Abstract
Background
To explore the efficacy of follitropin delta in ovarian stimulation of patients with the Rotterdam ESHRE/ASRM 2003 phenotypes of polycystic ovarian syndrome (PCOS) using a retrospective case series with an electronic file search in a reproductive medicine clinic.
Case presentation
Seventy-four patients with PCOS undergoing ovarian stimulation according to the individualized dosing algorithm of follitropin delta for in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI)/oocyte freezing were included. Follitropin delta resulted in a high number of pre-ovulatory follicles at the end of stimulation as expected in patients with PCOS. There was a large number of oocytes retrieved with an acceptable percentage of metaphase II (MII) oocytes. There were no cases of moderate or severe OHSS across all phenotypes.
Conclusion
Follitropin delta, using the individualized dosing algorithm, appears to be a safe method of ovarian stimulation with a low risk of OHSS in PCOS patients without sacrificing successful stimulation outcomes.
Similar content being viewed by others
Background
Polycystic ovary syndrome (PCOS) encompasses a spectrum of reproductive and metabolic abnormalities [5] with a prevalence between 4 and 21% [14]. The commonly used Rotterdam classification, set in 2003 by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine, divides PCOS into four main phenotypes based on features of clinical and or biochemical signs of hyperandrogenism (HA), ovulatory dysfunction (OD) and polycystic ovary morphology (PCOM) [7, 14, 18], highlighted in Table 1.
Women with PCOS have a high incidence of ovarian hyperstimulation syndrome (OHSS) due to the large cohort of follicles that can be recruited with gonadotropin therapy [11, 16]. This applies particularly to women with phenotypes A and B who have the highest anti-Mullerian hormone (AMH) levels [10].
OHSS is an iatrogenic complication of ovarian stimulation that is potentially life threatening [8]. A challenge for fertility specialists is prevention and finding a balance between ovarian stimulation for a successful outcome and reducing the patient’s risk of OHSS [8, 11]. As a result, certain characteristics and diagnostic markers of a patient’s ovarian reserve, namely AMH, are used to identify the patients at risk of developing OHSS and individualize the starting dose of gonadotropin [8, 15]. However, with conventional highly purified urinary gonadotropins or with recombinant follicle stimulating hormone (FSH or follitropin) alpha, clinicians must make an educated guess as to a starting dose that will produce an optimal number of oocytes while reducing the risk of OHSS.
Follitropin delta is a new recombinant FSH that is uniquely administered according to an individualized dosing algorithm including patient’s body weight and pretreatment AMH level with the goal of reducing risks of OHSS [2, 8, 12, 15]. Several published trials have established that follitropin delta has a similar safety profile and is non inferior with regards to ongoing pregnancy rates when compared to the more conventional gonadotropin, follitropin alfa [2, 8, 15].
No information is available in the literature regarding the outcomes of the use of follitropin delta for ovarian stimulation in regard to women who exhibit the different Rotterdam 2003 phenotypes of PCOS. In this study, the aim was to assess characteristics and responses of patients with different PCOS phenotypes when follitropin delta was used for ovarian stimulation with special focus on occurrence of OHSS.
Case presentation
This study was a retrospective case series of patients who were treated with follitropin delta (Rekovelle, Ferring Pharmaceuticals) for ovarian stimulation at Trio Fertility in Toronto, Ontario involving an electronic search of IVF charts of patients between February 2016 and March 2020.
Patient files included the standard investigation: the medical history, physical exam: including weight, height, antral follicle count (AFC) and AMH level. Sonohysterogram explored the patient’s uterine cavity. Also included was a detailed male partner history, physical exam and semen analysis interpreted according to the World Health Organization (WHO) criteria.
All patients who were administered follitropin delta for ovarian stimulation were included, regardless of age, body mass index (BMI), menstrual cycle duration, or AMH levels. All women were Caucasian. Once patients who received follitropin delta were identified from the electronic medical record, their data was anonymized for subsequent analysis and manuscript preparation and the authors were unaware of any identifying information. Exclusion criteria comprised missing data for the cycle in which follitropin delta was prescribed and also patients who did not meet the criteria for PCOS according to the 2003 Rotterdam consensus.
Follitropin delta was administered by a daily subcutaneous dose determined by AMH level and body weight before initiating treatment. AMH < 15 pmol/L: 12 μg; AMH ≥ 15 pmol/L: 0.10–0.19 μg/kg; maximum daily dose of 12 μg, shown in Table 2.
Seventy-two patients in this study were administered a gonadotropin releasing hormone (GnRH) antagonist while two patients received a long agonist protocol. The cycles were monitored by serum hormone levels and transvaginal ultrasound to record follicular size and number as well as endometrial thickness.
There were three methods of final triggering of oocyte maturation: human chorionic gonadotropin (hCG), GnRH agonist, or GnRH agonist and hCG (double trigger) which were administered 36 ± 2 h before oocyte pick-up. The retrieved oocytes then underwent fertilization by IVF/ ICSI with either fresh embryo transfer or freezing for frozen embryo transfer, or oocyte freezing, a plan that had been reviewed with each patient. For this study, a clinical pregnancy was defined as having a positive serum hCG and ultrasound confirmation of a positive fetal heart rate.
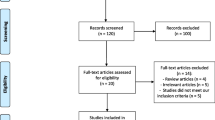
There were 127 patients who were prescribed follitropin delta for ovarian stimulation during the study period. Thirty three patient files were excluded either because the treatment algorithm was not used or because the files were missing certain key data required for this study. Also, twenty of the remaining patients did not meet the Rotterdam 2003 criteria for PCOS. This resulted in 74 patients eligible for this case series who were treated with follitropin delta for ovarian stimulation according to the algorithm. These patients were then categorized according to the phenotypes mentioned above; 47 women exhibited PCOS of Phenotype A, 6 patients of Phenotype B, 14 of C and 7 of D, shown in the participant flow in Fig. 1.
Table 3 displays the patient demographics according to each phenotype of PCOS. Patients; ages at cycle initiation were quite similar across the four phenotypes. The largest proportion - 63.5% - of patients included belonged to phenotype A which contained the highest body weights and BMI. The AMH levels and AFC were higher in phenotypes A and C compared to B and D. Approximately 40–50% of patients in each phenotype (except for phenotype C where it was 14%) had been prescribed supplementary treatments for infertility prior to initiation of ovarian stimulation including lifestyle modifications with a healthy diet, physical activity, acupuncture and medications including metformin and supplements such as coenzyme Q10.
All patients had an AMH within 12 months before initiating treatment with follitropin delta. As PCOS is a major risk factor for OHSS during treatment, 97.3% of patients were stimulated with the antagonist protocol and only 2 patients in phenotype C with the GnRH agonist protocol. Across all phenotypes, the majority of patients were undergoing their first IVF or ICSI cycle.
During this study, all patients were dosed according to the algorithm with adjustments made during the cycle if needed. The majority of patients were dosed entirely according to the algorithm, particularly in phenotypes B and C. Most dose adjustments involved a decrease in the dose with only two patients in phenotype A with an increase.
The daily dose of follitropin delta administered in patients dosed according to the algorithm was highest in phenotype B with a mean daily dosage of 11.7 mcg (Table 4). The mean daily dose in phenotypes A and C was the most similar in patients dosed according to the algorithm and those with dosing deviations. All patients had a similar length of treatment averaging 10 days.
Trigger of ovulation was individualized using: hCG, GnRH agonist or double trigger as shown in Table 5. Preventive measures for patients at risk of developing OHSS included administration of a dopamine agonist or an aromatase inhibitor after the oocyte retrieval and a request to return for an ultrasound for monitoring of pelvic fluid levels and ovary size. Four cycles were cancelled in phenotype A and 1 case in phenotype B and 1 cycle was converted to IUI, all for the reason of poor ovarian response.
Table 6 and Fig. 2 summarizes outcomes of the patients in the four phenotypes of PCOS. Phenotypes D, C and A had the greatest number of follicles at the end of stimulation as well as the highest number of oocytes retrieved and number of MII oocytes. Most patients underwent a frozen embryo transfer. OHSS occurred at a rate of 20% in patients in phenotype C, 14.3% in phenotype D and 12.7% in A. All cases of OHSS were grade 1 (abdominal distention and discomfort) and resolved without intervention. Phenotype C depicted the highest clinical pregnancy rate of 50%, followed by phenotypes D (42.8%) and A (40.4%).
Statistical analysis
This was basically a descriptive case series and no statistical analysis was performed with the exception of calculating the mean +/− SEM for certain results.
Discussion
This case series investigated ovarian stimulation with the use of follitropin delta in four phenotypes of PCOS women. The most common phenotype was A, which has also been reported in the literature to be the most prevalent phenotype [5]. Those in phenotype A exhibited the highest body weights and BMI as well as the highest AMH levels compared to the other phenotypes. Previous studies have shown that women with ‘classical’ and ‘severe’ PCOS have the highest AMH levels [17, 19].
Almost all patients were stimulated with the antagonist protocol. As per a Cochrane meta-analysis, treating patients with GnRH antagonists resulted in a lower rate of OHSS than with GnRH agonists despite no difference in clinical pregnancy rates between the two [1] and are therefore recommended to reduce the risk of OHSS [3].
According to Selcuk, patients with PCOS morphology display an easier stimulation whereas hyperandrogenemia results in better quality embryos and pregnancy rates [18]. In this study, the phenotypes with PCO morphology (phenotypes A, C and D) had the higher number of follicles formed at the end of stimulation, highest number of oocytes retrieved and almost double the number of MII oocytes compared to phenotype B. Thirty-seven patients across phenotypes chose oocyte or embryo freezing and so the clinical pregnancy rate in this study is not considered as a clinical outcome measure. Hence, the clinical pregnancy rate with follitropin delta could not be fully assessed.
The patients who are at the highest risk for OHSS are high responders, those with PCOS, a high AMH, with more than 18 oocytes retrieved and with serum estradiol concentrations > 5000 pmol/L [2, 16]. In addition, patients with a history of OHSS or elevated response to gonadotropins are also at risk [11]. According to Fischer et al., 10% of patients in all phenotypes of PCOS undergoing stimulation with follitropin alfa developed moderate and severe OHSS [9]. None of the patients stimulated with follitropin delta in this study developed grade 2 or greater OHSS [13] despite oocyte retrieval numbers up to a maximum of 37 and with some patients having more than 18 oocytes in all subgroups except Group B.
Follitropin delta resulted in absence of significant OHSS without sacrificing a good ovarian response when compared to the literature [4, 6]. The results showed a greater than average number of follicles at the end of stimulation, higher number of oocytes retrieved and higher number of MII oocytes in phenotypes A, C and D, i.e. patients with polycystic ovarian morphology.
The main limitations of this study are its retrospective nature and the small population size. There were confounding factors that could have affected the results. For example, there were three different triggers for final oocyte maturation. Also, the overall clinical pregnancy rate could not be determined since some patients did not undergo embryo transfer and opted for oocyte and embryo freezing.
A major weakness of this case study is the lack of a comparator group of matched controls resulting in the need for literature comparisons. An earlier trial demonstrated the non-inferiority of follitropin delta compared to follitropin alfa in regard to ongoing pregnancy and implantation rate in patients with ovulatory PCOS ovarian morphology. In addition, follitropin delta showed an increased predictability and a higher safety profile [11]. In the present study, there were no cases of moderate or severe OHSS in patients stimulated with follitropin delta despite high risk factors in many of the PCOS patients.
To our knowledge, this is the first study that explores the effects of follitropin delta on ovarian stimulation according to the different phenotypes of PCOS. Proper controlled trials are needed to explore the full safety profile and benefits of follitropin delta in the population of patients with PCOS.
Availability of data and materials
Anonymized data available on request from corresponding author.
Abbreviations
- ESHRE:
-
European Society for Human Reproduction and endocrinology
- ASRM:
-
American Society for Reproductive Medicine
- IVF:
-
In vitro fertilization
- ICSI:
-
Intracytoplasmic sperm injection
- PCOS:
-
Polycystic ovarian syndrome
- HA:
-
Hyperandrogenism
- OD:
-
Ovulatory dysfunction
- PCOM:
-
Polycystic ovary morphology
- AMH:
-
Anti-Mullerian hormone
- OHSS:
-
Ovarian hyperstimulation syndrome
- FSH:
-
Follicle stimulating hormone
- AFC:
-
Antral follicle count
- WHO:
-
World Health Organization
- BMI:
-
Body mass index
- hCG:
-
Human chorionic gonadotropin
- GnRH:
-
Gonadotropin releasing hormone
- MII:
-
Metaphase II
References
Al-Inany H, Youssef M, Ayeleke R, Brown J, Lam W, Broekmans F. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750.
Bosch E, Havelock J, Martin F, Rasmussen B, Klein B, Mannaerts B, et al. Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind phase 3 safety trial. Reprod Biomed Online. 2019;38:195–205.
Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020;(2):hoaa009.
Briggs R, Kovacs G, MacLachlan V, Motteram C, Baker H. Can you ever collect too many oocytes? Hum Reprod. 2014;30:81–7.
Clark N, Podolski A, Brooks E, Chizen D, Pierson R, Lehotay D, et al. Prevalence of polycystic ovary syndrome phenotypes using updated criteria for polycystic ovarian morphology. Reprod Sci. 2014;21:1034–43.
Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;2:370–6.
Fauser BCJM, Tarlatzis B, et al. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
Fernández-Sánchez M, Visnova H, Yuzpe A, Klein B, Mannaerts B, Arce J. Individualization of the starting dose of follitropin delta reduces the overall OHSS risk and/or the need for additional preventive interventions: cumulative data over three stimulation cycles. Reprod Biomed Online. 2019;38:528–37.
Fischer D, Reisenbüchler C, Rösner S, Haussmann J, Wimberger P, Goeckenjan M. Avoiding OHSS: controlled ovarian low-dose stimulation in women with PCOS. Geburts Frauenheilkunde. 2016;76:718–26.
Goverde A, van Koert A, Eijkemans M, Knauff E, Westerveld H, Fauser B, et al. Indicators for metabolic disturbances in anovulatory women with polycystic ovary syndrome diagnosed according to the Rotterdam consensus criteria. Hum Reprod. 2008;24:710–7.
Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94:389–400.
Koechling W, Plaksin D, Croston G, Jeppesen J, Macklon K, Andersen C. Comparative pharmacology of a new recombinant FSH expressed by a human cell line. Endocr Connect. 2017;6:297–305.
Kumar P, Sait FS, Shaema A, Kumar M. Ovarian hyperstimulation syndrome. J Hum Reprod Sci. 2011;4:70–5.
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6–15.
Nyboe Andersen A, Nelson S, Fauser B, García-Velasco J, Klein B, Arce J, et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107:387–396.e4.
Papanikolaou E, Pozzobon C, Kolibianakis E, Camus M, Tournaye H, Fatemi H, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85:112–20.
Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Müllerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol-Endocrinol Metab. 2009;296:E238–43.
Selçuk S, Özkaya E, Eser A, Kuyucu M, Kutlu H, Devranoğlu B, et al. Characteristics and outcomes of in vitro fertilization in different phenotypes of polycystic ovary syndrome. J Turkish Soc Obstet Gynecol. 2016;13:1–6.
Tal R, Seifer D, Khanimov M, Malter H, Grazi R, Leader B. Characterization of women with elevated antimüllerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211:59.e1–8.
Acknowledgements
Special thanks to the nursing and embryology staff for data entry into our electronic medical record.
Funding
Supported by a non-restricted grant from Ferring Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
S. Yacoub, data analysis, manuscript writing; K Cadesky, project development, manuscript writing; RF Casper, project development, supervision of data analysis, manuscript writing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Retrospective clinic quality review.
Consent for publication
Not applicable.
Competing interests
Operating grant from Ferring Pharmaceuticals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yacoub, S., Cadesky, K. & Casper, R.F. Low risk of OHSS with follitropin delta use in women with different polycystic ovary syndrome phenotypes: a retrospective case series. J Ovarian Res 14, 31 (2021). https://doi.org/10.1186/s13048-021-00773-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-021-00773-5