Abstract
Background
Congenital Disorders of Glycosylation (CDG) are a large group of inborn errors of metabolism with more than 140 different CDG types reported to date (1). The first characterized, PMM2-CDG, with an autosomal recessive transmission, is also the most frequent. The PMM2 gene encodes a phosphomannomutase. Here, a novel genetic variation causing PMM2-CDG is reported.
Case presentation
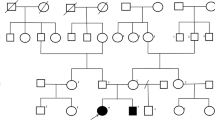
We report the case of a French child, from healthy and unrelated parents, presenting congenital ataxia with hypotonia, hyperlaxity, inverted nipples, as well as altered coagulation parameters and liver function. Transferrin isoelectrofocusing revealed a typical type I CDG profile. Direct Sanger sequencing and quantitative PCR of PMM2 revealed a unique and novel genotype. On one allele, the patient was heterozygote with a known missense variant NM_000303.3(PMM2):c.323C > T, p.Ala108Val in exon 4. On the second allele, whole genome sequencing (WGS) indicated the presence of a novel heterozygous 70 kb deletion.
Conclusion
We report in the present paper the largest known heterozygous deletion of a PMM2 gene. The observation reveals the impact of a precise diagnostic on genetic counselling: by using WGS, an erroneous conclusion of homozygosity in the case of a relatively rare variant could be avoided, and an index patient with healthy and unrelated parents correctly identified.
Similar content being viewed by others

Background
Congenital Disorders of Glycosylation (CDG) are a rapidly expanding family of genetic diseases. Today, 140 different CDG subtypes have been reported [1] categorized in 2 groups: CDG-I, affecting steps before the oligosaccharide precursor transfer in the endoplasmic reticulum, and CDG-II, affecting the steps following the transfer, mostly in the Golgi apparatus. The first patient cases were reported 40 years ago by Jaeken et al., [2]. Mutations in PMM2 (OMIM 601,785), a gene on chromosome 16p13 encoding a phosphomannomutase were shown to be responsible for the disease. This enzyme catalyzes the conversion in the cytosol of mannose-6-P to mannose-1-P, necessary for the synthesis of donor substrates for glycosylation, GDP-mannose, and Dol-P-mannose.
First named CDG-Ia then changed into PMM2-CDG in 2009 [3], the disease is thought to represent 70% of the total CDG cases, with an estimated incidence around 1:20 000 [4]. The spectrum of clinical phenotypes and severity is broad and is characterized with mainly a psychomotor development impairment associated with cerebellar hypoplasia, hypotonia, dysmorphia, and coagulopathy [5]. The lethality rate in the first 4 years of life is about 20%. Beyond childhood, PMM2-CDG patients have a good life expectancy [6]. The number of PMM2 mutations classified in HGMD (https://my.qiagendigitalinsights.com) is 142, most of these, 113, are missense mutations and the most frequent is p.Arg141His (R141H) [7]. We report the particular genotype of a PMM2-CDG patient with a heterozygous p.Ala108Val (A108V) mutation on one allele and a first-described > 70 kb-deletion on the other allele.
Case presentation
We report the case of a PMM2-CDG French child of Caucasian descent. She was born at term with a length of 48 cm for 3.110 kg, after uncomplicated pregnancy with vaginal delivery. The AGPAR score was 10 both at 1 min and 5 min and the newborn screening results were normal. The parents are unrelated and the 6-years older brother is healthy. Parents reported abnormal abrupt movements of the child after birth that stopped spontaneously.
Biologically, at 9 months of age, elevated transaminases TGO = 50 UI/ L, TGP = 51 [normal values 10–35 UI/ L] and normal coagulation parameters with ATIIIA = 63% [normal values 80–120%] and FXI 53% at the lower limit [normal values 50–150%] were assayed. The cytology and the thyroid function were normal.
The clinical examination at 9 months of age revealed ataxia, hypotonia, hyperlaxity, strabismus, esotropia, feeding difficulties, and inverted nipples. The child was calm and exclusively breastfed with an absence of facial dysmorphia and no sleeping disorders. At that time, the girl presented an inability to reach a seated posture. The diagnosis of CDG was oriented by an abnormal pattern in serum transferrin isoelectrofocusing [8] with an elevation of asialo- and disialo-transferrin, typical from a type I CDG (Fig. 1). Brain MRI revealed cerebellar abnormalities with vermis hypoplasia. The child finally reaches a seated posture at 11 months of age.
Genetic testing
Direct Sanger sequencing of the 8 exons of PMM2 reported a seemingly homozygous variant rs200203569 NM_000303.3(PMM2): c.323C > T in exon 4. The variant leads to a missense substitution of alanine 108 to proline (p.Ala108Pro), commonly named A108V, known to be pathogenic (ClinVar, SIFT, Mutation Taster). The A108V mutation is quite rare and is often associated with R141H, the most common deleterious PMM2 mutation, in compound heterozygous patients [9]. A homozygous presentation of R141H variant is thought to be incompatible with life as no case was reported so far [10]. For the A108V variant, the gnomAD (2.1) website reports a frequency of 0.0012% in the overall population. To our knowledge, no homozygous A108V patient is reported in the literature and, as the parents were unrelated, further genetic explorations were conducted. Direct Sanger sequencing of PMM2 of the paternal DNA reported a heterozygous A108V mutation while no mutation was found in the mother. At the time of the genetic exploration, the seemingly second variant could have been either due to the de novo variant or to the absence of sequence at the same location on the other allele. A quantitative PCR (qPCR) of the 13 exons was performed, showing a reduction of the DNA of the gene by 50% in the mother and the proband from exon 3 to exon 8, the last exon of PMM2 (Fig. 2). The hypothesis of the de novo variant was rejected as the heterozygous deletion in PMM2 gene was found. To evaluate the extent of the deletion that goes beyond PMM2 gene, Whole Genome Sequencing (WGS) was performed. WGS was preferred to CGH array to accurately determine the exact position of the breakpoints. WGS allowed to delineate the deletion of 70,453 bp in position chr16:8,897,826–8,968,278 (Fig. 3). In the HGMD database, the largest deletion reported is 28 kb-long.
The deletion also affects a part of CARHSP1 (Calcium Regulated Hear Stable Protein 1) (OMIM: 616,885) gene that plays a role in TNF mRNA stabilization, seemingly not affecting the phenotype.
Discussion and conclusions
In the present study, we described the case of a PMM2-CDG patient with congenital ataxia. The genotype identified in the child is novel. The clinical course was relatively mild for a child with PMM2-CDG as the child does not present facial dysmorphia. Given that the maternal mutation could not be detected upon Sanger sequencing, further investigations were performed to precise the genetic transmission of the disease.
A108V mutation was first described in France [7] and the effect on the enzyme activity is unknown. When associated to a mutation in R141H the remaining phosphomannomutase activity in leucocytes is 0.09% [11].
Quantitative PCR and WGS allowed to identify a large deletion on the maternal allele. A new deletion of 70,453 bp in position chr16:8,897,826–8,968,278 could be accurately detected with WGS including 6 exons of PMM2 and a part of CARHSP1 gene. Knockout of CARHSP1 has demonstrated the role of CARHSP1 as a TNF-α mRNA stability enhancer [12]. GnomAD database reports various loss of function heterozygote mutation for CARHSP1, indicating that the observed pathology is mainly due to the phosphomannomutase defect.
To our knowledge, this is the largest PMM2 deletion reported so far. Our example illustrates the usefulness of WGS in the case of an apparent homozygous variant in an unrelated family. Wherever possible, compound heterozygosity has to be confirmed with a parental genetic study. As the disease transmission for the couple is 25%, an antenatal diagnostic can now be proposed for future pregnancies.
This case underlines the importance of the correlation between the phenotype description and the genetic study, especially in disorders with a wide phenotypic spectrum like PMM2-CDG [13], where it is essential for a correct diagnosis to search thoroughly over the appearances.
In case of strong suspicion of PMM2-CDG based on the clinical phenotypes and despite the absence of transferrin abnormalities, the molecular analysis should be performed to avoid any diagnostic deadlocks.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CDG:
-
Congenital Disorder of Glycosylation
- PMM2:
-
Phosphomannomutase 2
- WGS:
-
Whole Genome Sequencing
- Bp:
-
Base pair
References
Ondruskova N, Cechova A, Hansikova H, Honzik T, Jaeken J. Congenital disorders of glycosylation: Still « hot » in 2020. Biochim Biophys Acta Gen Subj janv. 2021;1865(1):129751.
Jaeken J, Vanderschueren-Lodeweyckx M, Casaer P, Snoeck L, Corbeel L, Eggermont E, et al. Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG-deficiency, increased serum arylsulphatase A and increased CSF protein: a new syndrome?: 90. Pediatric Research févr. 1980;14(2):179–179.
Aebi M, Helenius A, Schenk B, Barone R, Fiumara A, Berger EG, et al. Carbohydrate-deficient glycoprotein syndromes become congenital disorders of glycosylation: an updated nomenclature for CDG. First International Workshop on CDGS. Glycoconj J. 1999;16(11):669–71.
Schollen E, Kjaergaard S, Legius E, Schwartz M, Matthijs G. Lack of Hardy-Weinberg equilibrium for the most prevalent PMM2 mutation in CDG-Ia (congenital disorders of glycosylation type Ia). Eur J Hum Genet mai. 2000;8(5):367–71.
Jaeken J, Stibler H, Hagberg B. The carbohydrate-deficient glycoprotein syndrome. A new inherited multisystemic disease with severe nervous system involvement. Acta Paediatr Scand Suppl. 1991;375:1–71.
Pajusalu S, Vals MA, Mihkla L, Šamarina U, Kahre T, Õunap K. The Estimated Prevalence of N-Linked Congenital Disorders of Glycosylation Across Various Populations Based on Allele Frequencies in General Population Databases. Front Genet. 2021;12:719437. https://doi.org/10.3389/fgene.2021.719437.
Matthijs G, Schollen E, Bjursell C, Erlandson A, Freeze H, Imtiaz F, et al. Mutations in PMM2 that cause congenital disorders of glycosylation, type Ia (CDG-Ia). Hum Mutat. 2000;16(5):386–94.
Stibler H, Beaugé F, Bjørneboe A, Aufrère G. Transferrin microheterogeneity in rats treated chronically with ethanol. Pharmacol Toxicol avr. 1989;64(4):383–5.
Le Bizec C, Vuillaumier-Barrot S, Barnier A, Dupré T, Durand G, Seta N. A new insight into PMM2 mutations in the French population. Hum Mutat mai. 2005;25(5):504–5.
Matthijs G, Schollen E, Van Schaftingen E, Cassiman JJ, Jaeken J. Lack of Homozygotes for the Most Frequent Disease Allele in Carbohydrate-Deficient Glycoprotein Syndrome Type 1A. Am J Human Genetics. 1998;62(3):542–50.
Matthijs G, Schollen E, Pardon E, Veiga-Da-Cunha M, Jaeken J, Cassiman JJ, et al. Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13 in carbohydrate-deficient glycoprotein type I syndrome (Jaeken syndrome). Nat Genet. 1997;16(1):88–92.
Pfeiffer JR, McAvoy BL, Fecteau RE, Deleault KM, Brooks SA. CARHSP1 is required for effective tumor necrosis factor alpha mRNA stabilization and localizes to processing bodies and exosomes. Mol Cell Biol janv. 2011;31(2):277–86.
Vuillaumier-Barrot S, Isidor B, Dupré T, Le Bizec C, David A, Seta N. Expanding the Spectrum of PMM2-CDG Phenotype. In: SSIEM, éditeur. JIMD Reports - Case and Research Reports, 2012/2 [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. p. 123‑5. (JIMD Reports; vol. 5). Available on:https://doi.org/10.1007/8904_2011_114 Cité 2022 avr 29
Acknowledgements
We thank the patient’s parents for their kind participation and support.
Funding
This work was supported by the Agence Nationale de la Recherche (ANR: Solv-CDG).
Author information
Authors and Affiliations
Contributions
Reported the case: N.D, R.A, Conducted the analyses: L.E, B.F, Analysed the data: L.E, B.F, Wrote the paper: L.E, FF, K.A, MG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent for genetic testing has been sign by the parent of the index case and is available on request. Not ethic committee is required in the context of a unique case report in the country were the analyses were made.
All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from parents to publish data on the patient.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lebredonchel, E., Riquet, A., Neut, D. et al. A PMM2-CDG caused by an A108V mutation associated with a heterozygous 70 kilobases deletion case report. Ital J Pediatr 48, 178 (2022). https://doi.org/10.1186/s13052-022-01355-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-022-01355-x