Abstract
EphrinB2, a membrane-tethered ligand preferentially binding to its receptor EphB4, is ubiquitously expressed in all mammals. Through the particular bidirectional signaling, EphrinB2 plays a critical role during the development of cardiovascular system, postnatal angiogenesis physiologically and pathologically, and cardiac remodeling after injuries as an emerging role. This review highlights the pivotal involvement of EphrinB2 in heart, from developmental cardiogenesis to pathological cardiac remodeling process. Further potential translational therapies will be discussed in targeting EphrinB2 signaling, to better understand the prevention and treatment of cardiovascular diseases.
Similar content being viewed by others
Background
Ephrins (erythropoietin-producing hepatocellular receptor interacting proteins) are membrane-tethered ligands for Eph tyrosine kinase receptors (erythropoietin-producing hatocellular receptors), ubiquitously expressed in mammal. According to the ways of anchoring cellular membrane, Ephrins were divided into type A and type B subfamilies. All five members of EphrinAs connected the extracellular Eph-binding domain to the transmembrane segment by utilizing a glycosyl phosphatidylinositol (GPI) anchor. Whereas, all three members of EphrinBs employed a single-pass transmembrane region to fuse the Eph-binding globular domain with a cytoplasmic tail followed by several phosphorylation sites and PDZ domains [1]. Classic mode of Ephrin/Eph signaling was bi-directional, involving Ephrin binding in trans to Eph which activated the receptor’s forward signaling, as well as Eph receptor eliciting Ephrin in trans which activated the ligand’s reverse signaling. Noteworthy, Ephrin reverse signaling, in certain circumstances, could be activated independently of the associated Eph receptors [2,3,4].
The interaction between Ephrin and Eph was critical in development and a variety of diseases. EphrinBs have been evidenced essential in a serious of developmental processes including vasculogenesis, neural and cardiac development. Meanwhile, EphrinBs were physiologically involved in regulating synaptic plasticity, axonal guidance and maintaining vascular homeostasis [5, 6]. Pathologically, EphrinBs were implicated in cancer progression and neurodegenerative diseases [7, 8]. Among the EphrinBs family, EphrinB2 and its preferentially paired receptor EphB4 played crucial roles during the development of cardiovascular system and postnatal angiogenesis. Beyond angiogenesis, EphrinB2 was emerging as an important regulator in cardiac remodeling after injuries, implying a potential therapeutic target in cardiovascular diseases. Thus, in this review, we provide an overview on the recent knowledge gained on the impact and function of EphrinB2 in heart from development to diseases. We also updated the potential therapeutic strategies that interfere with EphrinB2 signaling.
EphrinB2-mediated bi-directional signaling
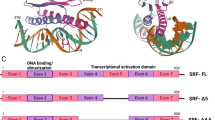
EphrinB2 was a highly conserved protein in mammal. Although human EphrinB2 was constituted 333 amino acids, while murine EphrinB2 had three more amino acids. They shared over 98% homology of amino acid sequence and roughly same structure. The domain structure of EphrinB2 consisted of ectodomain (1–229 aa in human, 1–232 aa in murine), transmembrane domain (230–250 aa in human, 233–253 aa in murine) and cytoplasmic domain (251–333 aa in human, 254–336 aa in murine), while the last three amino acids consisted a PDZ-binding motif in both human and murine [9] (Fig. 1). The forward signals originating from EphrinB2-binding Eph receptors mainly included the activation of PI3K and Rac-GTPases pathways [10]. The reverse signaling was transduced by a selfphosphorylation-dependent way and a PDZ-dependent way respectively due to the lack of intrinsic catalytic activity in EphrinB2. The tyrosine phosphorylation of EphrinB2 mediated by Src kinases [11] led to a recruitment of the SH2/SH3 domain-containing adaptor proteins such as Grb4 [12, 13], Stat1 [14] and Stat3 [15]. Grb4 regulated a serious of cytoskeleton signaling including FAK, PAK pathways affecting cell adhesion and migration [12], while Stat1 and Stat3, as critical transcription factors, transduced EphrinB2 signaling from cell membrane to nucleus. However, the exact phosphorylation sites of EphrinB2 remained obscure. The PDZ-dependent signaling was activated by binding proteins with PDZ domain to the PDZ motif, such as PDZ-RGS3 [16] and Dvl2 [17, 18], playing an important role in EphrinB2-mediated embryonic development and angiogenesis despite of the unclarified downstream efforts (Fig. 2). Notably, the crosstalk between phosphorylation signaling and PDZ signaling was existed. Phosphorylation of Tyr304 in human EphrinB2 conferred high-affinity and bifunctional binding activity to both Grb4 and PDZ-RGS3 [13].
EphrinB2 in heart development
EphrinB2 was of great importance in heart development. Conventional knockout of EphrinB2 was embryonic lethal in mice, which attributed to the defects of angiogenesis in yolk sac and myocardial trabeculation [19, 20]. Meanwhile, obvious arrest of heart development in these EphrinB2-null embryos was observed, including incompletion of cardiac looping, failure of endocardium expansion and formation of myocardial trabeculation. Afterwards, the endothelial specific knockout of EphrinB2 exhibited an indistinguishable angiogenic remodeling and cardiac defects from those of the global EphrinB2 knockout, further suggesting that EphrinB2 is affirmatively required in mouse embryonic endothelial and endocardial cells for proper cardiovascular development [21]. However, it was noteworthy, that whether EphrinB2 serves as a ligand or a receptor was still concealed. One study reported that the truncation of the majority of the EphrinB2 C-terminal cytoplasmic tail with or without HA tag (a loss-function approach of EphrinB2 reverse signaling) results in the similar embryonic lethality as the null, leading to a conclusion that EphrinB2 acts essentially as a receptor to transduce the reverse signaling in the early cardiac development [22]. In contrast, deletion of the same cytoplasmic residue but rather with βgal infusion (EphrinB2ΔV-βgal) allowed full embryonic development and birth of neonatal mice, in which EphrinB2-mutant protein preserved the surface binding activity and functioned as a proper ligand to stimulate EphB forward signaling [23]. Thus, the bidirectional effects of EphrinB2 may be both involved in embryonic cardiogenesis. To further elucidate the molecular mechanisms required for EphrinB2 transducing cellular signaling in vivo, two sets of knock-in mice were generated: (1) EphrinB2ΔV mice, which was lacking the C-terminal PDZ interaction residue to disrupt PDZ-dependent EphrinB2 signaling and (2) EphrinB25F mice, which was lacking phosphotyrosine-dependent EphrinB2 signaling with inactive mutation in 5 conserved tyrosine sites. Both of the mice survived the requirement of EphrinB2 in embryonic angiogenesis, while EphrinB25F mice survived to adulthood but EphrinB2ΔV mice died from failure of lymphatic vasculature maturation [17]. These data suggested that EphrinB2 reverse signaling may be complex and alternative in the embryo. Severe defects may only appear when both PDZ- and phosphorylation-dependent signalings are stripped off.
Additional data revealed that EphrinB2 reverse signaling is indispensable for cardiac valve maturation and the following neonatal survival. The EphrinB2ΔV-βgal mice eventually died within first day after birth, exhibiting extremely thickened aortic and pulmonary valves [23]. A massive accumulation of mesenchymal cells around the aberrant cardiac valves implied that loss of EphrinB2 reverse signaling possibly disrupts the mesenchymal-to-epithelial transition in cardiac valve maturation, while the mechanism remained a further investigation.
The genetic evidences in mice have identified that the cellular location of EphrinB2 was not restricted in endothelial cells, but was also in mesenchymal cells, vascular smooth muscle cells and pericytes surrounding the angiogenic vessles [19, 20, 24, 25]. It was supposed that EphrinB2 bridges the communication between these support cells and endothelial cells, as well as inter-endothelial interactions [26]. Mechanistically, EphrinB2 controlled the internalization of VEGF receptors-VEGFR2 and VEGFR3 in endothelial cells through PDZ-dependent reverse signaling [27, 28]. Within the trafficking of VEGFR, EphrinB2 was critically involved in VEGF signaling and thus promoted developmental angiogenesis and lymphangiogenesis. Lately, a soluble EphrinB2 (sEphrinB2) which was the ectodomain of EphrinB2 cleaved by A Disintegrin and Metalloprotease (ADAM) 10 was identified in both embryonic development and adult fibrotic diseases in mice [29, 30]. The existence of sEphrinB2 implied a potential approach of EphrinB2 during the inter-endothelial interaction. Since EphB4 was also capable of modulating VEGFR2 signaling through phospho-Erk1/2 [31], it is speculative that there may exist both autocrine and paracrine forms of EphrinB2 to activate the bidirectional signalings during the crosstalk between endothelial cells and the support cells.
Similarly, Sonia et al. reported that in avian embryos, both EphrinB2 and B1 were expressed in epicardium and then restricted in perivascular fibroblasts of the atrioventricular sulcus during the development. These EphrinBs regulated the migration of epicardial cells in the early stage and later participated in the coronary vascular development via perivascular fibroblasts [32].
Recently, accumulating researches uncovered the role of EphrinB2 in cardiomyocyte differentiation. In-vivo genetic data showed that the EphrinB2, directly induced by Notch1/RBPJK signaling in primitive myocardial epithelium during embryonic cardiogenesis, guided the endocardial cells towards the cardiomyocyte differentiation, leading to the transition of primitive myocardial epithelium into myocardial trabeculation and ventricular chamber formation [33]. Consistently, in-vitro data confirmed that EphrinB2/EphB4 signaling is involved in cardiac lineage development. Disrupting the interaction of EphrinB2 and EphB4 by utilizing specific antagonist peptide during murine embryonic stem cell (ESC) differentiation impaired the ESC derived-cardiac progenitor cells stepping towards cardiomyocytes [34, 35]. Interestingly, the expression of EphrinB2 in cardiomyocyte showed a dynamic alteration from neonatal to postnatal. Abundant expression of EphrinB2 was detected in neonatal murine cardiomyocyte, while adult murine cardiomyocyte only expressed EphrinB2 transcripts but failed to be detected at protein level physiologically [24, 25, 36]. Meanwhile, EphrinB2 expressed in murine ESC-derived cardiac progenitors but not the differentiated cardiomyocytes [34]. By employing human induced pluripotent stem cells (hiPS), we discovered that in hiPS-differentiated cardiomyocytes, EphrinB2 remarkably decreased during cardiomyocyte maturation (Fig. 3). The immature cardiomyocytes including fetal and neonatal ones contained proliferating ability and regenerated even after extensive injuries, whereas adult cardiomyocyte were mostly post-mitotic with rare proliferating capacity [37]. Likely, the alteration of EphrinB2 in cardiomyocyte from immature to adult suggested a possible role of EphrinB2 in cardiomyocyte proliferation and even cardiac regeneration in the pathological conditions such as heart failure.
EphrinB2 in heart diseases
EphrinBs in cardiac tissue architecture
In adult mouse heart ventricles, the EphrinB ligands are preferentially expressed in the vasculature and EphB receptors in cardiomyocytes [36]. Cardiomyocyte EphB4 which was activated by EphrinB2-Fc resulted in a significant inhibition of gap junctional intracellular communication between cardiomyocytes, suggesting that EphrinB/EphB signaling could modulate the electrical coupling of cardiomyocytes via gap junctions [36]. Noteworthy, another member of EphrinB family-EphrinB1 was discovered to be expressed in the lateral membrane of mouse cardiomyocyte, serving as a novel specific component to maintain the stability of cardiac tissue architecture [4]. Further investigations were urgently required to explain how EphrinB/EphB signaling functioned in cell-cell contraction to stabilize the cardiac architecture.
EphrinB2 in angiogenesis after cardiac injuries
Revascularization could therapeutically recover the injured myocardium in ischemic cardiovascular diseases such as myocardial infarction (MI) and ischemia/reperfusion (I/R) injury. Both the expression of EphrinB2 and EphB4 dramatically increased in MI, Brain IR injury and limb ischemic models in murine [38,39,40,41], suggesting the process of neovascularization occurred. Since EphrinB2/EphB4 controlled the endothelial spouting angiogenesis, which is essential to form a functional vessel [42], a certain activation of EphrinB2/EphB4 signaling in murine has been proved to induce angiogenesis in ischemic myocardium and brain where new blood vessels were urgently required [38, 40]. However, excessive expression of EphrinB2 in non-infarcted myocardium or physiologically-cultured endothelial cells did not work with few morphological alterations [40, 43]. Moreover, the mammalian inflammatory cells including neutrophil [44,45,46], macrophage [47] and T cell [48], all of which were evidenced to express EphrinB2, displayed a pro-angiogenic capacity during inflammatory angiogenesis which was beneficial for ischemic heart diseases. By using EphrinB2-Fc stimulation, Broqueres et al. have proved that the EphrinB2-activated peripheral blood mononuclear cells from diabetic patients could restore the impairment of postischemic neovascularization [49]. Thus, proper pathological/physiological stimulus seemed necessary to activate EphrinB2-regulated revascularization after cardiac injuries, whereas the conditions still need to be optimized and the mechanism was far from being resolved.
EphrinB2 in cardiac fibrosis
As expanded above, EphrinB2 was also expressed in mesenchymal cells, especially high in fibroblast. A decade before, pericyte-EphrinB2 knockout mice were observed with massive fibrosis surrounding aberrant vessels in skin [50], implying a potential role of EphrinB2 in organ fibrogenesis. Afterwards, EphrinB2 was proved to be involved in the fibrotic process of skin [51], retina [52] and kidney [53], respectively. Lately, two studies clarified the role and mechanism of EphrinB2 involved in lung and cardiac fibrosis. Although EphrinB2 emerged as a potent pro-fibrotic factor in the both organs after injury, EphrinB2 contributed to cardiac fibrosis through a phosphorylation-dependent signaling, leading to the transition of cardiac fibroblast to myofibroblast [15], while in lung fibrosis, fibroblast-derived EphrinB2 was cleaved as a soluble type to activate EphB forward signaling in an autocrine way [29]. Cardiac fibrosis, triggered by cardiac ischemia, hypoxia, overload-pressure, etc., was a critical pathological remodeling process after cardiac injury. EphrinB2 enhanced the TGF-β (Transforming Growth Factor-β) /Smad3 signaling-mediated cardiac fibrosis through regulating the interaction between Stat3 and Smad3 [15]. Interestingly, TGF-β also induced EphrinB2 expression in fibroblast, suggesting a positive feedback between EphrinB2 and TGF-β signaling [54]. Meanwhile, similar activation of EphrinB2 was observed in endothelial cells within TGF-β stimulation [55]. TGF-β-induced phenotype switch of endothelial cells towards mesenchymal cells was one of the mechanisms contributing to cardiac fibrosis [56, 57]. Thus, it is potential that EphrinB2 acts as a pivotal regulator in TGF-β-mediated organ fibrosis, as well as a promising therapeutic target.
EphrinB2 in human cardiac diseases
Up to now, the significant roles of EphrinB2 in human carcinogenesis have been clearly identified. High-level expression of EphrinB2 was associated with low-stage, progressive and metastable human solid tumors [58, 59], suggesting a certain therapeutic potential of EphrinB2 serving as a prognostic biomarker. Unlike the considerable achievements in clinical oncology, studies regarding on EphrinB2 in human cardiac diseases were scantly few. Lately, Levy et al. reported that a patient with a de novo variant in EFNB2 gene exhibited hypoplastic left ventricle and mild developmental delay [60]. EFNB2 mapped to 13q33.3 was located in the subtelomeric region of 13q chromosome. Recent cases have reported that patients with terminal deletion of 13q have developmental retardation and distinct congenital heart defects including tetralogy of fallot, ventricular septal defect, etc. EFNB2 was highly suspected as a candidate gene in these cases [61,62,63]. In another case with a familiar 13q33.2q33.3 deletion which was the smallest interstitial 13q33.3 deletion reported so far, only encompassing EFNB2 and ARGLU1, the patients exhibited congenital heart defects and neurological anomalies [60]. Levy et al. suggested that haploinsufficiency of the EFNB2 largely contributed to these developmental anomalies in heart and neural systems [60]. However, to make more precise phenotype-genotype correlations, further investigations with high-resolution SNP array data are encouraged.
Potential therapies targeting EphrinB2
Proteases cleaving EphrinBs
Several proteases can cleave EphrinBs from their extracellular, transmembrane and intracellular domains in a manner that can be dispensable of Eph interaction. Protease of ADAM family, especially ADAM10, has been confirmed to cleave EphrinB2 extracellularly [29], while ADAM8 and ADAM13 were proved to cleave other EphrinBs [64, 65]. Besides, extracellular cleavage of EphrinBs by matrix metalloproteases (MMPs) was reported. The cleavage could be enhanced by the interaction with EphBs such as the case of EphrinB1 cleaved by MMP8 [64, 66]. These proteolytic fragments from ectodomain of EphrinBs preserved the binding activity to EphBs, regulating embryonic development, angiogenesis and fibrosis. In addition, EphrinBs were also the substrates of a rhomboid transmembrane serine protease-RHBDL2, which cleaved the transmembrane segment of EphrinBs, with a preference for EphrinB3. Such interaction eventually led to the inactivation and degradation of EphrinB3 [67]. In contrast, the intracellular domain of EphrinB2 cleaved by γ-secretase exhibited an enhancing role on the reverse signaling through phosphorylation of the down-stream molecules-Src and FAK [68, 69]. Thus, protease regulation of EphrinBs has broad significance for development and diseases. Blockade of specific protease may be a useful approach to inhibit aberrant EphrinBs function.
Recombinant extracellular domain and antibodies
Recombinant extracellular domain (ECD) of Eph/ephrins were widely used in research, serving as soluble surrogates to activate as well as inhibit the bidirectional signaling [70]. The ECDs possessed high binding affinity to their counterparts. Coupling ECDs with Fc domain or albumin could further extend their half-life in vivo [71]. Numerous studies employing EphrinB2-Fc have illustrated its pro-angiogeneic role through activating EphB forward signaling [38, 72,73,74]. Meanwhile, a covalent coupling of EphrinB2 ECD with biomimetic fibrin vehicles could achieve the precise local delivery of EphrinB2. Such delivery managed to trigger angiogenic response locally, making it applicable in bioengineered tissues and therapeutic angiogenesis [75]. Recently, EphrinB2 ECD bio-conjugated with a soluble biopolymer and EphrinAs ECD embedded with nanoparticles were invented [76,77,78]. These nanoscaled technologies allowed an accurate modulation of Ephrin/Eph signalings in targeted cells, which has bright future in the emerging nanotherapy.
Although EphrinB2 ECD displayed as a soluble ligand activating EphBs, the soluble EphB4 ECD (sEphB4) [79], as well as its optimized form, sEphB4-HAS, which was fused of human serum albumin with sEphB4, blocked the EphrinB2/EphB4 bi-directional signaling. This blockage resulted in dysfunction of endothelium in-vitro and attenuation of pathological angiogenesis in vivo [80,81,82]. Furthermore, sEphB4 inhibited tumor invasion through blocking EphrinB2 [83]. Thus, sEphB4 could be utilized in anti-angiogenic therapy and the interference of EphrinB2 reverse signaling.
Additionally, inhibitory antibodies specifically targeting EphrinB2 and EphB4 have been applied in cancer research, showing high efficacy in inhibiting angiogenesis, lymphangiogenesis and tumor growth [84,85,86].
Small molecules interrupting the interaction of EphrinB2 with EphB4
To date, small molecule targeting EphrinB-EphB interaction has been synthesized though with limited binding affinities. The low affinity could be blamed by the flexibility and large size of Ephrin-binding domain in EphBs [87]. Hence, more efficient but larger compounds (> 500 Da) were created to improve the affinity of EphrinB/EphB interaction. Koolpe, et al. identified a 15 amino acid-long peptide of~ 1700 Da named TNYL-RAW, which antagonized the EphrinB2/EphB4 interaction through specific binding to the Ephrin-binding pocket of EphB4 with low nanomolar affinity [88]. Later, several modifications were employed in TNYL-RAW. By fusion with the Fc portion of human IgG1 coupling to a polyethylene glycol (PEG) polymer, the PEGylated TNYL-RAW remarkably increased stability and half-life in vivo, making it practicable for in-vivo applications with long-term interference of EphrinB2/EphB4 interaction [89]. Meanwhile, another two smaller molecules of ~ 600-700 Da, synthesized from the C-terminal portion of the TNYL-RAW peptide were also reported which could inhibit EphrinB2 binding to EphB4 at low micromolar concentrations [90].
Conclusion
EphrinB2 was a highly conserved protein ubiquitously expressed in all mammals, transducing signaling through a particular bidirectional pathway. EphrinB2-regulated bidirectional signaling was of great importance in cardiogenesis, guiding the completion of cardiac looping, expansion of endocardium, formation of myocardial trabeculation and cardiac valve and development of cardiac lineage differentiation as an emerging role. Lately, beyond cardiogenesis, EphrinB2 was identified critically involved in pathological cardiac remodeling process, such as angiogenesis after cardiac injury and cardiac fibrosis, which uncovered the tip of the iceberg regarding the roles of EphrinB2 under pathological conditions. Several proteases which were naturally existed in mammals, such as MMPs, ADAMs, γ-secretase, were capable of cleaving EphrinBs, leading to (de) activation of EphrinBs. Besides, synthesized recombinant ECD of Ephrin or Eph, inhibitory antibodies and small molecules were also able to (de) activate EphrinB2 signaling, providing a strongly potential target for further translational application in cardiovascular diseases.
Abbreviations
- ADAM:
-
A Disintegrin And Metalloprotease
- ECD:
-
Recombinant extracellular domain
- Eph:
-
Erythropoietin-producing hatocellular receptors
- Ephrin:
-
Erythropoietin-producing hepatocellular receptor interacting proteins
- ESC:
-
Embryonic stem cell
- hiPS:
-
Human induced pluripotent stem cells
- I/R:
-
Ischemia/reperfusion
- MI:
-
Myocardial infarction
- TGF-β:
-
Transforming Growth Factor-β
References
Yang D, Jin C, Ma H, Huang M, Shi GP, Wang J, Xiang M. EphrinB2/EphB4 pathway in postnatal angiogenesis: a potential therapeutic target for ischemic cardiovascular disease. Angiogenesis. 2016;19:297–309.
Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci. 2010;123:1235–46.
Daar IO. Non-SH2/PDZ reverse signaling by ephrins. Semin Cell Dev Biol. 2012;23:65–74.
Genet G, Guilbeau-Frugier C, Honton B, Dague E, Schneider MD, Coatrieux C, Calise D, Cardin C, Nieto C, Payre B, et al. Ephrin-B1 is a novel specific component of the lateral membrane of the cardiomyocyte and is essential for the stability of cardiac tissue architecture cohesion. Circ Res. 2012;110:688–700.
Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17:240–56.
Yoon J, Hwang YS, Lee M, Sun J, Cho HJ, Knapik L, Daar IO. TBC1d24-ephrinB2 interaction regulates contact inhibition of locomotion in neural crest cell migration. Nat Commun. 2018;9:3491.
Duffy P, Wang X, Siegel CS, Tu N, Henkemeyer M, Cafferty WB, Strittmatter SM. Myelin-derived ephrinB3 restricts axonal regeneration and recovery after adult CNS injury. Proc Natl Acad Sci U S A. 2012;109:5063–8.
Chen Y, Zhang H, Zhang Y. Targeting receptor tyrosine kinase EphB4 in cancer therapy. Semin Cancer Biol. 2017.
Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB. Crystal structure of an Eph receptor-ephrin complex. Nature. 2001;414:933–8.
Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13:39–62.
Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–37.
Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–9.
Su Z, Xu P, Ni F. Single phosphorylation of Tyr304 in the cytoplasmic tail of ephrin B2 confers high-affinity and bifunctional binding to both the SH2 domain of Grb4 and the PDZ domain of the PDZ-RGS3 protein. Eur J Biochem. 2004;271:1725–36.
Salvucci O, Ohnuki H, Maric D, Hou X, Li X, Yoon SO, Segarra M, Eberhart CG, Acker-Palmer A, Tosato G. EphrinB2 controls vessel pruning through STAT1-JNK3 signalling. Nat Commun. 2015;6:6576.
Su SA, Yang D, Wu Y, Xie Y, Zhu W, Cai Z, Shen J, Fu Z, Wang Y, Jia L, et al. EphrinB2 regulates cardiac fibrosis through modulating the interaction of Stat3 and TGF-beta/Smad3 signaling. Circ Res. 2017;121:617–27.
Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79.
Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410.
Mao Y, Huang X, Zhao J, Gu Z. Preliminary identification of potential PDZ-domain proteins downstream of ephrin B2 during osteoclast differentiation of RAW264.7 cells. Int J Mol Med. 2011;27:669–77.
Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53.
Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306.
Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–410.
Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69.
Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271:263–71.
Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–60.
Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MJ, Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–50.
Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–66.
Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–6.
Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–91.
Lagares D, Ghassemi-Kakroodi P, Tremblay C, Santos A, Probst CK, Franklin A, Santos DM, Grasberger P, Ahluwalia N, Montesi SB, et al. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat Med. 2017;23:1405–15.
Ji YJ, Hwang YS, Mood K, Cho HJ, Lee HS, Winterbottom E, Cousin H, Daar IO. EphrinB2 affects apical constriction in Xenopus embryos and is regulated by ADAM10 and flotillin-1. Nat Commun. 2014;5:3516.
Groppa E, Brkic S, Uccelli A, Wirth G, Korpisalo-Pirinen P, Filippova M, Dasen B, Sacchi V, Muraro MG, Trani M, et al. EphrinB2/EphB4 signaling regulates non-sprouting angiogenesis by VEGF. EMBO Rep. 2018.
Wengerhoff SM, Weiss AR, Dwyer KL, Dettman RW. A migratory role for EphrinB ligands in avian epicardial mesothelial cells. Dev Dyn. 2010;239:598–609.
Grego-Bessa J, Luna-Zurita L, Del MG, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–29.
Chen K, Bai H, Liu Y, Hoyle DL, Shen WF, Wu LQ, Wang ZZ. EphB4 forward-signaling regulates cardiac progenitor development in mouse ES cells. J Cell Biochem. 2015;116:467–75.
Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321–30.
Ishii M, Mueller I, Nakajima T, Pasquale EB, Ogawa K. EphB signaling inhibits gap junctional intercellular communication and synchronized contraction in cultured cardiomyocytes. Basic Res Cardiol. 2011;106:1057–68.
Yuan X, Braun T. Multimodal regulation of cardiac myocyte proliferation. Circ Res. 2017;121:293–309.
Ghori A, Freimann FB, Nieminen-Kelha M, Kremenetskaia I, Gertz K, Endres M, Vajkoczy P. EphrinB2 activation enhances vascular repair mechanisms and reduces brain swelling after mild cerebral ischemia. Arterioscler Thromb Vasc Biol. 2017;37:867–78.
Zhu L, Qian L, Wang S, Wang T, Jiang L. Expression of ephrinB2 and EphB4 in a neonatal rat model of periventricular white matter damage. J Perinat Med. 2015;43:367–71.
Mansson-Broberg A, Siddiqui AJ, Genander M, Grinnemo KH, Hao X, Andersson AB, Wardell E, Sylven C, Corbascio M. Modulation of ephrinB2 leads to increased angiogenesis in ischemic myocardium and endothelial cell proliferation. Biochem Biophys Res Commun. 2008;373:355–9.
Hayashi S, Asahara T, Masuda H, Isner JM, Losordo DW. Functional ephrin-B2 expression for promotive interaction between arterial and venous vessels in postnatal neovascularization. Circulation. 2005;111:2210–8.
Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–8.
Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–16.
Schruefer R, Sulyok S, Schymeinsky J, Peters T, Scharffetter-Kochanek K, Walzog B. The proangiogenic capacity of polymorphonuclear neutrophils delineated by microarray technique and by measurement of neovascularization in wounded skin of CD18-deficient mice. J Vasc Res. 2006;43:1–11.
Yuan K, Jin YT, Lin MT. Expression of Tie-2, angiopoietin-1, angiopoietin-2, ephrinB2 and EphB4 in pyogenic granuloma of human gingiva implicates their roles in inflammatory angiogenesis. J Periodontal Res. 2000;35:165–71.
Zamora DO, Babra B, Pan Y, Planck SR, Rosenbaum JT. Human leukocytes express ephrinB2 which activates microvascular endothelial cells. Cell Immunol. 2006;242:99–109.
Yuan K, Hong TM, Chen JJ, Tsai WH, Lin MT. Syndecan-1 up-regulated by ephrinB2/EphB4 plays dual roles in inflammatory angiogenesis. Blood. 2004;104:1025–33.
Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol. 2003;171:106–14.
Broqueres-You D, Lere-Dean C, Merkulova-Rainon T, Mantsounga CS, Allanic D, Hainaud P, Contreres JO, Wang Y, Vilar J, Virally M, et al. Ephrin-B2-activated peripheral blood mononuclear cells from diabetic patients restore diabetes-induced impairment of postischemic neovascularization. Diabetes. 2012;61:2621–32.
Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–73.
Avouac J, Clemessy M, Distler JH, Gasc JM, Ruiz B, Vacher-Lavenu MC, Wipff J, Kahan A, Boileau C, Corvol P, Allanore Y. Enhanced expression of ephrins and thrombospondins in the dermis of patients with early diffuse systemic sclerosis: potential contribution to perturbed angiogenesis and fibrosis. Rheumatology (Oxford). 2011;50:1494–504.
Umeda N, Ozaki H, Hayashi H, Oshima K. Expression of ephrinB2 and its receptors on fibroproliferative membranes in ocular angiogenic diseases. Am J Ophthalmol. 2004;138:270–9.
Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24:559–72.
Li Y, Chen Y, Tan L, Pan JY, Lin WW, Wu J, Hu W, Chen X, Wang XD. RNAi-mediated ephrin-B2 silencing attenuates astroglial-fibrotic scar formation and improves spinal cord axon growth. Cns Neurosci Ther. 2017;23:779–89.
Yan F, Wang Y, Wu X, Peshavariya HM, Dusting GJ, Zhang M, Jiang F. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014;5:e1010.
Yoshimatsu Y, Watabe T. Roles of TGF-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflam. 2011;2011:724080.
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61.
Oweida A, Bhatia S, Hirsch K, Calame D, Griego A, Keysar S, Pitts T, Sharma J, Eckhardt G, Jimeno A, et al. Ephrin-B2 overexpression predicts for poor prognosis and response to therapy in solid tumors. Mol Carcinog. 2017;56:1189–96.
Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Overexpression of ephrinB2 and EphB4 in tumor advancement of uterine endometrial cancers. Ann Oncol. 2007;18:485–90.
Levy J, Haye D, Marziliano N, Casu G, Guimiot F, Dupont C, Teissier N, Benzacken B, Gressens P, Pipiras E, et al. EFNB2 haploinsufficiency causes a syndromic neurodevelopmental disorder. Clin Genet. 2018;93:1141–7.
Wang YP, Wang DJ, Niu ZB, Cui WT. Chromosome 13q deletion syndrome involving 13q31qter: a case report. Mol Med Rep. 2017;15:3658–64.
Huang C, Yang YF, Yin N, Chen JL, Wang J, Zhang H, Tan ZP. Congenital heart defect and mental retardation in a patient with a 13q33.1-34 deletion. Gene. 2012;498:308–10.
Walczak-Sztulpa J, Wisniewska M, Latos-Bielenska A, Linne M, Kelbova C, Belitz B, Pfeiffer L, Kalscheuer V, Erdogan F, Kuss AW, et al. Chromosome deletions in 13q33-34: report of four patients and review of the literature. Am J Med Genet A. 2008;146A:337–42.
Dong Y, Harrington BS, Adams MN, Wortmann A, Stephenson SA, Lisle J, Herington A, Hooper JD, Clements JA. Activation of membrane-bound proteins and receptor systems: a link between tissue kallikrein and the KLK-related peptidases. Biol Chem. 2014;395:977–90.
Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013;5.
Tanaka M, Sasaki K, Kamata R, Sakai R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J Cell Sci. 2007;120:2179–89.
Pascall JC, Brown KD. Intramembrane cleavage of ephrinB3 by the human rhomboid family protease, RHBDL2. Biochem Biophys Res Commun. 2004;317:244–52.
Kemmerling N, Wunderlich P, Theil S, Linnartz-Gerlach B, Hersch N, Hoffmann B, Heneka MT, de Strooper B, Neumann H, Walter J. Intramembranous processing by gamma-secretase regulates reverse signaling of ephrin-B2 in migration of microglia. Glia. 2017;65:1103–18.
Atapattu L, Lackmann M, Janes PW. The role of proteases in regulating Eph/ephrin signaling. Cell Adhes Migr. 2014;8:294–307.
Barquilla A, Pasquale EB. Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2015;55:465–87.
Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–80.
Protack CD, Foster TR, Hashimoto T, Yamamoto K, Lee MY, Kraehling JR, Bai H, Hu H, Isaji T, Santana JM, et al. Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency. Sci Rep. 2017;7:15386.
Maekawa H, Oike Y, Kanda S, Ito Y, Yamada Y, Kurihara H, Nagai R, Suda T. Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol-3 kinase pathway and promotes angiogenesis in adult vasculature. Arterioscler Thromb Vasc Biol. 2003;23:2008–14.
Zheng LC, Wang XQ, Lu K, Deng XL, Zhang CW, Luo H, Xu XD, Chen XM, Yan L, Wang YQ, Shi SL. Ephrin-B2/fc promotes proliferation and migration, and suppresses apoptosis in human umbilical vein endothelial cells. Oncotarget. 2017;8:41348–63.
Zisch AH, Zeisberger SM, Ehrbar M, Djonov V, Weber CC, Ziemiecki A, Pasquale EB, Hubbell JA. Engineered fibrin matrices for functional display of cell membrane-bound growth factor-like activities: study of angiogenic signaling by ephrin-B2. Biomaterials. 2004;25:3245–57.
Conway A, Vazin T, Spelke DP, Rode NA, Healy KE, Kane RS, Schaffer DV. Multivalent ligands control stem cell behaviour in vitro and in vivo. Nat Nanotechnol. 2013;8:831–8.
Lohmuller T, Triffo S, O'Donoghue GP, Xu Q, Coyle MP, Groves JT. Supported membranes embedded with fixed arrays of gold nanoparticles. Nano Lett. 2011;11:4912–8.
Shaw A, Lundin V, Petrova E, Fordos F, Benson E, Al-Amin A, Herland A, Blokzijl A, Hogberg B, Teixeira AI. Spatial control of membrane receptor function using ligand nanocalipers. Nat Methods. 2014;11:841–6.
Kertesz N, Krasnoperov V, Reddy R, Leshanski L, Kumar SR, Zozulya S, Gill PS. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107:2330–8.
Shi S, Liu J, Joshi SB, Krasnoperov V, Gill P, Middaugh CR, Volkin DB. Biophysical characterization and stabilization of the recombinant albumin fusion protein sEphB4-HSA. J Pharm Sci. 2012;101:1969–84.
He S, Ding Y, Zhou J, Krasnoperov V, Zozulya S, Kumar SR, Ryan SJ, Gill PS, Hinton DR. Soluble EphB4 regulates choroidal endothelial cell function and inhibits laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2005;46:4772–9.
Ehlken C, Martin G, Lange C, Gogaki EG, Fiedler U, Schaffner F, Hansen LL, Augustin HG, Agostini HT. Therapeutic interference with EphrinB2 signalling inhibits oxygen-induced angioproliferative retinopathy. Acta Ophthalmol. 2011;89:82–90.
Scehnet JS, Ley EJ, Krasnoperov V, Liu R, Manchanda PK, Sjoberg E, Kostecke AP, Gupta S, Kumar SR, Gill PS. The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma and implications of EphrinB2 blockade. Blood. 2009;113:254–63.
Krasnoperov V, Kumar SR, Ley E, Li X, Scehnet J, Liu R, Zozulya S, Gill PS. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. Am J Pathol. 2010;176:2029–38.
Li JL, Sainson RC, Oon CE, Turley H, Leek R, Sheldon H, Bridges E, Shi W, Snell C, Bowden ET, et al. DLL4-notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–83.
Abengozar MA, de Frutos S, Ferreiro S, Soriano J, Perez-Martinez M, Olmeda D, Marenchino M, Canamero M, Ortega S, Megias D, et al. Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood. 2012;119:4565–76.
Tognolini M, Hassan-Mohamed I, Giorgio C, Zanotti I, Lodola A. Therapeutic perspectives of Eph-ephrin system modulation. Drug Discov Today. 2014;19:661–9.
Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280:17301–11.
Noberini R, Mitra S, Salvucci O, Valencia F, Duggineni S, Prigozhina N, Wei K, Tosato G, Huang Z, Pasquale EB. PEGylation potentiates the effectiveness of an antagonistic peptide that targets the EphB4 receptor with nanomolar affinity. PLoS One. 2011;6:e28611.
Duggineni S, Mitra S, Noberini R, Han X, Lin N, Xu Y, Tian W, An J, Pasquale EB, Huang Z. Design, synthesis and characterization of novel small molecular inhibitors of ephrin-B2 binding to EphB4. Biochem Pharmacol. 2013;85:507–13.
Funding
This study was supported from Provincial and Ministry Joint Major Projects of National Health Commission of China (WKJ-ZJ-1703 to Meixiang Xiang) and the National Natural Science Foundation of China (No. 81470384 and No. 81670259 to Meixiang Xiang).
Author information
Authors and Affiliations
Contributions
SS participated in the design, helped to draft the manuscript, and carried out documentation materials. YX and YZ participated in the design of schematic figures, respectively. JC participated in the design and helped to draft the manuscript. YX and MX participated in the design, coordinated the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not relevant.
Consent for publication
Not relevant.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Su, Sa., Xie, Y., Zhang, Y. et al. Essential roles of EphrinB2 in mammalian heart: from development to diseases. Cell Commun Signal 17, 29 (2019). https://doi.org/10.1186/s12964-019-0337-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-019-0337-3