Abstract
Despite a few breakthroughs in therapy for advanced disease in the recent years, pancreatic ductal adenocarcinoma continues to remain one of the most challenging human malignancies to treat. The overall prognosis for the majority of patients with pancreatic cancer is rather dismal, and therefore, more effective treatment options are being desperately sought. The practical goals of management are to improve the cure rates for patients with resectable disease, achieve a higher conversion rate of locally advanced tumor into potentially resectable disease, and finally, prolong the overall survival for those who develop metastatic disease. Our understanding of the complex genetic alterations, the implicated molecular pathways, and the role of desmoplastic stroma in pancreatic cancer tumorigenesis has increased several folds in the recent years. This has facilitated the development of novel therapeutic strategies against pancreatic cancer, some of which are currently under evaluation in ongoing preclinical and clinical studies. This review will summarize the existing treatment approaches for this devastating disease and also discuss the promising therapeutic approaches that are currently in different stages of clinical development.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related mortality in the US for both men and women [1]. In 2015 alone, it is estimated that 48,960 new cases of pancreatic cancer will be diagnosed in the US and 40,560 patients will die of this disease. As such, PDAC remains one of the most challenging malignancies with a dismal prognosis and limited therapeutic options. The 5-year survival rate for pancreatic cancer (all stages combined) is around 7%, which is the lowest among all different cancer sites. At the time of initial pancreatic cancer diagnosis, approximately 9% of patients present with localized disease, 28% have regional spread, and the remaining 53% of patients already have distant spread of their disease. There has been a very limited clinically meaningful improvement in survival rates for this disease during the past two decades. The poor prognosis of PDAC is largely attributed to delayed diagnosis due to nonspecific symptoms in the early stages of the disease, biological aggressiveness leading to rapid metastases, lack of effective screening methods, and resistance to radiation and chemotherapies.
It is now well established that PDAC is driven by alteration of multiple genes that regulate pathways and processes in the tumor cells and the neighboring microenvironment [2]. Despite our improved understanding of the molecular events underlying the multistep carcinogenesis of PDAC, the progress made towards improving the survival rates of these patients has been extremely slow. At present, multiple novel treatment strategies targeted against PDAC are under preclinical and clinical evaluation. This review will summarize the existing treatment approaches for this devastating disease and also discuss the promising therapeutic strategies that are currently in the different stages of clinical development.
Risk factors
The known risk factors that increase the likelihood of developing PDAC include cigarette smoking [3], alcohol abuse [4], high fat diet [5], and certain trace elements [6]. It is estimated that cigarette smoking doubles the risk of developing PDAC and accounts for approximately 20%–25% of the cases [3]. Chronic pancreatitis is also associated with an increased risk of PDAC, especially among smokers [7]. It has also been noted that the majority of patients with PDAC develop diabetes mellitus which is usually diagnosed in the preceding 1–2 years or concomitant with the new cancer diagnosis [7]. It is not entirely clear whether diabetes is a predisposing factor or a manifestation of PDAC itself. Obesity, which predisposes to insulin resistance, might be a common link between the two.
Approximately 5%–10% of patients with PDAC report a history of pancreatic cancer diagnosis in their family member [8]. The genetic syndromes such as familial breast cancer (BRCA2, BRCA1, and PALB2), the Peutz-Jeghers syndrome (LKB1/STK11), the familial atypical multiple mole melanoma (FAMMM) syndrome (p16/CDKN2A), hereditary pancreatitis (PRSS1), and the lynch syndrome (MLH1, MSH2, MSH6, PMS2) are also associated with an increased risk of developing PDAC [8,9]. Thus, patients with a family history of pancreatic cancer or these mutation carriers should undergo appropriate screening, as per the guidelines provided by the International Cancer of the Pancreas Screening Consortium [10].
Genetics and molecular pathogenesis
Pancreatic cancer most commonly originates in the exocrine cells of the pancreas [11]. Among the exocrine tumors, ‘ductal adenocarcinoma’ is the most frequently encountered pathological subtype and accounts for more than 90% of the cases. Initiation and development of PDAC involve a series of specific genetic alterations which promote growth and survival of aberrant precursors, initiation of a desmoplastic reaction in the stroma, and ultimately tissue invasion and metastases [12]. This oncogenic process begins with transformation of normal pancreatic duct epithelium into infiltrating cancer through a series of histologically defined precursors called pancreatic intraepithelial neoplasia (PanIN)-1, -2, and -3 [11]. These morphological changes occur in conjunction with several genetic alterations. Disease progression often involves development of distant metastases, which occurs late during the genetic evolution of pancreatic cancer [13]. Using genome sequencing, it has been determined that after the initiation of tumorigenesis, an average of 11.7 years is required for the birth of parental, non-metastatic founder pancreatic cancer clone, additional 6.8 years for the development of cancer cell subclones with metastatic potential, and an average of 2.7 years from then until the patient’s death [13].
It is now well established that pancreatic cancer cells contain one or more of the four primary genetic mutations that drive pancreatic cancer tumorigenesis [14]. These include KRAS, p16/CDKN2A, TP53, and SMAD4 mutations. KRAS plays a critical role in regulating important cellular functions including cell survival, cell differentiation, and proliferation [15]. Single point mutations in codon 12, 13, 59, or 61 of exon 2 and exon 3 of the KRAS oncogene lead to uncontrolled downstream signaling of RAF/MEK/ERK, leading to enhanced tumor cell proliferation and survival. It has been shown that these activating mutations in the KRAS are a necessary event for the initiation of pancreatic cancer and are therefore commonly found in the early precursor lesions (PanIN-1) [16,17]. With disease progression, the prevalence of oncogenic KRAS mutation increases and is present in over 90% of the tumors [17,18]. Inactivating mutation in the CDKN2A tumor suppressor gene results in the loss of p16 protein and thereby loss of regulation of the G1/S transition of the cell cycle. It is also thought to be a relatively early event in PDAC progression (PanIN-2 lesions) and is associated with larger tumors and early metastasis [17,19]. TP53 is a DNA checkpoint regulator in response to mutations from reactive oxygen species as well as telomere shortening. Abnormal TP53 gene allows cells to avoid DNA damage control checkpoints and subsequently apoptotic signals [20]. SMAD4 is a key component of the transforming growth factor-β (TGF-β) receptor signaling pathway and plays a role in activating transcription of cell cycle inhibitory factors. Inactivation of TP53 and SMAD4 occur at a later stage (PanIN-3) in pancreatic carcinogenesis [17].
A comprehensive genome analysis of 24 different human pancreatic cancers revealed an average of 63 genetic alterations per cancer, the majority of which were point mutations [2]. These mutations occur in several primary oncogenes and tumor suppressor genes and contribute to the genetic diversity of pancreatic cancer. This, in turn, leads to tumor heterogeneity, instability, and early metastasis. The genetic alterations associated with pancreatic cancer can be classified into a set of 12 core cellular signaling pathways: apoptosis, control of G1/S phase transition, sonic hedgehog (SHH) signaling, KRAS signaling, TGF-β signaling, Wnt/Notch signaling, DNA damage control, homophilic cell adhesion, integrin signaling, JNK signaling, invasion, and small GTPase signaling [2]. These pathways are responsible for some of the key cellular functions such as intracellular signaling, cell cycle regulation, metabolism, and DNA repair. Targeting these pathways has now become the main focus of drug development in pancreatic cancer.
A prominent histologic hallmark of PDAC is the presence of a desmoplastic reaction which consists of extracellular proliferation of leukocytes, fibroblasts, endothelial cells, neuronal cells, and collagen. This is mediated by paracrine signals from the pancreatic cancer cells which results in the formation of a dense stroma in the tumor microenvironment [21]. It is now established that the signals that promote this stromal reaction originate from the KRAS-mutant oncogene in the epithelium of pancreatic cancer cells. SHH signaling also acts in a paracrine fashion on the extracellular fibroblasts, resulting in their growth and differentiation [22]. The desmoplastic reaction not only acts as a mechanical barrier to the effective delivery of chemotherapeutic agents, it also provides an antiangiogenic and hypoxic microenvironment in which the pancreatic cancer cells like to grow and flourish.
Thus, it is now established beyond doubt that the wide range of genetic alterations and the stromal reaction play an important role in the initiation, progression, chemotherapeutic resistance, and recurrence of pancreatic cancer.
Clinical management
Localized disease
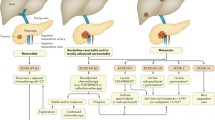
The recommended treatment for patients presenting with localized disease is surgery, since complete surgical resection with negative margins offers the only hope for cure in pancreatic cancer treatment [23]. Unfortunately, only 9% of the pancreatic cancer patients present with localized disease that is completely resectable at the time of initial diagnosis [1]. Depending upon the size and the location of the tumor, the operative procedure is either a cephalic pancreaticoduodenectomy (Whipple procedure), distal pancreatectomy, or total pancreatectomy [24]. The success of surgical resection depends on factors such as extent of lymph node involvement, tumor grade, tumor size, CA 19-9 levels, and the positivity of resection margins. Even following complete surgical resection, the 5-year survival rates are low at approximately 20%, and the overall prognosis remains discouraging [23]. Thus, postoperative treatment in the form of adjuvant chemotherapy [25] or chemoradiotherapy [26,27] is usually administered and is often gemcitabine- or 5-fluorouracil (5-FU)-based. The Charité Onkologie Clinical Studies in Gastrointestinal Cancers-001 (CONKO-001) trial randomized 354 patients with localized PDAC to receive either adjuvant gemcitabine or undergo observation after curative resection [28]. Gemcitabine arm was associated with a significant improvement in disease-free survival (DFS; 13.4 months vs. 6.9 months; P < 0.001). The median overall survival (OS) was however similar in the two arms (22.1 months vs. 20.2 months; P = 0.06) and was explained by the administration of gemcitabine to patients in the observation arm after disease progression. The European Study Group for Pancreatic Cancer (ESPAC)-3 trial compared gemcitabine vs. 5-FU in the adjuvant treatment of PDAC [25]. In this trial, 1,088 patients were randomized to receive either 5-FU/Leucovorin (LV) or gemcitabine. There was no difference in the median OS (23 months vs. 23.6 months; P = 0.39), progression-free survival (PFS; 14.1 months vs. 14.3 months; P = 0.53), and the global quality of life (QoL) scores between the treatment groups. In the Radiation Therapy Oncology Group (RTOG)-9704 trial, 451 patients with resected PDAC received either gemcitabine or 5-FU chemotherapy before and after 5-FU-based chemoradiation [29]. Among patients with pancreatic head tumors, a statistically non-significant improvement in median OS was seen in the gemcitabine containing arm (20.5 months vs. 16.9 months; P = 0.09). A phase III adjuvant trial (UNICANCER) comparing gemcitabine vs. modified FOLFIRINOX in surgically resected (R0 or R1) PDAC patients is currently ongoing (NCT01526135). Another phase III RTOG-0848 trial is comparing adjuvant gemcitabine with or without erlotinib in the first randomization and additional benefit of chemoradiation in the second randomization for patients with localized PDAC who have undergone R0 or R1 surgical resection (NCT01013649).
Another potential strategy for the treatment of localized pancreatic cancer is to administer chemotherapy in the neoadjuvant setting, and this approach has also been explored in multiple phase II clinical trials [30-34]. The rationale for this approach includes early treatment of micrometastatic disease, delivery of chemotherapy to an undisturbed tumor, and biological assessment of tumor aggressiveness [35]. An ongoing phase III study is comparing neoadjuvant gemcitabine/oxaliplatin combination vs. upfront surgery, to be followed by adjuvant gemcitabine in patients with resectable PDAC (NCT01314027).
Locally advanced pancreatic cancer
Locally advanced pancreatic cancer (LAPC) is the tumor that is confined locoregionally with some degree of involvement of the nearby major vascular structures but without any evidence of distant metastases. Approximately 30% of the patients are found to have locally advanced stage at the time of initial pancreatic cancer diagnosis [1]. Patients are usually classified as having either borderline-resectable or unresectable LAPC, depending upon the relationship of the tumor with the nearby vascular structures. Unfortunately, there are no standard guidelines for the management of LAPC. This is mainly due to the absence of a single standardized definition of LPAC, which limits a fair comparison of treatment strategies and results across the clinical trials.
The only potential way to achieve a cure in patients with LAPC is by maximizing upfront systemic and local therapy followed by a R0 surgical resection. For borderline-resectable LAPC patients that are deemed ineligible for upfront surgery, neoadjuvant treatment in the form of chemotherapy with or without radiation is usually performed [36-39]. Such neoadjuvant therapies have the potential to downstage borderline-resectable disease and make R0 resection feasible. For initially unresectable LAPC, neoadjuvant therapy should be offered as a bridge to potentially curative resection. Neoadjuvant therapy options include concurrent chemoradiation, chemotherapy alone, and chemotherapy followed by chemoradiation. A 1981 trial conducted by the Gastrointestinal Tumor Study Group (GITSG) established the superiority of combined 5-FU/radiation when compared with radiation therapy alone in unresectable LAPC [40]. Subsequently, gemcitabine-based chemoradiation was evaluated and established as an acceptable treatment option for LAPC, based on the results of the Eastern Cooperative Oncology Group (ECOG)-4201 trial [41] and the Taipei trial [42]. Chemotherapy alone is another management strategy in the neoadjuvant treatment of LAPC. Combination chemotherapy regimens consisting of gemcitabine backbone have failed to demonstrate survival advantage over single agent gemcitabine [43-45]. Non-gemcitabine-based regimens such as FOLFIRINOX and FOLFOX have also been explored in the neoadjuvant treatment of LAPC [46-50]. Yet another approach in the management of LAPC is chemotherapy followed by chemoradiotherapy prior to surgery. Such strategy has been looked at in retrospective series with encouraging results, but prospective phase II/III studies are needed before it can be incorporated into standard oncologic practice [50-52]. The four-arm phase III CONKO-7 trial will evaluate the efficacy of neoadjuvant chemotherapy plus chemoradiation vs. chemotherapy alone in an estimated 830 unresectable LAPC patients (NCT01827553). The chemotherapy options in this trial include either FOLFIRINOX or gemcitabine. A similar phase III trial in LAPC patients to evaluate neoadjuvant modified FOLFIRINOX followed by chemoradiation (with 5-FU as the radiosensitizer) is planned by the investigators from the Stanford University (NCT01926197).
Metastatic disease
Approximately 50%–60% of PDAC patients present with metastatic disease at the time of initial diagnosis [1], and the standard treatment option for these patients is chemotherapy. In 1997, gemcitabine monotherapy was established as the standard of care for metastatic disease. In this pivotal trial, single agent gemcitabine (1,000 mg/m2 weekly × 7 followed by 1 week of rest, then weekly × 3 every 4 weeks thereafter; n = 63) was compared with weekly bolus 5-FU (600 mg/m2; n = 63), and a modest survival benefit was demonstrated in the gemcitabine group (5.6 vs. 4.4 months; P = 0.0025) [53]. The primary efficacy measure was clinical benefit response (CBR), which was a composite of measurements of pain, Karnofsky Performance Status (KPS), and weight. The CBR was experienced by 23.8% of the gemcitabine-treated patients compared with 4.8% of 5-FU-treated patients (P = 0.0022). Despite the marginal improvement in 1-year survival, gemcitabine replaced 5-FU and was approved by the US Food and Drug Administration (FDA) as a standard treatment option for the treatment of metastatic pancreatic cancer largely based upon its efficacy in improving the disease-related symptoms [53]. Thereafter, gemcitabine continued as the chemotherapeutic standard of care in the management of patients with metastatic pancreatic cancer for a long time.
Erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), is the only biologic agent that has been approved for the treatment of advanced pancreatic cancer (APC). In the phase III PA.3 trial that randomized 569 patients with locally advanced and metastatic PDAC, the addition of erlotinib to gemcitabine resulted in a statistically significant improvement in median OS of approximately 10 days (6.24 months vs. 5.91 months; hazard ratio [HR] 0.82; P = 0.038) [54]. Despite the clinically insignificant benefit, the combination of gemcitabine plus erlotinib received the FDA approval in November 2005 due to a lack of more effective treatment options for this devastating disease at the time. However, due to the associated side effects and an exceedingly small clinical benefit, the use of this regimen in routine oncologic practice has remained virtually non-existent.
In recent years, the evidence has shifted from using single agent gemcitabine to combination regimens for front-line treatment of metastatic pancreatic cancer. FOLFIRINOX and gemcitabine plus nab-paclitaxel have now been established as the two standard combination chemotherapy options for metastatic disease. In the randomized phase III PRODIGE 4/ACCORD 11 trial that consisted of 342 metastatic PDAC patients, FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, LV 400 mg/m2, and 5-FU 400 mg/m2 given as a bolus followed by 2,400 mg/m2 given as a 46-hour continuous infusion every 2 weeks) when compared with gemcitabine alone (1,000 mg/m2 weekly for 7 of 8 weeks and then weekly for 3 of 4 weeks) demonstrated a significantly better median OS (11.1 vs. 6.8 months; HR 0.57; 95% confidence interval [CI] 0.45 to 0.73; P < 0.001), PFS (6.4 months vs. 3.3 months; HR 0.47; 95% CI 0.37 to 0.59; P < 0.001), and objective response rate (ORR; 31.6% vs. 9.4%; P < 0.001) [55]. Based on the statistically significant and clinically meaningful improvement in the survival, FOLFIRINOX was approved for the first-line treatment of metastatic PDAC. This improvement in OS with FOLFIRINOX is, however, associated with significant toxicity (febrile neutropenia, thrombocytopenia, peripheral neuropathy, vomiting, and diarrhea), and therefore, this regimen is only indicated for patients with good performance status. To improve the tolerability of FOLFIRINOX, a modified regimen was recently proposed in which the 5-FU bolus is removed and growth factor prophylaxis is administered routinely [56].
An important contributor to the chemoresistance of PDAC is the presence of a dense stroma in the tumor microenvironment. Nab-paclitaxel is albumin-bound paclitaxel that has been developed to diminish the stromal tissue network. The ‘albumin’ in nab-paclitaxel interacts with secreted protein acidic and rich in cysteine (SPARC), a matrix glycoprotein that has a role in tumor invasion and facilitates the uptake of paclitaxel by the tumor cells [57]. In a phase I/II trial of 67 patients with metastatic PDAC, nab-paclitaxel plus gemcitabine combination was associated with an ORR of 48%, median OS of 12.2 months, and 1-year survival of 48% [58]. Subsequently, in a large phase III MPACT trial, 861 metastatic PDAC patients were randomized to receive nab-paclitaxel (125 mg/m2) followed by gemcitabine (1,000 mg/m2) on days 1, 8, and 15 every 4 weeks, vs. gemcitabine monotherapy (1,000 mg/m2) weekly for 7 of 8 weeks (cycle 1) and then on days 1, 8, and 15 every 4 weeks (cycle 2 onwards) [59]. The combination of nab-paclitaxel plus gemcitabine significantly improved the median OS from 6.7 months to 8.5 months (HR 0.72; 95% CI 0.62 to 0.83; P < 0.001), PFS from 3.7 months to 5.5 months (HR 0.69; 95% CI 0.58 to 0.82; P < 0.001) and RR from 7% to 23% (P < 0.001) when compared with gemcitabine alone. In September 2013, the FDA approved nab-paclitaxel plus gemcitabine as a first-line treatment option for metastatic pancreatic cancer based on the results of this study. Currently, the choice between the two approved standard first-line chemotherapy options for metastatic disease (FOLFIRINOX or gemcitabine/nab-paclitaxel) is usually guided by the patient’s age, performance status, preference of treatment frequency, and toxicity profiles of the two regimens.
Emerging novel therapeutic targets and treatment strategies
Currently, a multitude of innovative therapeutic approaches are being developed to target the known molecular pathways involved in pancreatic tumorigenesis. Table 1 provides a list of the selected clinical trials that are currently recruiting patients to evaluate the safety and efficacy of novel agents in PDAC.
Source: http://www.clinicaltrials.gov; Accessed on January 01, 2015. NCT National Clinical Trial, EGFR epidermal growth factor receptor, TKI tyrosine kinase inhibitor, PTEN Phosphatase and tensin homolog, TGF transforming growth factor receptor, mAb monoclonal antibody, CRM1 chromosome region maintenance 1, DNA deoxyribonucleic acid, BET bromodomain and extra-terminal, SMAC second mitochondrial-derived activator of caspases, hTERT telomerase reverse transcriptase, CTLA-4 cytotoxic T-lymphocyte-associated protein 4, PBMC peripheral blood mononuclear cell, siRNA small interfering RNA, LA locally advanced.
RAS/RAF/MEK/ERK signaling pathway
Despite being the most common mutation associated with PDAC, attempts to target KRAS by inhibiting its post-translational modification have been unsuccessful so far. Tipifarnib (R115777) is an inhibitor of farnesyltransferase (FTase) which is a dominant enzyme involved in post-translational modification of RAS [60]. So far, it has not demonstrated any significant antitumor activity both as a single agent and in combination with gemcitabine [44,61,62].
Attempts are being made to identify downstream targets (mitogen-activated protein kinase [MAPK], phosphatidylinositide 3-kinase [PI3K]) to block KRAS-dependent signaling pathways. Towards this goal, a number of MEK inhibitors are currently being evaluated in clinical trials [63]. Selumetenib (AZD6244) is a selective MEK inhibitor that was found to have similar efficacy as capecitabine in a phase II clinical trial that enrolled APC patients after failing first-line gemcitabine therapy [64]. It is also being tested in combination with erlotinib in APC patients resistant to gemcitabine (NCT01222689). Based on the encouraging results of a phase I clinical trial, trametinib (another MEK inhibitor) was combined with gemcitabine in a phase II clinical trial of metastatic pancreatic cancer patients but failed to demonstrate a clinical benefit [65]. Combinations of other novel MEK inhibitors (pimasertib [MSC1936369B], refametenib [BAY86-9766]) with gemcitabine are currently under evaluation in clinical trials.
It is now known that expression of RAS oncogene up-regulates basal autophagy, which is required for cancer cell survival in starvation and in tumorigenesis [66]. Autophagy is therefore believed to be a significant mechanism for pancreatic cancer cell survival. Hydroxychloroquine, an anti-malarial drug is being evaluated as an autophagy inhibitor for the treatment of these aggressive cancers.
Epidermal growth factor receptor pathway
ErbB-1 (EGFR) and ErbB-2 (HER2/neu) expression is found in 90% and 21% of pancreatic cancers, respectively [67,68]. Therapies targeted against EGFR (both TKIs and monoclonal antibody [mAb]) in pancreatic cancer have yielded overall disappointing results so far. As discussed previously, the phase III PA.3 trial evaluated gemcitabine in combination with erlotinib in the first-line treatment of APC and was associated with a clinically insignificant improvement in median OS when compared with gemcitabine alone [54]. In another phase II study of gemcitabine-refractory APC patients, a combination of erlotinib and capecitabine was associated with only 10% radiological response and a median OS of 6.5 months [69]. The combination of cetuximab and gemcitabine has also been evaluated in the treatment of APC patients [70,71]. The initial phase II study demonstrated stable disease (SD) in 63% and partial response (PR) in 12% of the EGFR-expressing APC patients that were treated with cetuximab plus gemcitabine combination [70]. In a subsequent phase III study (Southwest Oncology Group [SWOG]-directed intergroup trial S0205), this combination was not associated with any survival benefit when compared with the single agent gemcitabine (6.3 months vs. 5.9 months; HR 1.06; 95% CI 0.91 to 1.23; P = 0.23) [71]. A randomized phase II study of panitumumab, erlotinib, and gemcitabine combination suggested a trend towards OS benefit when compared with erlotinib plus gemcitabine [72]. However, this three-drug combination with dual inhibition of the EGFR pathway was associated with significant toxicities leading to early termination of the study. Anti-HER2 agent trastuzumab has been combined with gemcitabine in a phase II study that included metastatic pancreatic cancer patients with 2+ (88% patients) or 3+ (12% patients) HER2/neu overexpression by immunohistochemistry [73]. The response rate of this combination was very similar to gemcitabine alone.
One of the probable explanations for the lack of a meaningful benefit from anti-EGFR TKIs in pancreatic cancer could be the development of acquired resistance to these agents, which is a mechanism well studied in lung cancer [74]. Clinical trials evaluating newer EGFR TKIs such as afatinib (NCT01728818) and dacomitinib (NCT02039336) in pancreatic cancer are currently underway.
Anti-angiogenesis
Targeting vascular endothelial growth factor (VEGF) pathway has shown promising results in the treatment of many solid cancers. However, anti-VEGF therapies have been ineffective clinically in treating patients with PDAC. The phase III Cancer and Leukemia Group B (CALGB) 80303 trial randomized 602 patients with APC to receive gemcitabine with or without bevacizumab in the first-line setting [75]. The addition of bevacizumab to gemcitabine was associated with increased toxicity and without any improvement in survival (5.8 months vs. 5.9 months; P = 0.95). In another large, randomized phase III trial (AVITA), 607 metastatic pancreatic cancer patients were randomized to receive gemcitabine plus erlotinib with either bevacizumab or placebo [76]. The bevacizumab arm was associated with statistically significant PFS advantage (4.6 months vs. 3.6 months; HR 0.73; P = 0.0002) but a non-significant improvement in median OS (7.1 months vs. 6 months; HR 0.89; P = 0.2087). Axitinib is a selective oral inhibitor of VEGF receptor-1, -2, and -3 that has been combined with gemcitabine in a phase II clinical trial of APC patients and showed a statistically non-significant gain in OS [77]. A subsequent phase III study that randomized 632 APC patients to receive gemcitabine plus axitinib or placebo was terminated early due to the lack of survival benefit (8.5 months vs. 8.3 months; HR 1.014; P = 0.5436) at the time of planned interim analysis [78]. Ziv-Aflibercept is an anti-VEGF recombinant fusion protein that has also been combined with gemcitabine in a phase III trial for the treatment of metastatic pancreatic cancer patients [79]. However, this trial was terminated early as well due to the lack of efficacy at the time of planned interim analysis. Sorafenib and masitinib are oral multikinase inhibitors with antiangiogenic properties. In the phase III BAYPAN trial, addition of sorafenib to gemcitabine did not improve PFS in APC patients [80]. The phase III study of gemcitabine plus masitinib also did not result in improvement of OS in patients with unresectable pancreatic cancer [81].
Insulin-like growth factor-1 receptor
Insulin-like growth factor (IGF)-1 receptor is highly expressed in PDAC and participates in downstream signaling pathways that are involved in cancer cell survival and proliferation. Several mAbs against IGF-1 receptor (cixutumumab, ganitumab, dalotuzumab) are currently being tested in clinical trials. Ganitumab (AMG 479) was studied in combination with gemcitabine in a phase II trial of metastatic pancreatic cancer patients and showed a slight improvement in 6-month survival rate when compared with gemcitabine plus placebo (57% vs. 50%) [82]. However, the phase III GAMMA trial of ganitumab plus gemcitabine combination was terminated early due to lack of efficacy at the preplanned interim analysis. In another phase II trial that evaluated cixutumumab plus gemcitabine and erlotinib in metastatic pancreatic cancer patients, the three-drug combination did not improve the PFS or OS when compared with gemcitabine plus erlotinib [83].
PI3K/AKT/mTOR pathway
This is one of the major downstream effector pathways of KRAS gene that is being evaluated as a potential target for pancreatic cancer treatment [84]. Rigosertib is a small molecular inhibitor of PI3K that was combined with gemcitabine in a phase II/III clinical trial (ONTRAC trial). The study was terminated early due to lack of demonstration of benefit at the time of interim analysis. Buparlisib (BKM120) is another PI3K inhibitor being evaluated in combination with mFOLFOX6 regimen in a study of advanced stage solid tumors including pancreatic cancer (NCT01571024). MK2206 is an AKT inhibitor currently under clinical evaluation in patients with pancreatic cancer (NCT01783171, NCT01658943). Archexin (RX-0201) is another AKT inhibitor that was evaluated in combination with gemcitabine in a phase II study (NCT01028495). BEZ235 is a combined inhibitor of PI3K and mTOR. A phase I study evaluating the activity of BEZ235 plus a MEK inhibitor (MEK162) in advanced solid tumor patients (including pancreatic cancer) with KRAS, NRAS and/or BRAF mutations has recently completed (NCT01337765). Everolimus (RAD001) is a mTOR inhibitor that was associated with a PFS of 1.8 months and OS of 4.5 months in a phase II study consisting of 33 gemcitabine-refractory, metastatic pancreatic cancer patients [85]. It is also being evaluated as a part of combination regimens with other agents in ongoing clinical trials.
Wnt/β-catenin pathway
Wnt signals are transduced through the frizzled receptor and lipoprotein-related protein to the β-catenin signaling cascade. There is evidence to suggest that Wnt pathway plays a role in pancreatic cancer formation via involvement in pancreatic cancer stem cells (CSCs) [86,87]. Phase I trials using mAbs (OMP-54 F28, OMP-18R5) against frizzled receptors to inhibit Wnt signaling in PDAC are currently ongoing (NCT02050178, NCT02005315).
Notch signaling pathway
Notch signaling has been shown to be upregulated in many human cancers including PDACs [88,89]. It mediates pancreatic CSC function and contributes to chemotherapy resistance, tumor recurrence, and metastases. Gamma secretase is an enzyme that causes proteolytic cleavage and release of the intracellular domain of the Notch, leading to activation of the Notch signaling pathway. In preclinical models, inhibition of Notch pathways with a gamma-secretase inhibitor (GSI) in combination with gemcitabine showed enhanced antitumor activity [90]. A phase II study evaluating an oral GSI (RO4929097) in pretreated metastatic pancreatic cancer patients was recently completed (NCT01232829). MK0752 is another GSI being tested in combination with gemcitabine for first-line treatment of stage III and IV PDAC patients (NCT01098344). Tarextumab (anti-Notch2/3 mAb, OMP-59R5) and demcizumab (anti-DLL4 mAb, OMP-21 M18) also inhibit Notch signaling and are being evaluated in clinical trials. ALPINE trial is studying the combination of tarextumab with nab-paclitaxel and gemcitabine (NCT01647828).
Targeting desmoplastic tumor microenvironment
The desmoplastic stroma in the tumor microenvironment is now regarded as a key component of pancreatic cancer biology which not only acts as a physical barrier to effective drug delivery inside the tumor but also facilitates tumor growth and promotes metastases. Strategies aimed at targeting the stromal compartment may enhance the delivery of chemotherapeutic agents to the tumor cells leading to improved efficacy. The most promising targets include the SHH signaling pathway, hyaluronic acid, and SPARC.
SHH pathway is an important signaling system that can activate the characteristic desmoplastic reaction present in the microenvironment of pancreatic tumors [22]. Sustained activation of this pathway enhances tumor growth during pancreatic oncogenesis [91]. Several clinical trials have been initiated to investigate the activity of SHH inhibitors in patients with PDAC. Vismodegib (GDC-0449) is a SHH inhibitor under clinical evaluation in combination with gemcitabine and nab-paclitaxel (NCT01088815). Saridegib (IPI-926) is another agent targeting the SHH pathway that was combined with gemcitabine in a phase II study of APC, but the trial was prematurely terminated since the combination was associated with a shorter survival than gemcitabine alone (NCT01130142). A study combining the SHH inhibitor sonidegib (LDE225) with FOLFIRINOX in untreated APC patients is ongoing (NCT01485744).
Hyaluronan is a glycosaminoglycan present in the extracellular matrix of PDAC, and high levels within the tumor are usually associated with a poor prognosis. Pegylated human recombinant PH20 hyaluronidase (PEGPH20) degrades hyaluronan and has been shown to decrease the hyaluronic acid content in a genetically-engineered PDAC mouse model, allowing for re-expansion of the PDAC blood vessels and enhanced intratumoral delivery of chemotherapeutic agents which leads to decreased tumor growth [92]. Clinically, the combination of PEGPH20 plus gemcitabine has shown promising activity in a phase Ib study of metastatic pancreatic cancer patients [93], and a phase II study of this combination is ongoing (NCT01453153). Another phase Ib/II study of PEGPH20 plus modified FOLFIRINOX combination in metastatic pancreatic cancer patients is currently under clinical evaluation (NCT01959139).
SPARC (osteonectin) is an extracellular matrix protein that plays a role in collagen turnover in the dense stroma. It is associated with invasion and metastasis in PDAC, and elevated levels are associated with poor prognosis. Nab-paclitaxel is albumin-bound paclitaxel that increases tumor accumulation of paclitaxel through binding of albumin to the stroma rich in overexpression of SPARC. As described previously, the efficacy of nab-paclitaxel plus gemcitabine combination in the first-line treatment of metastatic pancreatic cancer was demonstrated in the phase III MPACT trial which ultimately led to its FDA approval [59].
Some of the other novel strategies aimed at targeting the desmoplastic stroma within the pancreatic tumor include the use of matrix metalloproteinase (MMP) inhibitors, heparin derivatives, and hypoxia targeting agents. MMP inhibitors have been tried for the treatment of PDAC without much success to date. Marimastat (BB-2516) is a broad spectrum MMP inhibitor that was combined with gemcitabine in the treatment of APC but did not show any demonstrable clinical benefit [94]. Tanomastat (BAY12-9566) is another biphenyl MMP inhibitor with antiangiogenic and antimetastatic properties that was compared with gemcitabine for the treatment of APC patients and was found to be inferior to gemcitabine [95]. Heparin-derivative agents such as 2-0, 3-0 desulfated heparin (ODSH) and necuparanib (M402) are currently being studied in combination with gemcitabine and nab-paclitaxel for treatment of patients with metastatic pancreatic cancer (NCT01461915, NCT01621243). It is now well established the pancreatic tumor microenvironment is characterized by hypoxia. Consequently, hypoxia-targeting agents are being developed to evaluate this novel therapeutic strategy. TH-302 is a hypoxia-activated prodrug that is activated into a potent DNA-alkylating agent, bromo-isophosphoramide mustard selectively under hypoxic conditions. A recent phase II study of TH-302 plus gemcitabine showed a significant improvement in primary end point of PFS when compared with gemcitabine alone (5.6 months vs. 3.6 months; HR 0.61; 95% CI 0.43 to 0.87; P = 0.005) [96]. A phase III trial of this combination is currently in progress (MAESTRO study; NCT01746979).
TGF-β signaling pathway
TGF-β participates in stimulating stromal reaction, invasion, metastases, and angiogenesis in PDAC [97]. Examples of novel agents that target TGF-β signaling include trabedersen (AP-12009) and galunisertib (LY2157299). Trabedersen is a specific inhibitor of TGF-β2 that has demonstrated good safety and encouraging survival results in the phase I/II clinical study [98]. Galunisertib is being evaluated in combination with gemcitabine for the treatment of patients with APC in an ongoing phase Ib/II clinical trial (NCT01373164).
Epigenetic modification
Epigenetic changes such as histone deacetylation (HDAC) and DNA methylation (cytosine methylation within CG dinucleotides) can result in inactivation of the tumor suppressor genes leading to tumor growth and progression. Vorinostat is a HDAC inhibitor being tested in a phase I/II study of LAPC patients in combination with capecitabine and radiotherapy (NCT00948688). 5-Azacytidine is a cytosine analog that inhibits DNA methyltransferase, and a phase I study of its combination with gemcitabine in APC patients was recently terminated (NCT01167816).
Adenosine monophosphate-activated protein kinase pathway
The oral anti-diabetic drug metformin is an activator of adenosine monophosphate-activated protein kinase (AMPK) and disrupts the crosstalk between insulin receptor and G protein-coupled receptors (GPCR) signaling in pancreatic cancer cells, via inhibition of mTOR and suppression of its downstream effectors [99]. In xenograft mice models, metformin has been shown to inhibit pancreatic cancer growth [99]. Clinically, the available data surrounding the benefit of metformin in pancreatic cancer is conflicting and is mostly derived from retrospective studies. In a hospital-based case-control study, metformin was shown to decrease the risk of developing pancreatic cancer among diabetics [100]. In another retrospective analysis, metformin use was associated with an improvement in survival for the PDAC patients with diabetes [101]. In contrast, a more recent study from the UK failed to demonstrate a survival benefit from metformin use in PDAC patients [102]. There are multiple ongoing phase I and phase II trials that are evaluating the efficacy of metformin in PDAC. A phase I study of metformin plus erlotinib and gemcitabine in patients with APC has recently completed accrual (NCT01210911).
Synthetic lethality
The DNA double-strand breaks are repaired by a process of homologous recombination that is mediated via BRCA1 and BRCA2 proteins. Mutations in BRCA render this repair mechanism dysfunctional and are known to occur in both sporadic and familial cases of pancreatic cancer. Poly (ADP-ribose) polymerase (PARP) is another critical enzyme that mediates repair of DNA single-strand breaks. PARP pathway assumes the major role for DNA repair when BRCA mutation occurs. Consequently, inhibition of the PARP pathway results in ‘synthetic lethality’ via inhibition of DNA repair in BRCA-deficient tumor cells. A phase II study of veliparib alone or in combination with gemcitabine plus cisplatin for locally advanced and metastatic, BRCA 1–2, and PALB2-mutated pancreatic cancer patients is ongoing (NCT01585805). Other PARP inhibitors that are being evaluated in pancreatic cancer clinical trials include rucaparib (AG-14699; NCT02042378) and olaparib (AZD2281; NCT00515866).
Immunotherapy-based approaches
Despite significant efforts, no immunotherapeutic strategy against pancreatic cancer has demonstrated clinical benefit in a randomized phase III trial till date. This has been attributed to the immunologically quiescent microenvironment of pancreatic cancer. Currently, several approaches aimed at stimulating the host immune system against the pancreatic cancer tumor cells are under evaluation.
GI-4000, a form of RAS-specific immunotherapy, is heat-killed recombinant Saccharomyces cerevisiae yeast that expresses mutant RAS peptides [103]. A phase II trial of GI-4000 plus adjuvant gemcitabine is ongoing (NCT00300950). Reovirus is a tumor-targeted replication-competent virus with specificity for RAS-activated cells [104]. It is being combined with chemotherapy for the treatment of APC patients in two phase II clinical trials (NCT00998322, NCT01280058).
Developing vaccines against tumor antigens is another potential immunotherapeutic strategy to treat pancreatic cancer. Several antigens have been explored as potential targets for vaccine-based treatment in pancreatic cancer including carcinoembryonic antigen (CEA) [105], MUC1 [106,107], and heat shock proteins (HSP) [108]. Algenpantucel-L immunotherapy is a whole-cell allogeneic pancreatic cancer vaccine composed of two irradiated human pancreatic cell lines that have been genetically modified to overexpress murine alpha(1,3)-galactosyltransferase, resulting in expression of alpha-galactosyl (Alpha-Gal) epitopes on membrane glycoproteins and glycolipids. Since human cells do not express these epitopes, an immediate hyperacute rejection response ensues leading to the development of strong T-cell mediated antitumor immunity. This immunotherapeutic agent has demonstrated encouraging activity when combined with radiation and 5-FU plus gemcitabine in a phase II adjuvant trial of resected PDAC patients [109]. The phase III adjuvant trial that compares gemcitabine with or without algenpantucel-L, followed by chemoradiation has also completed recently (NCT01072981). Another phase III neoadjuvant trial is evaluating FOLFIRINOX with or without algenpantucel-L, followed by chemoradiation in borderline-resectable and unresectable LAPC patients (NCT01836432). GV1001 is a telomerase peptide vaccine shown to prolong survival when combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) in a phase I/II study of unresectable LAPC patients [110]. However, the phase III study comparing GV1001 and gemcitabine in sequential combination, vs. gemcitabine monotherapy in advanced unresectable pancreatic cancer was terminated early due to lack of survival benefit in the GV1001 arm (NCT00358566). G17D (Gastrimmune) is an antigastrin-17 immunogen that was evaluated in a randomized, multicenter, placebo-controlled study of APC patients and showed a non-significant improvement in OS when compared to placebo [111]. Another potential therapeutic strategy that has been explored is combining two vaccines (GVAX plus CRS-207) with the hope to achieve an enhanced efficacy. GVAX is composed of pancreatic cancer cells that have been genetically modified to secrete GM-CSF and can induce T-cell responses. CRS-207 is a live-attenuated Listeria-based vaccine that can induce listeriolysin O and mesothelin-specific T-cell responses. The combination of GVAX plus CRS-207 is being evaluated in a phase IIb clinical trial (ECLIPSE) that consists of previously treated metastatic pancreatic cancer patients (NCT02004262).
Prostate stem cell antigen (PSCA) is a glycosylphosphatidylinositol-linked cell surface antigen expressed in pancreatic cancers. AGS-1C4D4 is a fully human IgG1 mAb against PSCA that has been combined with gemcitabine for the treatment of metastatic pancreatic cancer patients and has shown encouraging results [112].
It is now established that CD40 activation can reverse immune suppression and drive antitumor T-cell responses. Utilizing this concept, agonist CD40 antibody has been used in combination with gemcitabine in a phase I study to shrink PDAC by stimulating tumor macrophages against pancreatic cancer stroma [113].
Immunoinhibitory checkpoint pathways (cytotoxic T lymphocyte-associated protein-4 [CTLA-4]/B7, programmed cell death-1 [PD-1]/programmed cell death ligand-1 [PD-L1]) are emerging as interesting immunotherapeutic targets for the treatment of cancer. Single agent ipilimumab was evaluated in a phase II trial of APC and failed to demonstrate an appreciable antitumor activity [114]. The combination of ipilimumab with gemcitabine is currently under phase I evaluation (NCT01473940).
Radioimmunotherapy with 90Y-clivatuzumab tetraxetan (radioimmunoconjugate comprised of the humanized mAb HuPAM4 that is radiolabeled with yttrium-90) is another potential therapeutic strategy that is being evaluated in clinical trials. The combination of 90Y-clivatuzumab tetraxetan with low-dose gemcitabine demonstrated a median OS of 7.7 months in a phase I study of untreated APC patients [115]. The phase III study (PANCRIT-1) of this combination in pretreated metastatic pancreatic cancer patients is ongoing (NCT01956812).
An additional therapeutic strategy is adoptive cell transfer (ACT) approach which utilizes introduction of engineered T-cells with chimeric antigen receptors (CARs) to specifically recognize a tumor antigen of interest. This personalized immunotherapy approach is still under preclinical stages of development in the field of pancreatic cancer [116].
Novel cytotoxic agents
PEP02 (MM-398) is a novel nanoparticle liposomal formulation of irinotecan. In a phase II study of gemcitabine-refractory metastatic PDAC patients, treatment with single agent PEP02 was associated with a median PFS and OS of 9 weeks and 21.6 weeks, respectively [117]. A phase III trial (NAPOLI 1) is evaluating the combination of PEP02 with 5-FU in metastatic pancreatic cancer patients who have failed prior gemcitabine-based therapy (NCT01494506).
S-1 is a fourth-generation oral fluoropyrimidine that contains tegafur (FT, a prodrug of 5-FU), 5-chloro-2,4-dihydropyrimidine (CHDP), and potassium oxonate (Oxo). It has been evaluated in the treatment of both resectable and advanced pancreatic cancer with encouraging results. In a phase II Japanese study (PC-01), 116 patients with unresectable APC were randomized to receive gemcitabine plus S-1 vs. gemcitabine alone [118]. There was significant improvement in the ORR (28.3% vs. 6.8%; P = 0.005) and median OS (13.7 months vs. 8.0 months; P = 0.035) in the S-1 arm. Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01) is a phase III non-inferiority trial that compared S-1 with gemcitabine as adjuvant chemotherapy for patients with curatively resected pancreatic cancer [119]. The interim analysis showed that S-1 was non-inferior to gemcitabine (OS at 2 years was 70% vs. 53%; HR 0.56; 95% CI 0.42 to 0.74; P < 0.0001 for non-inferiority) [120]. Based on the results of this study, the authors proposed that S-1 should be considered as a new standard treatment for patient with resected pancreatic cancer.
Identification of biomarkers
Development of more efficacious approaches for pancreatic cancer treatment would require identification of biomarkers that can predict the response and toxicity to various therapeutic agents. Research efforts geared towards this objective are underway.
The human equilibrative nucleoside transporter-1 (hENT1) plays an important role in the uptake of gemcitabine in cells and has been evaluated as a potential predictive biomarker of gemcitabine response. In a study that evaluated the expression pattern of genes involved in gemcitabine activity in 102 pancreatic tumor specimens, it was found that low hENT-1 expression levels were associated with a poorer prognosis [121]. However, in the pivotal phase II Low hENT1 and Adenocarcinoma of the Pancreas (LEAP) study, the hENT1 status was shown to have no clinical utility for predicting gemcitabine sensitivity [122]. Some additional potential biomarkers that have been evaluated in pancreatic cancer treatment include deoxycytidine kinase (dCK), ribonucleoside reductase-M1 (RRM1), and -M2 (RRM2) [123,124], KRAS status, SPARC staining [58], IGF-1R expression, and rs9582036 single nucleotide polymorphism (SNP) in the VEGF receptor-1 region [125]. Recently, pharmacogenomic profiling of circulating tumor and invasive cells (CTICs) isolated from patients with PDAC was evaluated as a predictor of tumor response, progression, and resistance [126].
Future directions and conclusion
The therapeutic advances in the field of pancreatic cancer have been painstakingly slow. Although we have seen a few breakthroughs in therapy for advanced disease in the recent years, the overall progress made in the field of pancreatic cancer has been relatively small in comparison to the some other tumor types. It should be noted that the majority of pancreatic cancer clinical trials over the past 5 years have failed to demonstrate any significant clinical benefit. A substantial fraction of these studies evaluated drug combinations using gemcitabine as the chemotherapy backbone. This should make us think that maybe it is time to move away from gemcitabine-based combinations and focus attention on developing innovative strategies to attack the pancreatic cancer oncogenesis. Selecting drug combinations with novel agents that target not only the primary tumor but also the surrounding stroma might be one such approach. Since our ability to safely combine drugs will be enhanced if the drug selection is based on biomarkers, we would also need prospective studies to validate potential biomarkers in well-defined patient populations in order to maximize the clinical efficacy while minimizing the toxicity of the therapeutic agents.
It is now well established that pancreatic cancer is a heterogeneous and a genetically diverse disease that results from successive accumulation of mutations over a long period of time, and these mutations affect multiple molecular pathways involved in pancreatic tumorigenesis. The presence of desmoplastic reaction in the tumor microenvironment and its role in pancreatic cancer initiation, invasion, and metastases is also being recognized. Consequently, multiple potential therapeutic approaches against pancreatic cancer are being developed and evaluated in several ongoing preclinical and clinical trials. We are hopeful that at least some of these novel strategies will demonstrate clinically meaningful benefit in future phase III studies and add to our armamentarium for treating this lethal malignancy.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6.
Blackford A, Parmigiani G, Kensler TW, Wolfgang C, Jones S, Zhang X, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009;69:3681–8.
Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765–76.
Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–58.
Amaral AF, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012;61:1583–8.
Wormann SM, Algul H. Risk factors and therapeutic targets in pancreatic cancer. Front Oncol. 2013;3:282.
Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365–74.
Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293–311.
Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–47.
Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72.
Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–5.
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7.
Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88.
Caldas C, Kern SE. K-ras mutation and pancreatic adenocarcinoma. Int J Pancreatol. 1995;18:1–6.
di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–9.
Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253–60.
Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–63.
Sasaki S, Yamamoto H, Kaneto H, Ozeki I, Adachi Y, Takagi H, et al. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21–5.
Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–22.
Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8.
Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106:4254–9.
Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–94.
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17.
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81.
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–26.
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77.
Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–26.
Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–9.
Pisters PW, Abbruzzese JL, Janjan NA, Cleary KR, Charnsangavej C, Goswitz MS, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. 1998;16:3843–50.
Pisters PW, Wolff RA, Janjan NA, Cleary KR, Charnsangavej C, Crane CN, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol. 2002;20:2537–44.
Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502.
Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–95.
Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26.
Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2001;5:27–35.
Small Jr W, Berlin J, Freedman GM, Lawrence T, Talamonti MS, Mulcahy MF, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942–7.
Stokes JB, Nolan NJ, Stelow EB, Walters DM, Weiss GR, de Lange EE, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–27.
Patel M, Hoffe S, Malafa M, Hodul P, Klapman J, Centeno B, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol. 2011;104:155–61.
Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–10.
Loehrer Sr PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–12.
Li CP, Chao Y, Chi KH, Chan WK, Teng HC, Lee RC, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol, Biol, Phys. 2003;57:98–104.
Rocha Lima CM, Green MR, Rotche R, Miller Jr WH, Jeffrey GM, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–83.
Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–8.
Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–16.
Ghosn M, Farhat F, Kattan J, Younes F, Moukadem W, Nasr F, et al. FOLFOX-6 combination as the first-line treatment of locally advanced and/or metastatic pancreatic cancer. Am J Clin Oncol. 2007;30:15–20.
Boone BA, Steve J, Krasinskas AM, Zureikat AH, Lembersky BC, Gibson MK, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108:236–41.
Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–8.
Gunturu KS, Yao X, Cong X, Thumar JR, Hochster HS, Stein SM, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol. 2013;30:361.
Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199.
Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–31.
Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55.
Burris 3rd HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Mahaseth H, Kauh JS, Brutcher E, Hawk NN, Kim S, Chen Z. Safety and efficacy of modified FOLFIRINOX in pancreatic cancer: a retrospective experience. In: ASCO Meeting. 2012.
Edmonds C, Cengel KA. Tumor-stroma interactions in pancreatic cancer: Will this SPARC prove a raging fire? Cancer Biol Ther. 2008;7:1816–7.
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–54.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–52.
Cohen SJ, Ho L, Ranganathan S, Abbruzzese JL, Alpaugh RK, Beard M, et al. Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol. 2003;21:1301–6.
Macdonald JS, McCoy S, Whitehead RP, Iqbal S, Wade 3rd JL, Giguere JK, et al. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23:485–7.
Akinleye A, Furqan M, Mukhi N, Ravella P, Liu D. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27.
Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216–23.
Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072–81.
Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–70.
Lemoine NR, Hughes CM, Barton CM, Poulsom R, Jeffery RE, Kloppel G, et al. The epidermal growth factor receptor in human pancreatic cancer. J Pathol. 1992;166:7–12.
Safran H, Steinhoff M, Mangray S, Rathore R, King TC, Chai L, et al. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24:496–9.
Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–92.
Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2004;22:2610–6.
Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–10.
Kim GP, Foster NR, Salim M, Flynn PJ, Moore DF, Zon R, et al. Randomized phase II trial of panitumumab, erlotinib, and gemcitabine (PGE) versus erlotinib-gemcitabine (GE) in patients with untreated, metastatic pancreatic adenocarcinoma. J Clin Oncol. 2011;29(suppl):abstr 4030.
Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706–12.
Niu FY, Wu YL. Novel agents and strategies for overcoming EGFR TKIs resistance. Exp Hematol Oncol. 2014;3:2.
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617–22.
Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–7.
Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101–8.
Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–62.
Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49:2633–42.
Goncalves A, Gilabert M, Francois E, Dahan L, Perrier H, Lamy R, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799–805.
Deplanque G, Demarchi M, Hebbar M, Flynn PJ, Melichar B, Atkins J, et al. Masitinib in nonresectable pancreatic cancer: results of a phase III randomized placebo-controlled trial. J Clin Oncol. 2013;31(suppl):abstr 158.
Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson Jr JJ, Rocha-Lima CM, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834–42.
Philip PA, Goldman BH, Ramanathan RK, Lenz H, Lowy AM, Philip PA, et al. Phase I randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib as first-line treatment in patients with metastatic pancreatic cancer (SWOG-0727). J Clin Oncol. 2012;30 suppl 4:abstr 198.
Akinleye A, Avvaru P, Furqan M, Song Y, Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6:88.
Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–8.
Cui J, Jiang W, Wang S, Wang L, Xie K. Role of Wnt/β-catenin signaling in drug resistance of pancreatic cancer. Curr Pharm Des. 2012;18:2464–71.
Farhana L, Dawson MI, Das JK, Murshed F, Xia Z, Hadden TJ, et al. Adamantyl retinoid-related molecules induce apoptosis in pancreatic cancer cells by inhibiting IGF-1R and Wnt/β-catenin pathways. J Oncol. 2012;2012:796729.
Sjolund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J C ancer. 2005;41:2620–9.
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, et al. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87.
Mizuma M, Rasheed ZA, Yabuuchi S, Omura N, Campbell NR, de Wilde RF, et al. The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical models. Mol Cancer Ther. 2012;11:1999–2009.
Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:5103–8.
Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20.
Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. A phase Ib study of gemcitabine plus PEGPH20 (pegylated recombinant human hyaluronidase) in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol. 2013;31(suppl):abstr 4010.
Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–7.
Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–302.
Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J, Jr., Chiorean EG, Rosen PJ, et al.: Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2014.
Fuxe J, Karlsson MC. TGF-β-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol. 2012;22:455–61.
Oettle H, Seufferlein T, Luger T, Schmid RM, Wichert GV, Endlicher E, et al. Final results of a phase I/II study in patients with pancreatic cancer, malignant melanoma, and colorectal carcinoma with trabedersen. J Clin Oncol. 2012;30(suppl):abstr 4034.
Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45.
Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8.
Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–12.
Hwang AL, Haynes K, Hwang WT, Yang YX. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas. 2013;42:1054–9.
Lu Y, Bellgrau D, Dwyer-Nield LD, Malkinson AM, Duke RC, Rodell TC, et al. Mutation-selective tumor remission with Ras-targeted, whole yeast-based immunotherapy. Cancer Res. 2004;64:5084–8.
Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–62.
Morse MA, Nair SK, Boczkowski D, Tyler D, Hurwitz HI, Proia A, et al. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer. 2002;32:1–6.
Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–64.
Rong Y, Qin X, Jin D, Lou W, Wu L, Wang D, et al. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exp Med. 2012;12:173–80.
Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, et al. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52:1964–72.
Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94–100. discussion p 100–101.
Bernhardt SL, Gjertsen MK, Trachsel S, Moller M, Eriksen JA, Meo M, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95:1474–82.
Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, et al. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas. 2012;41:374–9.
Wolpin BM, O’Reilly EM, Ko YJ, Blaszkowsky LS, Rarick M, Rocha-Lima CM, et al. Global, multicenter, randomized, phase II trial of gemcitabine and gemcitabine plus AGS-1C4D4 in patients with previously untreated, metastatic pancreatic cancer. Ann Oncol. 2013;24:1792–801.
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6.
Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33.
Ocean AJ, Pennington KL, Guarino MJ, Sheikh A, Bekaii-Saab T, Serafini AN, et al. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer. 2012;118:5497–506.
Chmielewski M, Hahn O, Rappl G, Nowak M, Schmidt-Wolf IH, Hombach AA, et al. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology. 2012;143:1095–107.
Ko AH, Tempero MA, Shan Y, Su W, Lin Y, Dito E, et al. A multinational phase II study of PEP02 (liposome irinotecan) for patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2011;29(suppl):abstr 4069.
Ozaka M, Matsumura Y, Ishii H, Omuro Y, Itoi T, Mouri H, et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol. 2012;69:1197–204.
Maeda A, Boku N, Fukutomi A, Kondo S, Kinoshita T, Nagino M, et al. Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01). Jpn J Clin Oncol. 2008;38:227–9.
Fukutomi A, Uesaka K, Boku N, Kanemoto H, Konishi M, Matsumoto I, et al. JASPAC 01: Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer. J Clin Oncol. 2013;31(suppl):abstr 4008.
Giovannetti E, Del Tacca M, Mey V, Funel N, Nannizzi S, Ricci S, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928–35.
Poplin E, Wasan H, Rolfe L, Raponi M, Ikdahl T, Bondarenko I, et al. Randomized, multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol. 2013;31:4453–61.
Xie H, Jiang W, Jiang J, Wang Y, Kim R, Liu X, et al. Predictive and prognostic roles of ribonucleotide reductase M1 in resectable pancreatic adenocarcinoma. Cancer. 2013;119:173–81.
Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–63.
Lambrechts D, Claes B, Delmar P, Reumers J, Mazzone M, Yesilyurt BT, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012;13:724–33.
Yu KH, Ricigliano M, Hidalgo M, Abou-Alfa GK, Lowery MA, Saltz LB, et al. Pharmacogenomic modeling of circulating tumor and invasive cells for prediction of chemotherapy response and resistance in pancreatic cancer. Clin Cancer Res. 2014;20:5281–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GG drafted the manuscript and revised it critically for important intellectual content. WS revised the manuscript critically for important intellectual content. Both authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Goel, G., Sun, W. Novel approaches in the management of pancreatic ductal adenocarcinoma: potential promises for the future. J Hematol Oncol 8, 44 (2015). https://doi.org/10.1186/s13045-015-0141-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-015-0141-5