Abstract
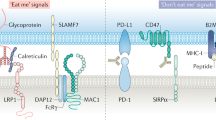
Cluster of differentiation 47 (CD47) (also known as integrin-associated protein) is a ubiquitously expressed glycoprotein of the immunoglobulin superfamily that plays a critical role in self-recognition. Various solid and hematologic cancers exploit CD47 expression in order to evade immunological eradication, and its overexpression is clinically correlated with poor prognoses. One essential mechanism behind CD47-mediated immune evasion is that it can interact with signal regulatory protein-alpha (SIRPα) expressed on myeloid cells, causing phosphorylation of the SIRPα cytoplasmic immunoreceptor tyrosine-based inhibition motifs and recruitment of Src homology 2 domain-containing tyrosine phosphatases to ultimately result in delivering an anti-phagocytic—“don’t eat me”—signal. Given its essential role as a negative checkpoint for innate immunity and subsequent adaptive immunity, CD47-SIRPα axis has been explored as a new target for cancer immunotherapy and its disruption has demonstrated great therapeutic promise. Indeed, CD47 blocking antibodies have been found to decrease primary tumor size and/or metastasis in various pre-clinical models. In this review, we highlight the various functions of CD47, discuss anti-tumor responses generated by both the innate and adaptive immune systems as a consequence of administering anti-CD47 blocking antibody, and finally elaborate on the clinical potential of CD47 blockade. We argue that CD47 is a checkpoint molecule for both innate and adaptive immunity for tumor evasion and is thus a promising target for cancer immunotherapy.
Similar content being viewed by others
Background
Cluster of differentiation 47 (CD47), also known as integrin-associated protein (IAP), is a ~50 kDa heavily glycosylated, ubiquitously expressed membrane protein of the immunoglobulin superfamily with a single IgV-like domain at its N-terminus, a highly hydrophobic stretch with five membrane-spanning segments and an alternatively spliced cytoplasmic C-terminus [1]. Each of the four alternatively spliced cytoplasmic tails exists in vivo at different frequencies (i.e., form 2 is the most abundant), but all lack a substantial signaling domain [2]. While CD47 was first identified as a membrane protein involved in β3 integrin-mediated signaling on leukocytes [3], it is now known to also interact with thrombospondin-1, signal regulatory protein-alpha (SIRPα), and others to regulate various cellular functions including cell migration, axon extension, cytokine production, and T cell activation [4–8]. However, recent studies have focused most on CD47-SIRPα axis for its inhibitory role in phagocytosis [9]. SIRPα, also known as Src homology 2 domain-containing protein tyrosine phosphatase substrate 1/brain Ig-like molecule with tyrosine-based activation motif/cluster of differentiation antigen-like family member A (SHPS-1/BIT/CD172a), is another membrane protein of the immunoglobulin superfamily that is particularly abundant in the myeloid-lineage hematopoietic cells such as macrophages and dendritic cells [10, 11]. The ligation of SIRPα on phagocytes by CD47 expressed on a neighboring cell results in phosphorylation of SIRPα cytoplasmic immunoreceptor tyrosine-based inhibition (ITIM) motifs, leading to the recruitment of SHP-1 and SHP-2 phosphatases. One resulting downstream effect is the prevention of myosin-IIA accumulation at the phagocytic synapse and consequently inhibition of phagocytosis [12–14]. Thus, CD47-SIRPα interaction functions as a negative immune checkpoint to send a “don’t eat me” signal to ensure that healthy autologous cells are not inappropriately phagocytosed. Consistent with this notion, CD47−/− cells are cleared rapidly when they are adoptively transferred to the congeneic wild-type mice [15]. However, it was recently shown that CD47-SIRPα axis, while crucial, represents just one mechanism that controls phagocytic behavior [16]. Indeed, CD47−/− mice do not manifest significant self-destruction phenotype unless they are in inflammatory conditions. Inflammatory cytokines stimulate protein kinase C-spleen tyrosine kinase (PKC-Syk) signaling pathway (which IL-10 negatively regulates), which then activates macrophage to target self cells [16]. Combined, these findings suggest a potential mechanism for anemia of chronic disease and that rhesus (Rh)-null individuals, who have <25% of normal CD47 levels, may be particularly vulnerable to anemia under inflammatory conditions and infections [17].
Research has demonstrated overexpression of CD47 in nearly all types of tumors, some of which include acute myeloid leukemia, non-Hodgkin’s lymphoma, bladder cancer, and breast cancer [18–25]. While CD47 is implicated in the regulation of cancer cell invasion and metastasis [18, 26], its most well-studied and important function related to tumor development is prevention of phagocytosis via ligating with SIRPα on the surrounding phagocytes [18, 27, 28]. Also, CD47 expression on cancer stem cells (CSCs) implies its role in cancer recurrence. Particularly, a study has shown that CSCs have increased CD47 expression to protect themselves from immune-mediated elimination during conventional anti-tumor therapies [29]. This increases the chance of CSC survival, which in turn could repopulate a new tumor mass and cause a tumor relapse.
CD47 blockade for direct cancer cell killing
Given the important inhibitory function of CD47 in phagocytosis of tumor cells, it has been extensively investigated as a potential target for tumor therapy. In various xenograft tumor models using NOD-scid-IL2Rgamma null (NSG) mice, use of human CD47-blocking monoclonal antibodies has demonstrated superb efficacy against human acute lymphocytic leukemia, acute myeloid leukemia, leiomyosarcoma, and solid tumors [18, 20, 27, 28, 30, 31]. Most work initially concluded that the therapeutic effects of anti-human CD47 were dependent on the direct killing of the tumor by phagocytes. However, it is important to note that xenograft models might have some unique features that favor innate immune-mediated tumor killings. First, human CD47 binds well to SIRPα of NSG mice, but not of other strains [32, 33]. This unique feature could put human tumor cells under CD47-SIRPα control more so in NSG mice than in other strains of mouse, making them more susceptible to signaling blockade. Thus, use of human SIRPα-transgenic recombination-activating gene (Rag)2−/− IL2Rgamma−/− mice may be necessary to accurately test such antibody’s therapeutic benefit [34]. Second, in xenograft models, only human tumor cells express human CD47. Hence, human CD47-blocking monoclonal antibodies can efficiently target human tumors without being “absorbed” by other normal cells (such as red blood cells) expressing mouse CD47. Third, xenograft tissue could come under strong innate immune attack. For example, lacking the mouse MHC class I “self” marker, xenograft human tumor cells might be attacked by natural killer (NK) cells if human leukocyte antigen (HLA) fails to mediate inhibitory signaling. Consistent with this notion, in syngeneic immunodeficient mouse models such as athymic nude mice or Rag-deficient mice, mouse anti-CD47 blockade resulted in less impressive efficacy after treatment [35]. Fourth, lymphocyte-deficient mice typically demonstrate stronger innate immune responses [36]. All reasons listed above suggest that contribution of direct killing by phagocytes to the therapeutic impact of CD47 blockade may be significantly different in an immunocompetent organism.
Role of CD8+ T cells upon CD47 blockade
Indeed, adaptive immune response, particularly that mediated by T cells, plays an important role in mouse anti-CD47 blockade-induced tumor control. In syngeneic immunocompetent mouse models, mouse anti-CD47 blockade shows impressive anti-tumor effect especially upon intratumoral delivery [35, 37]. Depletion of CD8+ T cells—but not CD4+ T cells—diminishes the therapeutic effect of anti-mouse CD47 antibody. Furthermore, after anti-mouse CD47 treatment, significantly more interferon (IFN)-γ spot-forming antigen-specific CD8+ T cells are present in the tumor, and T cell-mediated memory response is formed to protect mice from tumor re-challenge. All of these experimental results demonstrate that T cells are essential for anti-mouse CD47-mediated tumor regression. Thus, CD47 is a checkpoint molecule for both innate and adaptive immunity for tumor evasion.
Role of dendritic cells upon CD47 blockade
Since macrophages have been shown to play an important role in tumor cell phagocytosis in the xenograft model, they were assumed to be the major antigen-presenting cells for cytotoxic T lymphocyte (CTL) induction. Supporting this, enhancement of cross-priming by macrophages was observed in response to anti-human CD47 treatment [38]. However, using the syngeneic mouse model, we have recently shown that dendritic cells—not macrophages—appeared to play a more important role for CTL cross-priming and anti-tumor therapy based on the following observations [35]. First, in the presence of anti-mouse CD47 antibody, bone marrow-derived dendritic cells (BMDCs) were able to cross-prime CD8+ T cells to a greater extent than bone marrow-derived macrophages (BMDMs) in general. Second, ex vivo isolated dendritic cells (DCs) were more potent for cross-priming of CTL than macrophages after anti-mouse CD47 treatment. Third, the therapeutic effect of anti-mouse CD47 antibody was severely impaired following DC depletion but not macrophage depletion. Apparent contradiction between the two studies likely resulted from differences in experimental approaches. Indeed, when BMDCs were cultured without serum (similar to in vitro phagocytosis/priming assays in [38]), they demonstrated increased apoptosis (as measured by increased annexin V stain) which would likely impact their functional capacity. In contrast, macrophages demonstrated very minimal change in annexin V stain in the presence/absence of the serum [35].
Also, it seems that although macrophages can phagocytose more tumor cells, DCs are more potent than macrophages in antigen presentation [39]. Macrophages are good at scavenging and destroying phagocytosed tumor cells, but at the same time, tumor antigens and danger signals are overly degraded [39]. In contrast, DCs have developed means to preserve useful information from the ingested tumor cells that serve to initiate adaptive immune responses [39].
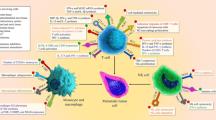
How anti-CD47 blockade boosts DC-mediated antigen cross-presentation and CTL induction is an intriguing question that we have started to answer. We found that after anti-mouse CD47 treatment, DCs—but not macrophages—express more Ifna mRNA [35]. Blocking type I IFN signaling by intratumoral injection of interferon alpha/beta receptor (IFNAR)-blocking antibody impaired the therapeutic effect of anti-mouse CD47, suggesting an important role of type I IFN signaling on DC activation. Supporting this, conditional deletion of Ifnar 1 in CD11c+ cells markedly reduced the therapeutic effect of CD47 blockade on tumor growth. These data also confirm the essential role of DCs as antigen-presenting cells (APCs) in vivo for CTL induction. Interestingly, our data further demonstrated that cytosolic DNA sensor stimulator of interferon genes (STING)—but not classical Toll-like receptor (TLR)-myeloid differentiation primary response gene 88 (MyD88) pathway—is required for type I IFN production and the therapeutic effect of anti-CD47. This raises a fascinating scenario that upon anti-CD47 treatment, DNA is released from tumor cells and taken up by DCs, resulting in the activation of STING and the production of type I IFN, which activates DCs for antigen cross-presentation (Fig. 1). The detailed mechanisms remain to be investigated in the future.
Working model of CD47 blockade for enhancing antigen cross-presentation by dendritic cells and increased T cell priming. Upon CD47-SIRPa blockade, tumor cells are phagocytosed and their DNA can gain access to the cytosol of intratumoral dendritic cells. Recognition of cytosolic DNA by cyclic GMP-AMP (cGAMP) synthase (cGAS) and generation of cGAMP lead to the activation of STING, resulting in the production of type I IFN. DCs are activated by type I IFN to cross-present tumor antigens to CD8+ T cells, which then proliferate and kill tumor cells
Targeting CD47-SIRPα signaling axis for therapy
As of November 13, 2016, there are eight phase I clinical trials that are investigating the effect of blocking CD47-SIRPα signaling axis in various cancer patients (summarized in Table 1). Among the six, NCT02216409, led by Forty Seven, Inc., is the first in-human trial and the only one yet whose data have been presented [40]. Briefly, in this study, humanized monoclonal anti-CD47 antibody (“Hu5F9-G4”) [41] was administered to patients with diverse solid tumors who are no longer candidates for conventional therapies. As a phase I clinical trial, it sought to determine the appropriate dosage of Hu5F9-G4 and to perform the initial pharmacodynamic and -kinetic studies. Patients tolerated priming (starting) dose of 0.1, 0.3, and 1 mg/kg well, while those receiving 3 mg/kg experienced a dose-limiting toxicity (abdominal pain, RBC hemagglutination, and headache). Hence, 1 mg/kg was decided as the priming dose, and currently, work is being done to determine the optimal maintenance dose. Hu5F9-G4-related adverse events, majority of which were reversible, included anemia, hyperbilirubinemia, headache, hemagglutination, nausea, and retinal toxicity. It would be interesting to see in the future how other two therapeutic agents compare to Hu5F9-G4 in terms of their safety profiles.
It is still unclear, however, if administration of Hu5F9-G4 alone will result in therapeutic benefits that are expected based on the promising results of many pre-clinical studies. Indeed, effective clinical responses are generally rare and statistically inconclusive in phase I trials, mainly due to small numbers of patients and inability to optimally administer the therapeutic agent (i.e., the dosage). Phase II and III trials will be critical for evaluating the ability to either delay disease progression or perhaps even cause its remission.
Given that blockade of CD47-SIRPα signaling axis has (and continues to) demonstrate success in more pre-clinical tumor models, more entries into clinical trials involving the CD47-SIRPα axis are anticipated. Below, we offer some suggestions and important considerations to potentially improve the specificity and efficacy of therapy.
Chemotherapy influences anti-mouse CD47 effects
Many patients might have previously received or continue to receive chemotherapy during anti-CD47 treatment. Since chemotherapy can suppress the immune system by killing recently activated immune cells [42, 43], it is possible that chemotherapy may blunt the therapeutic effects of CD47 blockade. However, on the other hand, chemotherapy may increase the release of tumor antigen and DNA from dying tumor cells, which may synergize with CD47 blockade. These possibilities have been experimentally evaluated [35]. It was found that chemotherapy administered after anti-CD47 therapy has a detrimental effect on the development of beneficial anti-tumor memory immune responses. In contrast, chemotherapy administered before anti-CD47 therapy not only synergized with anti-CD47 for tumor control but also preserved the host memory response against relapsing tumors. Several possibilities exist for the synergistic effect of chemotherapy and anti-CD47 treatment. First, chemotherapy may induce the release of tumor DNA from dying tumor cells, which could augment STING-mediated cytosolic DNA sensing. Second, chemotherapy may sensitize tumor cells by the upregulation of “eat me” signals, such as surface calreticulin, which could synergistically amplify the CTL induction in combination with “don’t eat me” blockade. Third, it is also possible that the chemotherapy preconditions the tumor microenvironment with more infiltrating inflammatory cells, allowing anti-CD47 blockade to work. Therefore, proper combination therapy of chemotherapeutic drugs and anti-CD47 antibody may depend on the type, timing, dose of these agents, and tumor types. Further studies are needed to uncover the underlying synergistic mechanisms for a rational combinational design.
Intratumoral CD47-SIRPα blockade
Given the ubiquitous expression of CD47 on normal cells, tumor-specific delivery of CD47 blockade would generate better anti-tumor effect with fewer side effects than systemic administration. Indeed, the possibility of attack against healthy self cells warrants a concern. For example, patients, especially those under chronic inflammatory conditions or infection, may become severely anemic upon CD47 blockade [16]. Thus, how to block CD47-SIRPα inside the tumor tissues specifically becomes the challenge. Tumor-targeting antibodies may be conjugated with anti-CD47 or SIRPα-Ig to increase specificity [44]. In the selection of a conjugation partner, two kinds of partners can be exploited. One is pro-phagocytic Fc receptor (FcR)-activating antibodies, such as the anti-CD20 antibody, since CD47-SIRPα interruption can synergize with antibody-dependent cellular phagocytosis [20, 44]. The other partner can be adaptive check point blockade antibodies including anti-programmed death ligand 1 (PDL1) for unleashing both an innate and adaptive anti-tumor response [45]. While cytotoxic T lymphocyte-associated protein 4 (CTLA4) or programmed cell death protein 1 (PD1) blockade monotherapy has gained enormous attention for its potential to result in a durable clinical response and prolonged overall survival with tolerable toxicity compared to standard chemotherapy, not all patients respond [46]. Discovery that nivolumab and ipilimumab dual therapy is more efficacious than ipilimumab monotherapy in patients with untreated metastatic melanoma highlights the importance of combination therapy and search for other molecular targets [47]. It is possible that combination therapy of anti-CD47 antibody, which increases the tumor cell phagocytosis and priming of anti-tumor CD8+ T cell responses, and anti-CTLA4/PD1, which reinvigorates exhausted T cells, may give greater synergism by improving different steps to generating effective anti-tumor immunity. Such idea that tumor-targeted delivery of the CD47 checkpoint antagonist can work as a potential booster to synergize with other tumor-targeting antibodies for better cancer immunotherapy is being actively investigated, as reflected by phase I clinical trials testing its combination therapy with cetuximab or rituximab (Table 1).
Conclusions
Many solid and hematologic malignancies express CD47 on their cell surface to display an anti-phagocytic signal to SIRPα-expressing myeloid cells and evade destruction by innate and adaptive immune system. Administration of anti-CD47 blocking antibodies has been enormously successful in various pre-clinical models, mechanism of which likely involves both phagocyte-mediated direct killing and their cross-priming of cytotoxic T cells. Our recent work has illustrated a critical role for dendritic cells and the STING pathway, as well as CD8+ T cells, to achieving therapeutic effect of CD47 blockade. Currently, there are eight clinical trials in progress related to CD47-SIRPα blockade and more entries are anticipated. In the future, a combinational design including anti-CD47 antibody with appropriate chemotherapy and immune-modulating agents such as anti-tumor antibodies, type I IFN, STING agonists, immune checkpoint modulators, and others should be intensely investigated for achieving synergistic and tumor-specific effect for clinical application.
Abbreviations
- APCs:
-
Antigen-presenting cells
- BMDC:
-
Bone marrow-derived dendritic cells
- CD47/IAP:
-
Cluster of differentiation 47/integrin-associated protein
- cGAMP:
-
Cyclic GMP-AMP
- cGAS:
-
cGAMP synthase
- CSC:
-
Cancer stem cell
- CTL:
-
Cytotoxic T lymphocyte
- CTLA4:
-
Cytotoxic T lymphocyte-associated protein 4
- DCs:
-
Dendritic cells
- DNA:
-
Deoxyribonucleic acid
- FcR:
-
Fc receptor
- GMP-AMP:
-
Guanosine-adenosine monophosphate
- HLA:
-
Human leukocyte antigen
- IFN:
-
Interferon
- IFNAR:
-
Interferon alpha/beta receptor
- Ig:
-
Immunoglobulin
- IL10:
-
Interleukin 10
- ITIM motifs:
-
Immunoreceptor tyrosine-based inhibition motifs
- MHC:
-
Major histocompatibility complex
- mRNA:
-
Messenger ribonucleic acid
- MyD88:
-
Myeloid differentiation primary response gene 88
- NK:
-
Natural killer
- NSG:
-
NOD-scid-IL2Rgamma null
- PD1:
-
Programmed cell death protein 1
- PDL1:
-
Programmed death ligand 1
- PKC:
-
Protein kinase C
- RAG:
-
Recombination-activating gene
- Rh:
-
Rhesus
- SIRPα/SHPS1/BIT/CD172a:
-
Signal regulatory protein-alpha/Src homology 2 domain-containing protein tyrosine phosphatase substrate 1/brain Ig-like molecule with tyrosine-based activation motif/cluster of differentiation antigen-like family member A
- STING:
-
Stimulator of interferon genes
- Syk:
-
Spleen tyrosine kinase
- TLR:
-
Toll-like receptor
References
Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11(3):130–5.
Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci. 1995;108(Pt 11):3419–25.
Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111(6 Pt 1):2785–94.
Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271(1):21–4.
Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276(43):40156–66.
Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274(5288):795–8.
Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT, Furuya N, Matozaki T. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol Biol Cell. 2004;15(8):3950–63.
Reinhold MI, Lindberg FP, Kersh GJ, Allen PM, Brown EJ. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J Exp Med. 1997;185(1):1–11.
Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2011;12(1):58–67.
Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O, Matozaki T. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174(4):2004–11.
van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175(12):7781–7.
van den Berg TK, van der Schoot CE. Innate immune ‘self’ recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29(5):203–6.
Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50.
Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180(5):989–1003.
Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–4.
Bian Z, Shi L, Guo YL, Lv Z, Tang C, Niu S, Tremblay A, Venkataramani M, Culpepper C, Li L, et al. Cd47-Sirpalpha interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc Natl Acad Sci U S A. 2016;113(37):E5434–5443.
Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95(2):375–87.
Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–85.
Kim MJ, Lee JC, Lee JJ, Kim S, Lee SG, Park SW, Sung MW, Heo DS. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. 2008;29(1):28–34.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713.
Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti Jr J, Chang HY, van de Rijn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106(33):14016–21.
Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64(3):1026–36.
Schulenburg A, Blatt K, Cerny-Reiterer S, Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC, Valent P. Cancer stem cells in basic science and in translational oncology: can we translate into clinical application? J Hematol Oncol. 2015;8:16.
Fan D, Li Z, Zhang X, Yang Y, Yuan X, Zhang X, Yang M, Zhang Y, Xiong D. AntiCD3Fv fused to human interleukin-3 deletion variant redirected T cells against human acute myeloid leukemic stem cells. J Hematol Oncol. 2015;8:18.
Fang X, Chen C, Xia F, Yu Z, Zhang Y, Zhang F, Gu H, Wan J, Zhang X, Weng W, et al. CD274 promotes cell cycle entry of leukemia-initiating cells through JNK/Cyclin D2 signaling. J Hematol Oncol. 2016;9(1):124.
Zhao H, Wang J, Kong X, Li E, Liu Y, Du X, Kang Z, Tang Y, Kuang Y, Yang Z, et al. CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep. 2016;6:29719.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs Jr KD, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–99.
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–7.
Soltanian S, Matin MM. Cancer stem cells and cancer therapy. Tumour Biol. 2011;32(3):425–40.
Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, Liu J, Majeti R, West RB, Fletcher JA, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A. 2012;109(17):6656–61.
Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360(2):302–9.
Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8(12):1313–23.
Yamauchi T, Takenaka K, Urata S, Shima T, Kikushige Y, Tokuyama T, Iwamoto C, Nishihara M, Iwasaki H, Miyamoto T, et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121(8):1316–25.
Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, Eynon EE, Manz MG, Flavell RA. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011;108(32):13218–23.
Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209–15.
Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13(10):1248–52.
Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, Berzofsky JA, Roberts DD. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74(23):6771–83.
Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110(27):11103–8.
Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–56.
Sikic BI, Narayanan S, Colevas AD, Padda SK, Fisher GA, Supan D, Wakelee HA, Aoki R, Pegram MD, Villalobos VM et al: A first-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2016, 34:(suppl; abstr 3019). http://meetinglibrary.asco.org/content/162472-176.
Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, Willingham S, Howard M, Prohaska S, Volkmer J, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10(9):e0137345.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61.
Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34.
Piccione EC, Juarez S, Tseng S, Liu J, Stafford M, Narayanan C, Wang L, Weiskopf K, Majeti R. SIRPalpha-antibody fusion proteins selectively bind and eliminate dual antigen-expressing tumor cells. Clin Cancer Res. 2016;22:5109–19.
Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113(19):E2646–2654.
Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.
Acknowledgements
The authors thank the members of Dr. Fu and Li laboratories for helpful suggestions and comments on the project. We also thank Mr. Daryl Harmon for editing.
Funding
This work was supported by the Ministry of Science and Technology of China grant (No. 2011DFA31250) to Y-XF and the US National Institutes of Health grants CA141975 to Y-XF. HK was supported by a scholarship from the Abney Foundation. ZL is supported by the US National Institutes of Health grants DK105033, CA186866, CA188419, and AI070603.
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Authors’ contributions
XL and HK drafted the manuscript and are co-first authors. XL, HK, Y-XF, and ZL discussed and revised the manuscript. All authors read and approved final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
This is not applicable for this review.
Ethics approval and consent to participate
This is not applicable for this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, X., Kwon, H., Li, Z. et al. Is CD47 an innate immune checkpoint for tumor evasion?. J Hematol Oncol 10, 12 (2017). https://doi.org/10.1186/s13045-016-0381-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-016-0381-z