT and ULTs) for T. absoluta adults ranged from − 1 to − 12 °C and 37 to 43 °C respectively for 2 h treatments. In addition, the CTmax for larvae and adults were 47.9 ± 1.25 and 44.1 ± 0.43 and CTmin were 3.5 ± 0.07 and − 5.2 ± 0.23 respectively. Field temperature recorded during 2015/2016 season show that highest maximum temperatures were below both ULT and CTmax for both T. absoluta larvae and adults. In addition, LLTs and CTmin for adults were relatively lower than the lowest minimum field temperatures (Fig. 4b). This, added to the high TSM and WT, indicates that T. absoluta may not be at risk of cold and heat stress both of which has an implication on the invasion succession pathway. These results supports that T. absoluta is highly temperature tolerant at both extremes and may survive in arid/semi-arid sub-Saharan Africa whenever hosts plants are available. Its high basal thermal tolerance, coupled by favourable climates in Botswana (Fig. 4a and b), may mean that T. absoluta survives all-year-round temperature conditions, in the absence of diapause, a characteristic likely aiding successful establishment. Furthermore [38], showed rapid cold hardening may also aid invasion success in insects, and indeed T. absoluta has been shown to rapidly cold-harden [see 2], a phenomenon likely aiding the invasion pathway. The absence of native coevolved natural enemies has also been reported to promote invasion success in novel environments [5, 64]. It is highly likely that the rapid spread and establishment of T. absoluta in Botswana may have been facilitated by the absence of biological control agents. We thus recommend that native fortuitous natural enemies need be identified and promoted coupled with a campaign against the instinctive overuse of pesticides by small scale farmers [19] to preserve potential native natural enemies, reduce cost of production and protect public health. Further work needs to determine T. absoluta insecticide resistance to establish a controlled effective spraying program, coupled with the identified effective natural enemies to establish an efficacious tailor-made integrated pest management (IPM) program. Overall, an area-wide approach to T. absoluta management is recommended, and one that involves a coordinated Southern African region, to prevent further spread and establishment of the species [2].
Conclusion
Current results support the rapid spread and establishment of T. absoluta in Botswana following its first detection. This continued invasion by T. absoluta in tropical climates is a real concern for the horticultural industry, as well as African food and nutrition security. Host plant availability, climate suitability and high thermal tolerance may to a larger extent have contributed to the successful invasion, rapid spread and establishment of T. absoluta in the semi-arid tropical Botswana. In addition, intensive monocultures, continuous irrigation and unrestricted trade of Solanaceous crops coupled with strong winds and a lack of natural enemies may also be contributory factors. Furthermore, absence of efficient and coordinated area-wide management practices may have exacerbated the successful rapid invasion. A significant long-term management strategy would be necessary to optimize surveillance and monitoring of T. absoluta in the region for developing sustainable management options. Similarly, introduction of egg-targeting parasitoids (Trichogramma spp.) and predators as well as larval parasitoids (mostly belonging to Braconidae families) and predators (Miridae) [2, 14] could improve management of African suppression programmes, more especially in non-agroecosystem and natural environments.
References
Tonnang HEZ, Mohamed SA, Khamis F, Ekesi S. Correction: identification and risk assessment for worldwide invasion and spread of Tuta absoluta with a focus on Sub-Saharan Africa: implications for phytosanitary measures and management. PLoS ONE. 2015;10:e0138319. https://doi.org/10.1371/journal.pone.0138319.
Biondi A, Guedes RNC, Wan FH, Desneux N. Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, present and future. Ann Review Entomol. 2018;63:239–58.
Renault D, Laparie M, McCauley SJ, Bonte D. Environmental adaptations, ecological filtering, and dispersal central to insect invasions. Ann Rev Entomol. 2018;63:345–68.
Germain JF, Lacordaire AI, Cocquempot C, Ramel JM, Oudard E. Un nouveau ravageur de la tomate en France: Tuta absoluta. PHM-Revue Horticole. 2009;512:37–41.
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J, Catalán Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbaneja A. Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci. 2010;83:197–215.
Brévault T, Sylla S, Diatte M, Bernadas G, Diarra K. Tuta absoluta Meyrick (Lepidoptera: Gelechiidae): A new threat to tomato production in sub-Saharan Africa. African Entomol. 2014;22:441–4.
Pfeiffer D, Muniappan R, Sall D, Diatta P, Diongue A, Dieng EO. First record of Tuta absoluta (Lepidoptera Gelechiidae) in Senegal. Fla Entomol. 2013;96:661–2.
Chidege M, Al-zaidi S, Hassan N, Julie A, Kaaya E, Mrogoro S. First record of tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Tanzania. Agric. Food Sec. 2016;5:17. https://doi.org/10.1186/s40066-016-0066-4.
Tumuhaise V, Khamis FM, Agona A, Sseruwu G, Mohamed SA. First record of Tuta absoluta (Lepidoptera: Gelechiidae) in Uganda. Int J Trop Insect Sci. 2016;36:135–9. https://doi.org/10.1017/S1742758416000035.
Visser D, Uys VM, Nieuwenhuis RJ, Pieterse W. First records of the tomato leaf miner Tuta absoluta (Meyrick. (Lepidoptera: Gelechiidae) in South Africa. Biol Invasions. 1917;2017:6.
Chidege M, Abel J, Afonso Z, Tonini M, Fernandez B. Tomato Leaf Miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Detected in Namibe Province Angola. J. Appl. Life Sci. Int. 2017;12:1–5.
Mutamiswa R, Machekano H, Nyamukondiwa C. First Report of Tomato Leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Botswana. Agric. Food Sec. 2017;6:49. https://doi.org/10.1186/s40066-017-0128-2.
Desneux N, Luna MG, Guillemaud T, Urbaneja A. The invasive South American tomato pinworm, Tuta absoluta continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci. 2011;84:403–8.
Urbaneja A, Gonzalez-Cabrera J, Arno J, Gabarra R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean Basin. Pest Manag Sci. 2012;68:1215–22.
Bawin T, Dujeu D, De Backer L, Francis F, Verheggen FJ. Ability of Tuta absoluta (Lepidoptera: Gelechiidae) to develop on alternative host plant species. Can Entomol. 2016;148:434–42.
Toševski I, Jović J, Mitrović M, Cvrković T, Krstić O, Krnjajić S. Tuta absoluta (Meyrick, 1917) (Lepidoptera, Gelechiidae): a new pest of tomato in Serbia. Pestic. Phytomed. 2011;26(3):197–204.
Abbes K, Harbi A, Elimem M, Hafsi A, Chermiti B. Bioassay of three solanaceous weeds as alternative hosts for the invasive tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae) and insights on their carryover potential. African Entomol. 2016;24(2):334–42.
FAO. The State of Food Insecurity in the World: Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Rome, FAO. 2012.
Machekano H, Mvumi BM, Nyamukondiwa C. Diamondback Moth, Plutella xylostella (L.) in Southern Africa: research trends, challenges and insights on sustainable management options. Sustainability. 2017;9:91. https://doi.org/10.3390/su9020091.
IPCC. Climate change 2014: synthesis report. In: Core Writing Team, Pachauri RK, Meyer LA, editors. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC; 2014. p. 151.
Richardson DM, Pysek P. Plant invasions-merging the concepts of species invasiveness and community invisibility. Prog Phys Geogr. 2006;30:409–31.
Blackburn TM, Pysek P, Bacher S, Carlton JT, Duncan RP, Jarosic V, Wilson JR, Richardson DM. A proposed unified framework for biological invasions. Trends Ecol Evolut. 2014;26:333–9.
Duncan RP, Blackburn TM, Rossinelli S, Bacher S. Quantifying invasion risk: the relationship between establishment probability and founding population size. Methods Ecol Evol. 2014;5:1255–63.
Szucs M, Melbourne BA, Tuff T, Hufbauer RA. The roles of demography and genetics in the early stages of colonization. Proc R Soc B. 2014;2014(28):20141073.
Dixon AF, Honek A, Kell P, Kotela MAA, Sizzling AL, Jarosik V. Relationship between the minimum and maximum temperature thresholds for development of insects. Funct Ecol. 2009;23:257–64.
Kelley AL. The role thermal physiology plays in species invasion. Conserv Physiol. 2014. https://doi.org/10.1093/conphys/cou04527.
Kumschick S, Gaertner M, Vila M, Essl F, Jeschke JM, Pysek P, Ricciardi A, Bacher S, Blackburn TM, Dick JT, Evans T. Ecological impacts of alien species: quantification, scope, caveats, and recommendations. Bioscience. 2015;65:55–63.
Nentwig W, Bacher S, Pysek P, Vila M, Kumschick S. The generic impact scoring system (GISS): a standardised tool to quantify the impact of alien species. Environ Monit Assess. 2016;188:1–13.
Biber-Freudenberger L, Ziemacki J, Tonnang HEZ, Borgemeister C. Future risks of pest species under changing climatic conditions. PLoS ONE. 2016;11(4):e0153237. https://doi.org/10.1371/journal.
Perkins LB, Leger EA, Nowak RS. Invasion triangle: an organizational framework for species invasion. Ecol. Evol. 2011;1:610–25.
Hoffmann AA. Physiological climatic limits in Drosophila: patterns and implications. J Exp Biol. 2009;213:870–80.
Karadjova O, Ilieva Z, Krumov V, Petrova E, Ventsislavov V. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): Potential for entry, establishment and spread in Bulgaria. Bulg J Agric Sci. 2013;19:563–71.
Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE. The spread of invasive species and infectious disease as drivers of ecosystem change. Front Ecol Environ. 2008;6:238–46.
Olyarnik SV, Bracken ME, Byrnes JE, Hughes AR, Hultgren KM, Stachowicz JJ. Ecological factors affecting community invasibility. In: Rilov G, Crooks J, editors. Biological invasions in marine ecosystems. Berlin: Springer; 2009. p. 215–38.
Chown SL, Terblanche JS. Physiological Diversity in Insects: ecological and Evolutionary Contexts. Adv. Insect Physiol. 2007;33:50–152.
Gerhardt F, Collinge SK. Abiotic constraints eclipse biotic resistance in determining invasibility along experimental vernal pool gradients. Ecol Appl. 2007;17:922–33.
Andrew NR, Hill SJ. Effect of climate change on insect pest management. In: Coll M, Wajnberg E, editors. Environmental pest management: challenges for agronomists, ecologists, economists and policymakers. Oxford: Wiley; 2017.
Nyamukondiwa C, Kleynhans E, Terblanche JS. Phenotypic plasticity of thermal tolerance contributes to the invasion potential of Mediterranean fruit flies (Ceratitis capitata). Ecol Entomol. 2010;35:565–75.
Gotcha N, Terblanche JS, Nyamukondiwa C. Plasticity and cross-tolerance to heterogeneous environments: divergent stress responses co-evolved in an African fruit fly. J Evol Biol. 2017;31:98–110.
Kelley AL. The role thermal physiology plays in species invasion. Conserv Physiol. 2014. https://doi.org/10.1093/conphys/cou045.
IPPC. Reporting pest presence: Preliminary surveillance reports on Tuta absoluta in Zambia. International Plant Protection Convention (IPPC). 2016a https://www.ippc.int/en/countries/zambia/pestreports/2016/09/reporting-pest-presence-preliminary-surveillance-reports-on-tuta-absoluta-in-zambia/. Accessed 14 Sep 2016.
IPPC. First detection of Tuta absoluta in South Africa. International Plant Protection Convention (IPPC). 2016b. https://www.ippc.int/en/countries/south-africa/pestreports/2016/09/first-detection-of-tuta-absoluta-in-south-africa. Accessed 1 Sept 2016.
Hayden JE, Lee S, Passoa SC, Young J, Landry JF, Nazari V, Mally R, Somma LA, Ahlmark KM. Digital Identification of Microlepidoptera on Solanaceae. USDA-APHIS-PPQ.2013.
Machekano H, Mvumi BM, Nyamukondiwa C. Loss of coevolved basal and plastic responses to temperature may underlie trophic level host-parasitoid interactions under global change. Biol Control. 2017. https://doi.org/10.1016/j.biocontrol.2017.12.005.
Nyamukondiwa C, Terblanche JS. Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): effects of age, gender and feeding status. J Therm Biol. 2009;34:406–14.
Bertoli CI, Scannapieco AC, Sambucetti P, Norry FM. Direct and correlated responses to chill-comma recovery selection in Drosophilla buzzatii. Entomol Exp Appl. 2010;134:154–9.
Chang XQ, Ma CS, Zhang S, Lu L. Thermal tolerance of Diamondback moth, Plutella xylostella. J Appl Ecol. 2012;23:772–8.
Chidawanyika F, Terblanche JS. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera:Totricidae). J Insect Physiol. 2011;57:108–17.
Nyamukondiwa C, Weldon CW, Chown SL, le Roux PC, Terblanche JS. Thermal biology, population fluctuations and implications of temperature extremes for the management of two globally significant insect pests. J Insect Physiol. 2013;59:1199–211.
Chown SL, Nicolson SW. Insect physiological ecology: mechanisms and patterns. Oxford: Oxford University Press; 2004.
Terblanche JS, Deere JA, Clussella-Trullas S, Janion C, Chown SL. Critical thermal limits depend on methodological context. Proc R Soc B. 2007;274:2935–42.
R Development Core Team. R: A language and environment for statistical computing. Vienna. Austria R Foundation for Statistical Computing. 2016.
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;108:6668–72.
Martins JC, Picanço MC, Bacci L, Guedes RNC, Santana PA Jr, Ferreira DO, Chediak M. Life table determination of thermal requirements of the tomato borer Tuta absoluta. J Pest Sci. 2016;89:897–908.
Mutamiswa R, Chidawanyika F, Nyamukondiwa C. Dominance of spotted stemborer Chilo partellus Swinhoe (Lepidoptera: Crambidae) over indigenous stemborer species in Africa’s changing climates: ecological and thermal biology perspectives. Agric For Entomol. 2017. https://doi.org/10.1111/afe.12217.
Lux SA, Copeland RS, White IM, Manrakhan A, Billah MK. A new invasive fruit fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. Insect Sci Appl. 2003;23:355–61.
Carey JR. Biodemography of the mediterranean fruit fly: aging, longevity and adaptation in the wild. Exp Gerontol. 2011;46:404–11.
dos Santos LA, Mendes MF, Krüger AP, Blauth ML, Gottschalk MS, Garcia FRM. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0174318.
Ferracini C, Ingegno BL, Navone P, Ferrari E, Mosti M, Tavella L, Alma A. Adaptation of indigenous larval parasitoids to Tuta absoluta (Lepidoptera: Gelechiidae) in Italy. J Econ Entomol. 2012;105:1311–9.
Cocco A, Deliperi S, Lentini A, Mannu R, Delrio G. Seasonal phenology of Tuta absoluta (Lepidoptera: Gelechiidae) in protected and open-field crops under Mediterranean climatic conditions. Phytoparasitica. 2015;43:713–24.
CFIA. Tuta absoluta (Tomato Leafminer)—Fact Sheet. Ontario. Government of Canada Publications. 2016.
Kruger AC, Goliger AM, Retief JV. Strong wind climatic zones in South Africa. Wind Struct. 2010;13:1.
Henneberry TJ. Integrated systems for control of the pink bollworm Pectinophora gossypiella in cotton 567–579. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-wide control of insect pests. Arizona: Western Cotton Research Laboratory USDA/ARS; 2007.
Manenti T, Sørensen JG, Loeschcke V. Environmental heterogeneity does not affect levels of phenotypic plasticity in natural populations of three Drosophila species. Ecol Evol. 2017;7:2716–24.
Farji-Brener AG, Corley JC. Successful invasions of hymenopteran insects into NW Patagonia. Ecol Austral. 1998;8:237–49.
Jarošík V, Kenis M, Honěk A, Skuhrovec J, Pyšek P. Invasive insects differ from non-invasive in their thermal requirements. PLoS ONE. 2015;10:e0131072. https://doi.org/10.1371/journal.pone.0131072.
Khadioli N, Tonnang ZEH, Ongamo G, Achia T, Kipchirchir I, Kroschel J, Le Ru B. Effect of temperature on the life history parameters of noctuid lepidopteran stemborers, Busseola fusca and Sesamia calamistis. Ann Appl Biol. 2014;165:373–86. https://doi.org/10.1111/aab.12157.
Son D, Bonzi S, Somda I, Bawin T, Boukraa S, Verheggen F, Francis F, Legreve A, Schiffers B. First record of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) in Burkina Faso. African Entomol. 2017;25:259–63.
Bale JS. Implications of cold-tolerance for pest management. In: Denlinger DL, Lee RE, editors. Low temperature biology of insects. Cambridge: Cambridge University Press; 2010. p. 342–72.
Dillon ME, Wang G, Huey RB. Global metabolic impacts of recent climate warming. Nature. 2010;467:704–6.
Chown SL, Slabber S, McGeoch MA, Janion C, Leinaas HP. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc R Soc Lond B Biol Sci. 2007;274:2661–7.
Authors’ contributions
HM and CN contributed to conceptualization and methodology; CN contributed to funding acquisition, project administration, resources and supervision; HM and RM contributed to investigation and writing of the original draft; and HM, RM and CN contributed to data curation, validation, formal analysis, writing, review and editing. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge Botswana International University of Science and Technology for funding and Russell IPM for T. absoluta pheromone lures. We acknowledge assistance from the Department of Crop Protection (Ministry of Agriculture), on site selection, the Botswana National Botanical Gardens for data on distribution of wild Solanaceous plants and Department of Meteorological Services for climate data. We are also grateful to Dr. Tharina L. Bird for map drawing and assistance with trapping in the Okavango Delta and Mmabaledi Buxton and Mphoeng Ofitlhile for assistance with trap monitoring in some districts.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Collected and analysed data during the current study are available upon request from the corresponding author.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable since the study involved tomato plants and the invasive insect pest (T. absoluta), both of which are not endangered or protected species. The permission to enter National Parks and other any protected areas was obtained from Department of Wildlife and National Parks (Ministry of Environment, Wildlife and Tourism).
Funding
The project was funded through Botswana International University of Science and Technology (BIUST) Research Office grant.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Machekano, H., Mutamiswa, R. & Nyamukondiwa, C. Evidence of rapid spread and establishment of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in semi-arid Botswana. Agric & Food Secur 7, 48 (2018). https://doi.org/10.1186/s40066-018-0201-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40066-018-0201-5