Abstract
Background
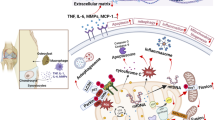
Osteoarthritis (OA), the most common form of arthritic disease, results from destruction of joint cartilage and underlying bone. It affects animals, including Asian elephants (Elephas maximus) in captivity, leading to joint pain and lameness. However, publications regarding OA pathogenesis in this animal are still limited. Therefore, this study aimed to investigate the effect of proinflammatory cytokines, including interleukin-1 beta (IL-1β), IL-17A, tumor necrosis factor-alpha (TNF-α), and oncostatin M (OSM), known mediators of OA pathogenesis, and lipopolysaccharides on the expression of cartilaginous degrading enzymes, matrix metalloproteinase (MMP)-3 and MMP-13, in elephant articular chondrocytes (ELACs) cultures. Anti-arthritic drugs and the active compounds of herbal plants were tested for their potential attenuation against overproduction of these enzymes.
Results
Among the used cytokines, OSM showed the highest activation of MMP3 and MMP13 expression, especially when combined with IL-1β. The combination of IL-1β and OSM was found to activate phosphorylation of the mitogen-activated protein kinase (MAPK) pathway in ELACs. Lipopolysaccharides or cytokine-induced expressions were suppressed by pharmacologic agents used to treat OA, including dexamethasone, indomethacin, etoricoxib, and diacerein, and by three natural compounds, sesamin, andrographolide, and vanillylacetone.
Conclusions
Our results revealed the cellular mechanisms underlying OA in elephant chondrocytes, which is triggered by proinflammatory cytokines or lipopolysaccharides and suppressed by common pharmacological or natural medications used to treat human OA. These results provide a more basic understanding of the pathogenesis of elephant OA, which could be useful for adequate medical treatment of OA in this animal.
Similar content being viewed by others
Background
Osteoarthritis (OA), the most prevalent arthritic disease, is characterized by cartilage degradation and consequent joint pain and disability [1, 2]. OA affects many species, including elephants, especially Asian elephants (Elephas maximus) kept in captivity. Excessive body weight along with the captive environment and trained behaviors are critical factors of OA pathogenesis in elephants [3, 4]. These factors disturb the equilibrium between the synthesis and degradation of the extracellular matrix (ECM) by chondrocytes, leading to further degradation of the ECM by matrix-degrading enzymes, especially matrix metalloproteinases (MMPs) [5]. The disturbance of this equilibrium is found particularly among captive elephants [6].
MMPs are a group of zinc-dependent endopeptidases that, when in excess, cause degeneration of the cartilage ECM. There has been a reported increase in MMP-3 and MMP-13 in humans and animals with OA, suggesting that these MMPs play a pivotal role in OA cartilage destruction [7,8,9,10]. It has previously been shown that the production of matrix-degrading enzymes is activated by proinflammatory cytokines, including interleukin-1 beta (IL-1β), IL-17A, tumor necrosis factor-alpha (TNF-α), and oncostatin M (OSM) [11,12,13,14]. In addition, the combination of OSM with other proinflammatory cytokines causes the greatest loss of cartilage matrix in OA [15,16,17]. Moreover, lipopolysaccharides (LPS), i.e., outer-membrane components of Gram-negative bacteria, contribute to septic arthritis and cartilage degeneration by upregulating the synthesis of catabolic factors, including proinflammatory cytokines and matrix-degrading enzymes [18, 19]. In OA pathogenesis, cytokine-induced signal transduction involves the activation of several pathways, including those of the mitogen-activated protein kinase (MAPK) family [20].
OA in elephants is caused by an imbalance of pressure on joints, which in turn is caused by a lack of exercise or an excessive body weight. This damages the cartilage, releasing inflammatory mediators and enzymes and, consequently, leading to joint inflammation. Affected elephants show signs of lameness and joint swelling and are reluctant to lay down because it will be difficult to stand up again. Swimming in a big pool to reduce weight bearing and administration of anti-inflammatory drugs are considered suitable treatments [21].
Current pharmacologic approaches for OA treatment aim at reducing inflammation and pain, improving joint function, and delaying disease progression. Commonly used medicines include steroids, non-steroidal anti-inflammatory drugs (NSAIDs), and disease-modifying OA drugs (DMOADs) [22], among which the most common agents are dexamethasone, indomethacin, etoricoxib, and diacerein, which have been shown to inhibit the expression of MMPs such as MMP1, MMP2, MMP3, MMP9, and MMP13 [23,24,25,26]. However, these substances are associated with a high incidence of adverse effects, including gastrointestinal damage and heart failure [27]. Thus, natural product-derived compounds with anti-inflammatory activity and low toxicity have become alternative treatments for OA. Among such compounds, sesamin, andrographolide, and vanillylacetone or zingerone have been reported to exhibit chondroprotective activity by inhibiting the expression of MMP1, MMP3, and MMP13 in chondrocytes [28,29,30].
It was reported that IL-1β stimulated the degradation of elephant cartilage in an explant culture model [31]. However, the existence of published studies on the cellular mechanisms of OA in elephants is limited. Therefore, the present study aimed to investigate the molecular mechanisms underlying the activation of expression of MMP-3 and MMP-13 by proinflammatory cytokines and LPS in elephant articular chondrocytes (ELACs). Additionally, the ability of commonly used anti-OA medications and natural compounds to inhibit these mechanisms was investigated. The information gained from this study will be useful in improving the treatment of elephants with OA and in supporting further research on elephant degenerative arthritis, both of which are important for a better quality of life for the elephants and contribute to vital elephant conservation.
Results
Proinflammatory cytokines induced upregulation of MMP3 and MMP13 expression in ELACs culture
Treatment with OSM alone resulted in a slight increase in MMP3 mRNA levels and a marked elevation of MMP13 levels. However, IL-1β, IL-17A, and TNF-α did not influence the expression of these genes in the monolayer culture model (Fig. 1). The combination of cytokines OSM and TNF-α significantly induced MMP13 expression, whereas the combination of OSM and IL-1β or IL-17A tended to induce MMP3 expression. In the pellet culture model (Fig. 2), the results of individual cytokine treatments show that only TNF-α could significantly activate the expression of MMP13. Meanwhile, the results of treatments with combined cytokines demonstrate that OSM combined with IL-1β dramatically increased the expression of both MMP3 and MMP13, whereas OSM combined with TNF-α slightly induced the expression of MMP13 but not that of MMP3.
Proinflammatory cytokines upregulate the mRNA expression of MMP3 (a) and MMP13 (b) in ELACs. The chondrocytes were treated with individual proinflammatory cytokines as follows: IL-1β (2.5 ng/mL); IL-17A (5 ng/mL); and TNF-α (5 ng/mL), or their combination with OSM (2 ng/mL) or IL-17A (5 ng/mL), for 24 h. mRNA levels were assessed by real-time RT-PCR. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05), whereas # signifies statistical significance in relation to single-cytokine treatment (#p < 0.05)
IL-1β in combination with OSM stimulates expression of MMP3 (a) and MMP13 (b) in ELAC pellets culture. ELAC pellets were treated with IL-1β or TNF-α, alone or in combination with OSM, for 3 days. The mRNA levels were assessed by real-time RT-PCR. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05), whereas # signifies statistical significance in relation to single-cytokine treatment (#p < 0.05)
Drugs and active compounds of medicinal plants inhibited cytokine-induced expression of MMP3 and MMP13 in ELACs culture
The results show that medications used to treat OA in humans, such as diacerein, dexamethasone, indomethacin, and etoricoxib, significantly attenuated MMP3 and MMP13 mRNA levels in the ELACs culture (Fig. 3a and b). Likewise, natural active compounds, including sesamin, andrographolide, and vanillylacetone, significantly suppressed the MMP3 and MMP13 mRNA levels in a dose-dependent manner (Fig. 4a and b).
Anti-arthritic drugs decrease the cytokines-induced expressions of MMP3 (a) and MMP13 (b) in ELACs. Chondrocytes were pre-treated with a combination of IL-1β (2.5 ng/mL) and OSM (2 ng/mL) for 2 h, after which they were treated with various concentrations of DIA (diacerein; 2.5–10 μM), DEX (dexamethasone; 5–20 nM), INDO (indomethacin; 2.5–10 μM), and ETORI (etoricoxib; 2.5–10 μM), for 24 h. mRNA levels were assessed by real-time RT-PCR. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05), whereas # signifies statistical significance in relation to the cytokines treatment group (#p < 0.05)
Natural active compounds reduce the cytokines-induced mRNA levels MMP3 (a) and MMP13 (b) in ELACs. The chondrocytes were pre-treated with a combination of IL-1β (2.5 ng/mL) and OSM (2 ng/mL) for 2 h, after which they were treated with various concentrations of SE (sesamin; 0.25–1 μM), AD (andrographolide; 1.25–5 μM), and VA (vanillylacetone; 20–80 μM), for 24 h. The mRNA levels were assessed by real-time RT-PCR. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05), whereas # signifies statistical significance in relation to the cytokines treatment group (#p < 0.05)
LPS induced the expression of MMP3 and MMP13 along with proinflammatory cytokine genes in ELACs culture
The results show that LPS at a 0.125 μg/mL concentration significantly increased MMP3 and MMP13 mRNA levels as well as the levels of IL1B and IL6 while increasing the expression of the TNF-α gene (TNFA) at a concentration of only 0.25 μg/mL (Fig. 5).
LPS induces expression of MMP3 and MMP13 (a), and proinflammatory cytokines (b) in ELACs culture. The chondrocytes were treated with LPS at various concentrations (0.125–1 μg/mL) for 24 h, then mRNA levels were assessed by real-time RT-PCR. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05)
Co-treatment with LPS and anti-arthritic drugs such as diacerein, dexamethasone, indomethacin, and etoricoxib significantly suppressed MMP3 and MMP13 mRNA levels in a dose-dependent manner (Fig. 6a and b). Figure 6c illustrates the LPS-induced increase of MMP-13 protein levels in the culture media, which was significantly suppressed by dexamethasone and indomethacin. However, the level of MMP-3 in the culture media could not be assessed using a human MMP-3 CLIA kit (data not shown).
Anti-arthritic drugs suppressed mRNA levels of MMP3 (a) and MMP13 (b) and decreasing MMP13 protein levels (c). The chondrocytes were pre-treated with 0.5 μg/mL LPS for 2 h, after which they were treated with various concentrations of DIA (diacerein; 2.5–10 μM), DEX (dexamethasone; 5–20 nM), INDO (indomethacin; 2.5–10 μM), and ETORI (etoricoxib; 2.5–10 μM) for 24 h. mRNA levels were then assessed by real-time RT-PCR. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05), whereas # signifies statistical significance in relation to the cytokines treatment group (#p < 0.05)
Activation of the MAPK pathway in ELACs by IL-1β combined with OSM
The MAPK pathway, one of the molecular mechanisms involved in OA pathogenesis, was activated in ELACs treated with a combination of IL-1β and OSM. The results show that the combined proinflammatory cytokines activated the maximum phosphorylation of p38, ERK, and JNK from 5 to 10 min, followed by its gradual decrease after 15 min (Fig. 7).
Activation of the MAPK pathway in ELACs by IL-1β combined with OSM. ELACs were stimulated by the combination of IL-1β (2.5 ng/mL) and OSM (2.5 ng/mL) at the indicated time points. Cell lysates were immunoblotted to investigate the total and phosphorylated molecular forms, which indicated an active MAPK pathway. Immunoblots are represented in (a) and bar graphs (b) show the proportion between the band intensities of phosphorylated p38, ERK, and JNK over their total forms. Results are presented as mean ± SEM. * signifies statistical significance compared with control (*p < 0.05)
Discussion
OA is the most prevalent musculoskeletal disorder in both humans and animals. Most studies on OA have focused on humans, with few reports available on animals, especially elephants. Asian elephants kept in captivity frequently suffer from OA caused primarily by residing in damp buildings and being overworked by humans as well as by restricted movement, which leads to cartilage degeneration and lameness [3, 4]. Reports on the mechanisms underlying OA in elephants are rare.
The present study used monolayer and pellet cultures of elephant chondrocytes as in vitro models to investigate the mechanisms underlying OA pathogenesis. Although the pellet culture, a three-dimensional culture model, mimicked the chondrocytes’ microenvironment within cartilage tissue more accurately [32], two-dimensional monolayer cultures are a faster and simpler model for cell-based studies. They allowed for quick evaluation of the effects of several proinflammatory cytokines known to be involved in OA pathogenesis on the expressions of MMP3 and MMP13 in ELACs.
The present results clearly demonstrate that ELACs are sensitive to activation by proinflammatory cytokines. Among the proinflammatory cytokines, the treatment with OSM alone strongly induced the expression of MMP13 in the monolayer cultures; TNF-α, which has been previously reported to induce the expression of MMP1, MMP3, and MMP13 in equine chondrocytes [11], caused a significant upregulation of MMP13 in the elephant chondrocyte pellet culture. IL-17A, alone or in combination with IL-1β or TNF-α, did not alter the expression of MMP3 or MMP13. The treatment with a combination of IL-17A and OSM caused a slight upregulation of MMP3 with no effect on MMP13. This result is inconsistent with previous studies on human cartilage cultures, which showed that the combination of IL-17A with TNF-α and OSM synergistically upregulates the expression of enzymes MMP-1 and MMP-13 [33]. This cytokine is known to be increased in the serum of OA patients, suggesting its involvement in human OA pathogenesis [34].
Although IL-1β has been reported to play a key role in the OA pathogenesis of large animals by upregulating the expression of MMP-1, MMP-3, and MMP-13 enzymes [13, 35, 36], our results clearly demonstrate that in the elephant chondrocyte pellet culture model, this cytokine could only induce the expression of MMP3 and MMP13 in combination with OSM. This result is consistent with a recent report suggesting that IL-1α and IL-1β are not crucial mediators of murine OA, which may explain the lack of success of IL-1-targeted therapies for OA [37]. Nevertheless, a previous report by our team demonstrated a great loss of hyaluronan from elephant cartilage explants treated with human recombinant IL-1β, suggesting the catabolic potential of this cytokine via accelerating the processes of cleavage and release of ECM biomolecules from the affected cartilage tissue, leading to degenerative cartilage in OA [31].
OSM, which belongs to the IL-6 family, is one of the proinflammatory cytokines that contribute to inflammation and cartilage destruction in degenerative arthritis [38]. OSM induces the expression of MMP1, MMP3, and MMP13 in bovine chondrocytes [12]. This cytokine has also been reported to synergize the action of other proinflammatory cytokines such as IL-1β, TNF-α, and IL-17A, resulting in acceleration of cartilage degeneration [15,16,17]. In this study, in elephant chondrocytes, the combination of OSM with IL-1β exerted the strongest induction of MMP3 and MMP13 expression in both the monolayer and pellet culture models, whereas the combined OSM with TNF-α only influenced the expression of MMP13. Our results suggest a cell-type specificity in response to the activation of cytokines. Additionally, all cytokines used in the present study were human recombinant proteins, implying that their actions on elephant chondrocytes may not represent the actions of species-specific cytokines. Nevertheless, the significant enhancement of MMP3 and MMP13 expression achieved by the combination of OSM and IL-1β provides important information regarding the action of these cytokines in the catabolic processes of elephant OA, which are similar to OA pathogenesis in other animals [17, 39].
Enzymes MMP-3 and MMP-13 are members of a zinc-dependent group of endopeptidases and considered crucial for the destruction process of cartilage ECM that occurs in OA [7,8,9,10]. The present study reveals that the expression of elephant MMP13 is more sensitive to induction by cytokines than MMP3. Among MMPs, most studies have focused on MMP-13, a collagenase-3, which is suggested to play a critical role in both the early stages and progression of OA [9, 40]. It is overexpressed in patients with OA but not in healthy patients. MMP-13 involves in cartilage degradation and also acts as a regulatory factor. It has been suggested that it plays a key role in controlling the onset of OA by leading chondrocytes from a normal to a pathological state [41]. MMP-3, stromelysin-1, is a matrix-degrading enzyme found to be increased in the serum and plasma of humans with OA, although its levels are not directly associated with OA severity [42]. Immunohistochemical assay of the synovium tissue of OA shows a high expression of MMP-3, which is positively correlated to the severity of the disease [10].
Likewise, in this study, the high expression of these enzymes in elephant chondrocytes was demonstrated under activation by the proinflammatory cytokines responsible for OA pathogenesis. Our results suggest that these enzymes, especially MMP-13, which exerts a strong response to cytokine activation, may be one of the key catabolic enzymes involved in elephant cartilage degeneration. Cytokine-induced upregulation of MMP13 mRNA levels was accompanied by an increase of MMP-13 protein levels in the culture media. This protein was successfully measured by a test kit designed to determine the level of human MMP-13, suggesting that the structures of elephant and human MMP-13 is closely related. However, another test kit designed to analyze human MMP-3 levels could not successfully be applied to measure the level of MMP-3 protein in elephant chondrocytes. Therefore, we postulate that the MMP-3 protein structure similarity between humans and elephants falls below the threshold of the recognizable capability of the human MMP-3 monoclonal antibody provided with the test kit.
Currently, scientific evidence on OA pathogenesis in elephants is limited. Expanding information regarding the biomechanisms of the disease as well as the effectiveness of drugs will support the development of therapeutic interventions that may be helpful to treat elephant OA. As such, the present study selected four drugs commonly prescribed to treat OA in humans and other animals, namely, dexamethasone, indomethacin, etoricoxib, and diacerein. Dexamethasone is a synthetic corticosteroid previously shown to inhibit the expression of MMP3 and MMP13 in IL-1α-induced bovine chondrocytes and suppress cytokine-induced inhibition of matrix biosynthesis in bovine cartilage [26]. NSAIDs are generally used to reduce pain and inflammation in arthritis through inhibition of cyclooxygenase (COX) [43]. Indomethacin is a non-selective inhibitor, whereas etoricoxib is in the COX2 selective class of NSAIDs. The former has been reported to reduce the expression of MMP1 and MMP3 in IL-1α-induced bovine chondrocytes [23], whereas the latter has been found to decrease the levels of MMP-2 and MMP-9 [25]. Diacerein, a DMOADs, has been reported to decrease the production of IL-1-converting enzyme and IL-1β in human osteoarthritic cartilage [44] as well as suppress the expression of MMP1, MMP3, MMP13, ADAMTS-4, and ADAMTS-5 in IL-1β-induced bovine chondrocytes [24]. Our results show that these drugs effectively suppress the expression of MMP3 and MMP13 induced by the combination of IL-1β and OSM or LPS, suggesting that they exhibited an anti-arthritic potential in the elephant chondrocytes culture model.
Moreover, this study demonstrates the protective effect of natural compounds previously reported to have anti-arthritic properties such as sesamin, andrographolide, and vanillylacetone against cytokine-induced expression of MMP3 and MMP13 in elephants, suggesting similarities in human and elephant OA pathogenesis, which is ameliorated by the action of these natural compounds. The concentration ranges of the natural compounds used in this study did not cause cell mortality but still effectively reduced the expression of MMP3 and MMP13 and were selected based on the results of the MTT cytotoxic assay [see Additional file 1]. However, the therapeutic dose of these agents on human or animal arthritis remains unclear. Therefore, the application of these agents to human or animal arthritis must be further investigated to achieve the maximum therapeutic effect.
It was reported that supplementation of sesame seed in patients with knee OA at a dose of 40 g daily for 2 months, along with standard medical therapy, improved the disease activity by reducing serum IL-6 [45]. In papain-induced rat OA, intra-articular injection of 20 μL of 1 or 10 μM sesamin reduced cartilage distortion [28]. This compound is the most prominent lignan in sesame seed oil [46] and has been reported to exert anti-arthritic effects by reducing IL-1β-induced production of proinflammatory mediators and cartilage-degrading enzymes MMP-1, MMP-3, and MMP-13, in human osteoarthritic chondrocytes via suppressing phosphorylation of NF-κB p65 and IκB and activation of the Nrf2 signaling pathway [28, 47].
Vanillylacetone, also called zingerone, is the major component of ginger root and has known antioxidant and anti-inflammatory properties [48]. In cytokine-induced degradation of porcine cartilage explant, this compound decreased the release of MMP-13 and cartilage matrix biomolecules into the culture media by suppressing the p38 and JNK MAPK signaling pathways [30]. Patients receiving one ginger extract capsule prepared from 2500 to 4000 mg of dried ginger rhizomes twice daily for 6 weeks showed a significant reduction of OA symptoms [49]. However, reports on the usage of vanillylacetone for anti-arthritic purposes in humans or animals are still limited.
Andrographolide is a major bioactive compound of Andrographis paniculata (Burm.f.) that was found to inhibit the expression of MMPs and inducible nitric oxide synthase in an IL-1β-induced OA model [29]. This agent reduced the productions of proinflammatory cytokines in vitro by suppressing the p38 MAPK and ERK1/2 pathways and alleviated arthritis severity in mice treated by oral administration of andrographolide 100 mg/kg/d [50]. It was reported that a combined administration of andrographolide 50 mg/kg/d and methotrexate 2 mg/kg/week in rat arthritis induced by complete Freund’s adjuvant significantly attenuated inflammatory symptoms and reduced liver injury caused by methotrexate [51]. Andrographolide has been proposed as a new potential anti-arthritic agent [52]. Therefore, it is worth further investigating the optimal dose of this agent for arthritis treatments in animals or humans. LPS are known to induce infectious arthritis and contribute to low-grade inflammation in OA pathogenesis [19, 53, 54]. They enhance the production of MMP-1, MMP-3, MMP-13, nitric oxide, and prostaglandin E2 in OA patients, leading to an increase in the area of cartilage destruction [55]. Likewise, the present study on elephant chondrocytes demonstrated a strong inducing effect of bacterial LPS on the expression of proinflammatory cytokine genes, including IL1B, TNFA, and IL6, together with matrix-degrading enzymes MMP3 and MMP13. These results shed light on the in vitro mechanisms of septic arthritis in an elephant chondrocyte culture model, which, when induced by LPS, showed an increased expression of proinflammatory cytokines and matrix-degrading enzymes. These effects were mitigated by dexamethasone, indomethacin, etoricoxib, and diacerein. Our findings suggest that these drugs attenuate LPS-induced inflammation and catabolic factors in both elephant and human chondrocytes.
MAPK is one of the most important signaling pathways regulating OA pathogenesis [56]. It is activated by proinflammatory cytokines, including IL-1β and OSM [12, 57], with consequent upregulation of cartilage-degrading enzyme production, including that of MMP-3 and MMP-13 [56, 58]. This study investigated the mechanisms underlying elephant OA by treating elephant chondrocytes with a combination of IL-1β and OSM via a commercial test kit commonly used to detect cellular activation in human cells via the MAPK signaling pathway. The present study shows that this test kit was successful in revealing the effects of these cytokines on the activation of p38, ERK, and JNK phosphorylation within 5–10 min before the phosphorylated forms gradually weakened. Our results support the notion that signal transduction in elephants is similar to that in humans and that this test kit is applicable to elephant chondrocytes.
Conclusions
Overall, the findings of this study provide insight into the molecular mechanisms of OA pathogenesis in ELACs, which share similarities with those occurring in humans and other animals. In addition, anti-arthritic drugs commonly used to treat OA in humans and other animals were found to ameliorate the expression of factors associated with arthritis, including proinflammatory cytokines and enzymes responsible for cartilage degeneration. The present study provides data that contribute to the development of treatments for elephants with OA and support research into arthritis in this species.
Methods
Preparation of primary ELACs
A stillborn elephant calf was caused by dystocia with no clinical appearance of joint disease in an elephant camp in Chiang Mai, Thailand. Cartilage samples from the femoral head of the stifle joint were aseptically collected within 6 h postmortem during the necropsy process, which was consented by the owner. Primary ELACs were isolated by overnight digestion with type II collagenase at 37 °C. The ELACs were washed with phosphate-buffered saline and grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% v/v fetal calf serum (FCS), penicillin (100 U/mL), and streptomycin (100 μg/mL) in a humidified incubator at 37 °C with 5% CO2 until confluence.
Monolayer culture and cytokine treatment of ELACs
ELACs at a 3 × 105 cells/well density were grown to confluence in DMEM containing 10% FCS. The ELACs were sustained in serum-free DMEM for 24 h, after which they were treated with proinflammatory cytokines (ProSpec, Rehovot, Israel), IL-1β (2.5 ng/mL), IL-17A (5 ng/mL), and TNF-α (5 ng/mL), either alone or in combination with OSM (2 ng/mL) for 24 h or with IL-17A (5 ng/mL) for 24 h. The ELACs were also treated with various concentrations of 0.125–1 μg/mL LPS (Sigma-Aldrich, U.S.A.). After 24 h, the cells were harvested, and the expression of MMP3 and MMP13 was investigated by real-time RT-PCR.
Pellet culture and cytokine treatment of ELACs
ELACs at 1 × 106 were centrifuged in 15 mL conical culture tubes at 1500 rpm for 5 min. The pellets that formed at the bottom of the tube were cultured for seven days in 500 μl of chondrogenic medium (DMEM containing 10% FCS, 1X Insulin-Transferrin-Selenium [59], 25 μg/mL ascorbic acid-2 phosphates, 10− 7 M dexamethasone) in a humidified incubator at 37 °C and 5% CO2 to allow for spherical shape formation of each pellet. The pellets were then further treated with IL-1β (5 ng/mL) and TNF-α (10 ng/mL), alone or in combination with OSM (4 ng/mL), for 3 days before being harvested for MMP3 and MMP13 mRNA expression analysis by real-time RT-PCR.
Treatment with drugs and natural compounds
ELACs in monolayer cultures were treated with a combination of 2.5 ng/mL IL-1β and 2 ng/mL OSM or 0.5 μg/mL LPS for 2 h [60]. Following this, they were treated with drugs, including diacerein (2.5–10 μM; TRB Chemidica, Italy), dexamethasone (5–20 nM; Sigma-Aldrich, U.S.A.), indomethacin (2.5–10 μM; Sigma-Aldrich, U.S.A.), and etoricoxib (2.5–10 μM; Zuelling, Philippines) or with natural bioactive compounds (Sigma-Aldrich, U.S.A.), including sesamin (0.25–1 μM), andrographolide (1.25–5 μM), and vanillylacetone (20–80 μM), for 24 h. The cells were then harvested to investigate the expression of MMP3 and MMP13 by real-time RT-PCR, and the culture media were analyzed for protein levels of MMP-3 and MMP-13.
Real-time RT-PCR
Total RNA was extracted from the ELACs obtained from the monolayer or pellet cultures using the Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare Life Sciences, U.K.), according to the manufacturer’s protocol. The total RNA of the monolayer (0.5 μg) and pellet (0.25 μg) cultures was reverse transcribed into complementary DNA using the ReverTra Ace® qPCR RT Master Mix (TOYOBO, Japan). The elephant primer sequences were designed based on the NCBI Primer-BLAST tool in association with GenBank accession numbers and synthesized by Bio Basic, Canada (Table 1). Real-time RT-PCR was performed using the SensiFAST™ SYBR No-ROX Kit (Bioline, U.K.). Gene expression quantification was based on the 2−∆∆Ct method against the expression of the glyceraldehydes-3-phosphate dehydrogenase gene (GAPDH) as a housekeeping gene [61].
Measurement of MMP-3 and MMP-13 levels in the culture media
The levels of MMP-3 and MMP-13 enzymes in the culture media were measured using human MMP-3 (catalog number: E-CL-H0931) and MMP-13 (catalog number: E-CL-H0127) sandwich ELISA kits (Elabscience, China), according to the manufacturer’s instructions. Briefly, 100 μl of MMP-3 or MMP-13 standard and sample (culture media) was added to the monoclonal antibody against the proteins (MMP-3 or MMP-13) pre-coated micro CLIA plate well, then incubated at 37 °C. After 90 min of incubation, the standard and sample were discarded, and 100 μl of a biotinylated detection antibody working solution was added to each well. The plate was incubated for 1 h at 37 °C, followed by three washings. A horseradish peroxidase conjugate (HRP) working solution was then added to each well (100 μl/well) and left to incubate at 37 °C for 30 min. After washing, 100 μl of substrate mixture solution was added to each well before being incubated in the dark for 5 min at 37 °C. The luminescence value was detected using a Synergy H4 hybrid multi-mode microplate reader (BioTek, U.S.A.), and the protein concentrations were calculated by comparing the samples with standard curves.
Western blot analysis of intracellular signaling molecules
ELACs were treated with a combination of the cytokines IL-1β (2.5 ng/mL) and OSM (2.5 ng/mL) at various time points. To investigate the activation of the MAPK pathway, the cells were collected in a radioimmunoprecipitation assay buffer. The cell lysates were vortexed every few minutes before centrifugation at 14,000 g for 10 min at 4 °C, after which the supernatants of the cell lysate were transferred into new tubes. The cells were lysed with a sample buffer containing 5% mercaptoethanol. Equal amounts (25 μg protein) of the cell lysates were heated for 10 min at 95 °C then subjected to 13% SDS-PAGE and transferred to a nitrocellulose membrane. After blocking non-specific proteins with 5% skim milk in TBS containing 0.1% Tween 20 (TBS-T) for 1 h, the membranes were washed with TBS-T and probed with primary antibodies (Cell Signaling Technology, U.S.A.), including rabbit anti-phosphorylated-p38 MAPK antibody, rabbit anti-phosphorylated-p44/42 MAPK antibody, rabbit anti-phosphorylated-SAPK/JNK antibody, rabbit anti-p38 MAPK antibody, rabbit anti-p44/42 MAPK antibody, rabbit anti-SAPK/JNK antibody, and mouse anti-β-actin (Biolegend, CA), at 4 °C overnight. After being washed with TBS-T, the membranes were incubated for 1 h with the secondary antibody conjugated with HRP anti-rabbit IgG or anti-mouse IgG at room temperature. The positive bands were visualized by enhanced chemiluminescence using the ChemiDoc system (Bio-Rad, U.S.A.). The intensity of the immuno-positive bands was calculated using the TotalLab TL120 software.
Statistical analysis
The results are presented as the mean ± standard error of the mean of three independent experiments. The statistical analysis was performed using one-way analysis of variance followed by LSD for post-hoc multiple comparisons. A level of p < 0.05 was considered statistically significant.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ELACs:
-
Elephant articular chondrocytes
- FCS:
-
Fetal calf serum
- IL-17A:
-
Interleukin-17A
- IL-1β:
-
Interleukin-1beta
- LPS:
-
Lipopolysaccharides
- MAPK:
-
the mitogen-activated protein kinase
- MMP:
-
Matrix metalloproteinase
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- OSM:
-
Oncostatin M
- TNF-α:
-
Tumor necrosis factor-alpha
References
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:1.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:6.
Hittmair KM, Vielgrader HD. Radiographic diagnosis of lameness in African elephants (Loxodonta africana). Vet Radiol Ultrasound. 2000;41:6.
Clubb R, Mason G. A review of the welfare of zoo elephants in Europe: RSPCA Horsham, UK; 2002.
Im HJ, Li X, Muddasani P, Kim GH, Davis F, Rangan J, et al. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J Cell Physiol. 2008;215:2.
Egger GF, Witter K, Weissengruber G, Forstenpointner G. Articular cartilage in the knee joint of the African elephant, Loxodonta africana, Blumenbach 1797. J Morphol. 2008;269:1.
Brama PA, TeKoppele JM, Beekman B, van El B, Barneveld A, van Weeren PR. Influence of development and joint pathology on stromelysin enzyme activity in equine synovial fluid. Ann Rheum Dis. 2000;59:2.
Clements DN, Carter SD, Innes JF, Ollier WE, Day PJ. Analysis of normal and osteoarthritic canine cartilage mRNA expression by quantitative polymerase chain reaction. Arthritis Res Ther. 2006;8:6.
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:1.
Chen JJ, Huang JF, Du WX, Tong PJ. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac J Trop Med. 2014;7:4.
Richardson DW, Dodge GR. Effects of interleukin-1β and tumor necrosis factor-α on expression of matrix-related genes by cultured equine articular chondrocytes. Am J Vet Res. 2000;61:6.
Li WQ, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol. 2001;166:5.
Tung J, Fenton J, Arnold C, Alexander L, Yuzbasiyan-Gurkan V, Venta P, et al. Recombinant equine interleukin-1ß induces putative mediators of articular cartilage degradation in equine chondrocytes. Can J Vet Res. 2002;66.
Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, −13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal. 2004;16:4.
Koshy PJ, Henderson N, Logan C, Life PF, Cawston TE, Rowan AD. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis. 2002;61:8.
Hui W, Rowan AD, Richards CD, Cawston TE. Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003;48:12.
Durigova M, Roughley PJ, Mort JS. Mechanism of proteoglycan aggregate degradation in cartilage stimulated with oncostatin M. Osteoarthr Cartil. 2008;16:1.
Campo GM, Avenoso A, Campo S, D'Ascola A, Traina P, Sama D, et al. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 2009;106:1.
Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr Cartil. 2016;24:10.
Loeser RF, Erickson EA, Long DL. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr Opin Rheumatol. 2008;20:5.
West G. Musculoskeletal system. Biology, medicine, and surgery of elephants Ames. IA: Blackwell Publishing; 2006.
Qvist P, Bay-Jensen AC, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): is it in the horizon? Pharmacol Res. 2008;58:1.
Sadowski T, Steinmeyer J. Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthr Cartil. 2001;9:5.
Legendre F, Bogdanowicz P, Martin G, Domagala F, Leclercq S, Pujol JP, et al. Rhein, a diacerhein-derived metabolite, modulates the expression of matrix degrading enzymes and the cell proliferation of articular chondrocytes by inhibiting ERK and JNK-AP-1 dependent pathways. Clin Exp Rheumatol. 2007;25:4.
Yang SF, Hsieh YS, Lue KH, Chu SC, Chang IC, Lu KH. Effects of nonsteroidal anti-inflammatory drugs on the expression of urokinase plasminogen activator and inhibitor and gelatinases in the early osteoarthritic knee of humans. Clin Biochem. 2008;41:1–2.
Li Y, Wang Y, Chubinskaya S, Schoeberl B, Florine E, Kopesky P, et al. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23:2.
Varga Z, Sabzwari SRA, Vargova V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: an under-recognized public health issue. Cureus. 2017;9:4.
Phitak T, Pothacharoen P, Settakorn J, Poompimol W, Caterson B, Kongtawelert P. Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry. 2012;80.
Ding QH, Ji XW, Cheng Y, Yu YQ, Qi YY, Wang XH. Inhibition of matrix metalloproteinases and inducible nitric oxide synthase by andrographolide in human osteoarthritic chondrocytes. Mod Rheumatol. 2013;23:6.
Ruangsuriya J, Budprom P, Viriyakhasem N, Kongdang P, Chokchaitaweesuk C, Sirikaew N, et al. Suppression of cartilage degradation by Zingerone involving the p38 and JNK MAPK signaling pathway. Planta Med. 2017;83:3–04.
Tangyuenyong S, Viriyakhasem N, Aungsuchawan S, Peansukmanee S, Thitaram C, Kongtawelert P, et al. Catabolism of Asian elephant cartilage matrix biomolecules in explant culture. KKU Vet J. 2012;22:2.
Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:4.
Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, Fitzgerald O, et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11:4.
Askari A, Naghizadeh MM, Homayounfar R, Shahi A, Afsarian MH, Paknahad A, et al. Increased serum levels of IL-17A and IL-23 are associated with decreased vitamin D3 and increased pain in osteoarthritis. PLoS One. 2016;11:11.
Dvorak LD, Cook JL, Kreeger JM, Kuroki K, Tomlinson JL. Effects of carprofen and dexamethasone on canine chondrocytes in a three-dimensional culture model of osteoarthritis. Am J Vet Res. 2002;63:10.
Cortial D, Gouttenoire J, Rousseau C, Ronzière M-C, Piccardi N, Msika P, et al. Activation by IL-1 of bovine articular chondrocytes in culture within a 3D collagen-based scaffold. An in vitro model to address the effect of compounds with therapeutic potential in osteoarthritis. Osteoarthr Cartil. 2006;14:7.
Nasi S, Ea H-K, So A, Busso N. Revisiting the role of Interleukin-1 pathway in osteoarthritis: interleukin-1α and-1β, and NLRP3 Inflammasome are not involved in the pathological features of the murine Menisectomy model of osteoarthritis. Front Pharmacol. 2017;8.
Plater-Zyberk C, Buckton J, Thompson S, Spaull J, Zanders E, Papworth J, et al. Amelioration of arthritis in two murine models using antibodies to Oncostatin M. Arthritis Rheum. 2001;44:11.
Rowan AD, Hui W, Cawston TE, Richards CD. Adenoviral gene transfer of interleukin-1 in combination with oncostatin M induces significant joint damage in a murine model. Am J Pathol. 2003;162:6.
Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil. 2005;13:7.
Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19:1.
Naito K, Takahashi M, Kushida K, Suzuki M, Ohishi T, Miura M, et al. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology (Oxford). 1999;38:6.
Yoon JB, Kim SJ, Hwang SG, Chang S, Kang SS, Chun JS. Non-steroidal anti-inflammatory drugs inhibit nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes independent of cyclooxygenase activity. J Biol Chem. 2003;278:17.
Moldovan F, Pelletier J, Jolicoeur F-C, Cloutier J-M, Martel-Pelletier J. Diacerhein and rhein reduce the ICE-induced IL-1β and IL-18 activation in human osteoarthritic cartilage. Osteoarthr Cartil. 2000;8:3.
Khadem Haghighian M, Alipoor B, Malek Mahdavi A, Eftekhar Sadat B, Asghari Jafarabadi M, Moghaddam A. Effects of sesame seed supplementation on inflammatory factors and oxidative stress biomarkers in patients with knee osteoarthritis. Acta Med Iran. 2015;53:4.
Murata J, Matsumoto E, Morimoto K, Koyama T, Satake H. Generation of triple-transgenic Forsythia cell cultures as a platform for the efficient, stable, and sustainable production of Lignans. PLoS One. 2015;10:12.
Kong P, Chen G, Jiang A, Wang Y, Song C, Zhuang J, et al. Sesamin inhibits IL-1beta-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget. 2016;7:50.
Kim MK, Chung SW, Kim DH, Kim JM, Lee EK, Kim JY, et al. Modulation of age-related NF-κB activation by dietary zingerone via MAPK pathway. Exp Gerontol. 2010;45:6.
Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44:11.
Li ZZ, Tan JP, Wang LL, Li QH. Andrographolide benefits rheumatoid arthritis via inhibiting MAPK pathways. Inflammation. 2017;40:5.
Li F, Li H, Luo S, Ran Y, Xie X, Wang Y, et al. Evaluation of the effect of andrographolide and methotrexate combined therapy in complete Freund's adjuvant induced arthritis with reduced hepatotoxicity. Biomed Pharmacother. 2018;106.
Hidalgo MA, Hancke JL, Bertoglio JC, Burgos RA. Andrographolide a new potential drug for the long term treatment of rheumatoid arthritis disease. Innovative Rheumatology: IntechOpen; 2013.
Blasioli DJ, Kaplan DL. The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev. 2014;20:4.
Huang Z, Kraus VB. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016;12:2.
Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Oh HJ, et al. The catabolic pathway mediated by toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:7.
Shi J, Zhang C, Yi Z, Lan C. Explore the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early stage of osteoarthritis. IUBMB Life. 2016;68:4.
Li X, Guo Y, Huang S, He M, Liu Q, Chen W, et al. Coenzyme Q10 prevents the Interleukin-1 Beta induced inflammatory response via inhibition of MAPK signaling pathways in rat articular chondrocytes. Drug Dev Res. 2017;78:8.
Hellman NE, Spector J, Robinson J, Zuo X, Saunier S, Antignac C, et al. Matrix metalloproteinase 13 (MMP13) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1), regulated by the MAPK pathway, are both necessary for Madin-Darby canine kidney tubulogenesis. J Biol Chem. 2008;283:7.
Su SC, Tanimoto K, Tanne Y, Kunimatsu R, Hirose N, Mitsuyoshi T, et al. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22:6.
Zheng X, Xia C, Chen Z, Huang J, Gao F, Li G, et al. Requirement of the phosphatidylinositol 3-kinase/Akt signaling pathway for the effect of nicotine on interleukin-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. Biochem Biophys Res Commun. 2012;423:3.
Al-Shanti N, Saini A, Stewart CE. Two-step versus one-step RNA-to-CT 2-step and one-step RNA-to-CT 1-step: validity, sensitivity, and efficiency. J Biomol Tech. 2009;20:3.
Acknowledgements
The authors gratefully acknowledge all general support throughout the research process from Thailand Excellence Center for Tissue Engineering and Stem Cells, Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Thailand. In addition, we wish to thank Miss Pianghathai Yavirach for her valuable suggestions in some parts of molecular analysis.
Authors‘contributions
SO and SC designed the experiments and applying for Grants; S.O. contributed as a project administrator; ST, WT, CS, and CT worked out for the ethical approval and collected the animal tissues; N.S. performed the experiments; SO and N.S. analyzed the data; N.S. and S.O. prepared the original draft of manuscript; SC, ST, WT, CS, and CT, NS and SO revised and edited the manuscript. All the authors mentioned in this manuscript have agreed for authorship, read and approved the manuscript, and given consent for submission and subsequent publication of the manuscript.
Funding
This research work was supported by Thailand and the National Research Council of Thailand (Government Budget; 2015). The funder had no role in study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Animal use and all procedures in the present study were approved by the Animal Care and Use Committee, Faculty of Veterinary Medicine, Chiang Mai University, Thailand (FVM–ACUC; Ref. No. R22/2559). We obtained written informed consent to use the deceased elephant from the owner of the elephant camp.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
The effect of natural compounds on elephant articular chondrocytes viability by using MTT assay.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sirikaew, N., Chomdej, S., Tangyuenyong, S. et al. Proinflammatory cytokines and lipopolysaccharides up regulate MMP-3 and MMP-13 production in Asian elephant (Elephas maximus) chondrocytes: attenuation by anti-arthritic agents. BMC Vet Res 15, 419 (2019). https://doi.org/10.1186/s12917-019-2170-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-019-2170-8