Abstract
Background
Therapeutic use of leaves of M. oleifera has been evaluated in diabetes because of its possible capacity to decrease blood glucose and lipids concentration after ingestion, as result of the polyphenols content and others compounds. Nevertheless most results have been obtain from leaf extract, therefore this study would use leaf powder as the regular way of consumption of population to know effects over toxicity glucose, triglycerides, cholesterol, corporal weight, and predominant groups of microbiota.
Methods
Powdered leaf was administrated in different doses to know toxicity and genotoxicity using LD50 and micronuclei assay. Hyperglycemia was induced by alloxan on Sprague Dawley rats. Glucose and body weight were measured once a week meanwhile cholesterol and triglycerides were analyzed at the end of the study by commercial kits. Different organs were examined by hematoxylin-eosin technique. Lactic acid bacteria and Enterobacteriaceae were enumerated from stool samples.
Results
The tested doses revealed no lethal dose and no significant differences in genotoxicity parameter. The consumption of the leaves showed a hypoglycemic effect (< 250 mg/dL in diabetic M. oleifera treated group), however in corporal weight showed an increased (> 30 g over no M. oleifera treated groups). There was no change in enumeration of lactic acid bacteria (8.4 CFU/g) but there were differences in the predominance of type of lactobacillus and enterobacteria enumeration.
Conclusions
These results help to increase information over the most popular use of M. oleifera and its safety. However there are needed more studies over the hypoglycemic mechanisms and effects over intestinal microbiota.
Similar content being viewed by others
Background
Diabetes mellitus is a disease characterized by hyperglycemia caused by the impairment of insulin secretion, insulin action or both. A chronic increase in glucose levels can lead to macro- and microvascular complications, such as heart disease, hypertriglyceridemia, nephropathy, and neuropathy. In diabetes mellitus 2, insulin resistance can also lead to gastroparesis as a secondary effect, causing abdominal pain, nausea, emesis, meteorism, and an alteration in intestinal microbiota marked by enterobacteria predominance and a decrease in the number of beneficial bacteria [1, 2].
Glycemic control through diet is necessary for preventing or limiting the consequences of diabetes mellitus. Therefore, the consumption of functional foods and nutraceutical or bioactive compounds derived from plants used as food can be used as nutritional tools because of their clinical effects. For example, the ingestion of Moringa oleifera could bring biological benefits due to the nutritional content of its leaves, such as protein, glucoside, vitamins (A, C), and minerals [3, 4].
Moringa oleifera is an Indian tree that has been cultivated in diverse regions of Mexico, and it is referred to as “drum stick tree” or the “horse riding tree.” It belongs to the family Moringaceae, the order Brassicales, and the genus Moringa, which contains 13 species ranging in height from 5 to 10 m. It has an open crown of drooping, feathery foliage, flowers with distinctive green patches at the tips of the petals and sepals, tripinnate leaves and trunk. This tree is important because its flowers, pods, and leaves have medicinal uses. It has been reported that the flower contains a stimulant and is used to treat inflammation; the spots and seeds have liver-protective and antihypertensive properties, while the leaves have been used to treat microbial infections and to control glucose levels.
The leaves are eaten as vegetables of food ingredient because of the high content of vitamins, antioxidants and macronutrients to improve nutritional deficiencies [5]. However the study of effect of biological compounds of different part of Moringa oleifera plant have brought different action mechanism, functional benefits and toxicity profile that have not yet been elucidated. Is in that reason that it has been suggested that the use of new pharmaceutical and nutraceutical products, as M. oleifera, must be tested first for safety in appropriate in vitro and in vivo models, before being used in human health, in order to ensure the absence of toxicity and for better understanding of action mechanisms [6, 7]. In spite of nutraceutical beneficial properties, the different compounds of the plant present distinct pharmacological effects, including toxicity profiles, which have not yet been completely elucidated. Also, international regulations relating to human health demand that all kind of pharmaceutical and nutraceutical products are tested for their safety, and the way to ensuring this is to conduct toxicity tests in appropriate in vitro and in vivo models [5]. Therefore for toxicological evaluation it has been used animal models to reveal histopathological damage [8].
There have been used in biological assay aqueous and ethanol extract of leaf in different doses, meanwhile leaf powder studies have been most done in clinical research [9]. Thus it can be use in vivo models to bring more information about powder leaf consumption effect on different diseases. For example, induction of experimental diabetes in rats is a convenient model to study activity of hypoglycemic agents over hyperglycemia and it is consequences where it has been observed the possible antioxidant and antidiabetic effects through plasma glucose, triacylglycerol and cholesterol monitoring, microscopic lesion observation, marker enzyme, serum and lipid peroxidation measuring and for understanding the pathophysiology [9, 10]. Also the experimental animal model of diabetes mellitus can be done by chemical induction using streptozotocin or alloxan which diabetogenic action has been employed and proven in different animal species, with different rout of administration or nutritional stratus [11]. Humans and mouse genotype is very similar between them, this influences metabolism, as it becomes highly comparable with humans, and different strains of mice differ in their response to high fat diet and sensitivity to metabolic diseases. BALB/c mice are naturally resistant to the development of high fat diet induced obesity and development of diabetes as a result [12]. Mouse is a good biological tool that allows the analyses of different tissues with little limitation on the amount of biological materials available. This animal model provide an important tool for study of the pathogenesis, prevention and treatment of diabetic complications [13]. Mouse is economical and requires simple husbandry compared to larger mammals, and there is a huge volume of literature on the physiology, behavior, and biochemistry of such rodents. Importantly, it is possible to modify the diet of mouse or treat them with drugs to mimic specific diseases and/or to improve their health status [14].
There have been used in biological assay aqueous and ethanol extract of leaf in different doses, meanwhile leaf powder studies have been most done in clinical research [15]. Thus it can be use in vivo models to bring more information about powder leaf consumption effect on different diseases. For example, induction of experimental diabetes in rats is a convenient model to study activity of hypoglycemic agents over hyperglycemia and it consequences where it have been observed the possible antioxidant and antidiabetic effects through plasma glucose, triacylglycerol and cholesterol monitoring, microscopic lesion observation, marker enzyme serum and lipid peroxidation measuring [15, 16].
The therapeutic use of M. oleifera leaves has been evaluated in diabetes because of their possible capacity to decrease blood glucose concentrations after ingestion because they contains polyphenols such as quercetin-3-glycoside, rutin, kaempferol and glycosides [17,18,19]. Decrease in blood sugar because of M. oleifera therapy can be noticed in different tests as: fasting blood glucose, oral glucose tolerance test and post prandial glucose on diabetic rats, in an average decrease of 25% or more [20]. The antidiabetic activity of Moringa seed powder has been observed in rat models with the decreased glucose and the amelioration of levels of lipid peroxide, the diminish levels of IL6, and immunoglobulins A in comparison with diabetic positive control in both insulin resistant and insulin deficient bioassays [21, 22]. Meanwhile Anudeep et al., showed the Moringa oleifera contain soluble fiber that enhance amelioration of levels of glucose, proliferation of lymphocytes and induced nitric oxide from macrophages. In other study observed the fortification of M. oleifera in diabetes can lead to fasting blood glucose dropped, which can help to reduce the entrance of glucose to mitochondria and diminish the release reactive oxygen species and advance glycated end products (AGEs) which can enhance cell adhesion and inflammation in diabetic patient [23]. Treatment with Moringa has showed after histological examination of pancreas from diabetic rats, a significantly damage reversed in the histoarchitectural of the islet cells [24].
Elevation of lipid concentration is part of the physiopathology of diabetes mellitus which increased the risk of premature arterosclerosis. Therefore the use of M. oleifera is clamed to possess hypolipidemic properties. It was found that crude leaf extract along with high-fat diet decrease cholesterol and triacylglycerol in serum [26]. When bioassay was done with Moringa in comparison with lovastatin, it was found that both decreased the lipid profile on liver, heart and aorta [25]. In other study it was observed that consumption of M. oleifera for 15 weeks can decrease total cholesterol, triglycerides, LDL-cholesterol, atherogenicity index in HIV negative patients [26].
However most results have been obtained in studies of leaf extracts; therefore, this study investigates the use of leaf powder, which is the common method of consumption, to investigate the effects on glucose, triglycerides, cholesterol and corporal weight and the predominant groups of microbiota. To expand the knowledge on biological properties of Moringa oleifera powder leaf in this study clinical and toxicological effect on alloxan-induced diabetic rats were assessed, specially to know variability in acute toxicity and microbiota impact using one route of administration the is intended in human.
Methods
Plant material
Moringa oleifera leaves were collected from a private herbarium at the Universidad Autónoma de Sinaloa, Sinaloa, México, with registration code UAS-MO-10, Lot Number 5. The leaves were washed and oven dried at 40 °C for 24 h, until the moisture content reached 10%. The dry leaves were pounded, and the powder was kept at room temperature until use [27].
Lethal dose 50 and genotoxicity
The lethal dose 50 was determined in Balb-C56 male mice by the administration of different doses of leaf powder (mg/kg) according to Lorke (1983) [28], with a modification in the number of animals (n = 5) according to Mann-Whitney U chart to get the two tails critical value with the minimal animal number use. There were four randomly assigned groups (5 mice/group/per cage): Group 1: control group (saline solution); Group 2: administration of 100 mg/kg of Moringa oleifera; Group 3: 200 mg/kg of M. oleifera; and Group 4: 500 mg/kg of M. oleifera.
Animals were under observation for signs and symptoms of toxicity or death from 24 h until 7 days after the administration of M. oleifera to determine the lethal dose. To investigate genotoxicity, a day 7 blood sample was taken from the tip of the tail of each animal, and a peripheral blood smear was performed on the samples. The blood smears were dried at room temperature, fixed with absolute ethanol for 10 min, and stained with acridine orange for analysis. Erythrocytes were micronucleated according to the procedure detailed by Zúñiga-González et al. [29]. All samples were enumerated manually without previous knowledge of group assignment. Observations were performed with a BX51 microscope with epifluorescence using a 100X immersion objective. Cumulative damage was determined by quantifying the frequency of micronucleated erythrocytes (MNEs) over 10,000 total erythrocytes (TEs), composed of polychromatic erythrocytes (PCEs) and normochromatic erythrocytes (NCEs). To determined recent damage, mononucleated polychromatic erythrocytes (MNPCEs) over 1000 PCEs were counted. The proportion of PCEs/1000 TEs was quantified to determine the cytotoxic potential of the diet [30].
Induction of hyperglycemia
Hyperglycemia was induced by applying 150 mg/kg alloxan monohydrate (A7413, Sigma-Aldrich®, St. Louis, MO, USA). After 72 h, a blood test confirmed hyperglycemia (> 200 mg/dL) [31], after which M. oleifera was orally administered.
Experimental design
In the present study, thirty healthy male Sprague Dawley rats with an average body weight of 180–200 g were used in a special cage with water ad libitum and 12 h light/dark cycle in a room temperature controlled at 25 °C. The animals were distributed into 5 groups (n = 6), which included one control group, one healthy group treated with M. oleifera, one untreated diabetic group, one diabetic group treated with M. oleifera, and one diabetic group treated with glibenclamide (Sigma-Aldrich®, St. Louis, MO, USA). The administered dose of Moringa oleifera was 50 mg/day, and the glibenclamide dose was 600 μ/kg/day [32]. Both were administered orally for 8 weeks using a cannula needle (VWR, 20068–642, West Chester, PA, USA) at the laboratory. Animals were wuthanized by exsanguination after isoflurane anesthesia.
Clinical parameters
Body weight (g) and glucose were measured weekly using a triple-arm scale (730-SW, Ohaus, Pine Brook, NJ, USA) with a basket to hold the animals and an Accu-Check Active® (Roche Diagnostics®, Indianapolis, IN, USA; range 10–600 mg/dL), respectively. At the end of the experimental period, a 12-h fast was imposed on the animals, after which they were sacrificed. Triglycerides (11503), cholesterol (11505), LDL (11585) and HDL (11557) were measured with commercial kits (BioSystems® S.A, Barcelona).
Histopathology
At the end of the biological assay, different organs, including the intestine, liver, and kidney, were extracted and placed in a sterile container. Organs were placed in 10% neutral buffered formalin (HT501128, Sigma-Aldrich®, St. Louis, MO, USA), sectioned for histological analysis, stained with hematoxylin-eosin, and examined using light microscopy. Diagnosis of abnormalities was based on histological examination of the biopsies. For this purpose, the animals were sacrificed using CO2.
Lactic acid bacteria (LAB) and Enterobacteriaceae
Lactic acid bacteria and Enterobacteriaceae were enumerated in 1 g stool samples. Briefly, 9 ml of 0.85% sodium chloride (Promega H5271, Madison, WI, USA) was added to the sample and homogenized. Three additional decimal dilutions (1:10, 1:100, and 1:1000, v/v) were performed. For LAB enumeration, a 0.1 ml sample was taken from each dilution, placed on an agar plate containing MRS medium (Man, Rogose and Sharpe, BD Difco Laboratories®, Sparks Maryland, MD, USA), and cultured using the spread plate method with an incubation at 35°C for 48 ± 2 h in a candle jar, under an approximately 15% oxygen atmosphere [33]. Three replicate plates were prepared for each dilution. Colonies were counted after incubation. Gram-positive colonies, which were catalase negative and oxidase positive, were isolated, purified, subcultured on MRS agar and treated as presumptive LAB. The isolates were characterized and identified using API 50CH and API 50CHL medium (bioMe’rieux®, Marcy-l’E’toile, France) following the manufacturer’s instructions.
The Enterobacteriaceae were enumerated in the samples from this solution and placed on five different media: eosin methylene blue agar (EMB), MacConkey, brilliant green agar (BG), Salmonella–Shigella agar (SS), and Hektoen enteric agar. After incubation at 35 °C for 24 h, representative colonies were isolated, purified, and subcultured on selective agar. They were then subjected to Gram staining.
Statistical analysis
Differences between treatments were identified with an analysis of variance (ANOVA; SPSS-PC, version 20.0). In cases where the standard deviation differed between groups, Mann-Whitney U and Kruskal Wallis multiple comparison nonparametric tests were applied with a 95% (p < 0.05) confidence level.
Ethical considerations
All procedures performed in studies involving animal models were in accordance with the ethical standards of the institutional ethics committee of Centro Universitario de Tonalá and the Mexican Official Standard NOM-062-ZOO-1999, which were approved by the same committee.
Results
Toxicity
The tested doses of M. oleifera revealed no adverse effects in the experimental animals, including possible alterations such as food intake, unusual body growth, reduced activity, diarrhea, bleeding, or death. Therefore, no lethal dose was determined. As for genotoxicity, it was observed that the control group showed no significant difference in any genotoxicity parameter after intra and inter group comparisons of MNEs, MNPCEs and PCEs, as expected (Fig. 1). M. oleifera treatment groups did not show differences (p > 0.05) between the various doses (100, 250 and 500 mg/kg) in MNEs. A slight rise was seen in MNEs compared to the control group, but the difference was not significant (p > 0.05). Intra and inter group comparisons of MNPCEs detected no significant (p > 0.05) difference between doses of M. oleifera or between treatment groups and the control group. PCE values, similar to previous groups, showed no statistically significant difference (p > 0.05) between intra and inter group doses or between treatment groups and the control group (Table 1).
Data are expressed as the mean ± standard deviation per group. Moringa solution was administered interrogatorily to rats. MNE: micronucleated erythrocytes/10000 TE; MNPCE: micronucleated polychromatic erythrocytes/1000 PCE; PCE: polychromatic erythrocytes/1000 TE; TE: total erythrocytes; n: simple size, 5 rats per group; NS: not significant. *: intra-group significance (p > 0.05) (repeated measures ANOVA and LSD test post hoc for multiple comparisons); **: inter-group significance (p > 0.05) (one-way ANOVA and Dunnett t-test post hoc for multiple comparisons).
Lethal dose
The doses of M. oleifera powder leaf tested reveled no adverse effects in experimental animals, including possible alterations such as food intake, unusual body growth, reduced activity, diarrhea, bleeding or death.
Histopathology
Intestinal tissue sections (38 × 0.6 × 0.6 cm) showed the control group and the healthy group treated with M. oleifera to have intestines lined by intestinal cylindrical epithelium forming glands, lamina propria with a transmucosal lymphoplasmacytic inflammatory infiltrate, and regular lymphocytes with no histopathological indicator of metaplasia, dysplasia, or malignancy.
All diabetic groups were shown to have an epithelium with severe atrophy covering large areas, flattened villi, inflammation, and a lamina propria with lymphoplasmacytic inflammatory infiltrate negative for malignancy. One untreated diabetic rat showed necrosis (Fig. 2).
Clinical parameters
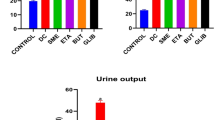
The initial body weight for all groups was ±200 g. After alloxan induction, diabetic groups decreased in weight. During the 8 weeks, the body weight of the control group was lower than that of the healthy group treated with M. oleifera (246 g vs 263 g, respectively), but there was no significant difference. The diabetic group treated with M. oleifera showed an increased body weight in comparison with both the diabetic group treated with glybenclamide and the untreated diabetic group (229 g, 190 g, and 173 g, respectively) (Fig. 3). The diabetic group treated with M. oleifera was different than the untreated diabetic group (p < 0.05).
Glucose levels of 100 mg/dL were measured in the experimental animals at the beginning of the study (p > 0.05). After hyperglycemia induction, the diabetic groups showed glucose values of ±300 mg/dL (p > 0.05). In the second week, glucose levels in the diabetic group treated with M. oleifera diminished in comparison to the untreated diabetic group. On the other hand, the control group and the healthy group treated with M. oleifera did not show differences over this time period (p > 0.05). Triacylglycerol values were not different between diabetic groups (Fig. 4). The healthy group treated with M. oleifera showed lower values (24 mg/dL) in comparison to the control group (53 mg/dL).
Lactic acid bacteria (LAB) and Enterobacteriaceae
There were not significant differences between study groups in LAB enumeration (8.4 UFC/g). The predominant genus identified in the control group, the healthy group treated with M. oleifera, and the diabetic group treated with M. oleifera was Lactobacillus fermentum. On the other hand, the untreated diabetic group and the diabetic group treated with glyburide revealed the presence of Lactobacillus acidophilus and Leuconostoc lactis. Enterobacteria enumeration produced lower counts in the healthy group treated with Moringa oleifera in comparison to the other diabetic groups (p < 0.05).
Discussion
Moringa oleifera possesses bioactive compounds, the qualities of which have been studied in recent years to establish a more scientific basis for its use and to elucidate its biological activity. The leaves have been used as antidiabetic, antibacterial, and anti-inflammatory herbal drugs [21, 34,35,36,37,38]. There are studies that show no risk in using M. oleifera leaves at various doses; however, most of these data have been derived from studies on plant extract. Therefore, the study of leaf powder consumption brings new knowledge about the safety of this plant while providing options for plant preservation without the loss of nutrients, especially since the leaf of this plant can be used as a vegetable in soup preparations, cooked and mixed in with ground peanut cake or packaged in powder pills [21, 36,37,38].
Furthermore, M. oleifera is a source of antioxidants, vitamins, and a protease-resistant glycoprotein that functions as dietary fiber [39,40,41]. Moringa oleifera has antioxidant activity because it contains phenolic compounds and flavonoids, specifically three classes of phytochemicals: glucomoringin, flavonoids (quercetin and kaempferol) and phenolic acids (chlorogenic acid) [41]. These bioactive compounds can exert antioxidant and anti-inflammatory effects that could induce cellular protection [42, 43], as can be observed in M. oleifera regulation of the formation of micronuclei in response to damage to the genetic material in cells. Micronuclei are chromosomal fragments or entire chromosomes that were not included into the daughter cell nuclei at mitosis. The erythrocyte micronucleus assay is a simple and minimally invasive method that detects in vivo structural or numerical chromosome damage [5]. The results of this study show that M. oleifera regulates the formation of micronuclei, maintaining the basal values through an antioxidant effect that neutralizes free radicals that would otherwise affect DNA. Moreover, M. oleifera did not increase MNE frequency and maintains the ratio of PCEs and MNPCEs. In fact, these values were the same as the values in the control group. The doses tested in this study did not reach the LD50, and there was no observed histopathological damage in different organs. Previous studies have shown no toxicity or adverse effects in body organs for aqueous leaf extract in rats [36, 44]. However, Assare et al., (2012) [45] observed that supra-supplementation with 3000 mg/kg of aqueous leaf extract reduced albumin serum levels and total protein. Differences in results for aqueous extracts could stem from differences in extraction and purification processes or source location of the plant. Therefore, these new findings based on leaf powder, as well as previous results for leaf extract, could help to provide an enhanced understanding of the acute and chronic toxicity of Moringa oleifera.
Hypoglycemic activity in the bioassay could be a result of phytochemicals, which are bioactive substances with antioxidant activity that have been associated with a protective effect against chronic degenerative diseases. These hypoglycemic effects have been tested with doses of seed powder (50–100 mg/kg body weight) in diabetes mellitus, where in decreases in fasting blood sugar and serum hemoglobin A1c compared with positive controls have been observed [46]. Previous studies suggest that kaempferol stimulates glucose uptake in the rat soleus muscle via the PI3K and PKC pathways. Orally administered kaempferol significantly decreased fasting blood glucose and serum HbA1c levels while improving insulin resistance. Quercetin inhibits the transport of fructose and glucose by GLUT2 in the brain and stimulates GLUT4 translocation and expression in skeletal muscle [27, 47]. This could explain the tendency towards lower blood glucose levels in the diabetes group treated with Moringa oleifera compared to the positive control group in the present study. Furthermore, the effect of leaf consumption on triglyceride levels showed a tendency to decrease in healthy animals, which is similar to other studies in which M. oleifera has been shown to have hypolipidemic potential [28].
Diabetic M. oleifera treated rats increased in body weight. This result is consist with previous studies. Olayaki et al., (2015) observed that oral administration of extract of M. oleifera significantly reduces blood glucose concentration and inhibits weight loss in alloxan-induced diabetic rats. Another previous study showed a significant increase in the body weight of rats treated with 50 mg and 100 mg of seed powder [46]. The increase in weight may be due to the content of seed powder, specifically essential amino acids and vitamins A, B, C and E. In addition, antioxidants and antimicrobial compounds (phenols, tannins, alkaloids and cumarins) can act as growth promoters [48].
Consumption of M. oleifera leaves did not show differences in lactic acid bacteria enumeration, although there was an effect on enterobacteria enumeration in the healthy group treated with M. oleifera. It has been reported that M. oleifera may have antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Aspergillus niger, and Candida albicans. Additionally, less antimicrobial activity has been observed against Gram-positive than Gram-negative microorganisms [17]. M. oleifera leaves contain biocomponents whose antibacterial potentials are comparable to that of the antibiotic oxytetracycline against gram-negative organisms, which may be the result of the presence of natural antioxidants (ascorbic acid, flavonoids, phenolics and carotenoids, and even because off the presence of saponin, tannic, phenolic and alkaloid phyto constituents) [48]. Some of these compounds can lead to cell membrane perturbations and exert a β-lactam action on the transpeptidation of the cell wall. Leaf extract contains small peptides that can increase the permeability of cell membranes or cell walls. Some compounds may interact with the lipid bilayers in cell membranes, leading to the separation of the two membranes, thus leading to cellular swelling and cell death [49].
Leaf administration needs more scientific basis. Most studies of therapeutic use of M. oleifera are with aqueous, hydroalcohol or alcohol leaf extract against hyperlipidemia and hyperglycemia. Nerveless studies of the use of leaf powder are limited. There are reports of high-fat diet containing Moringa leaf powder to diminish lipid profile in dyslipidemic rats [26], over glucose tolerance in Wistar rats and Goto-Kakizaki rats modeled type 2 diabetes [48], as antioxidant or supplement as nutrition counseling [49]. However diabetes mellitus is a disease which leads to raised level of glucose, triglycerides, corporal weight and a gut microbiota disbiosis, therefore this study would bring information about relationships between leaf powder M. oleifera consumption in different doses with gut microbiota changes and clinical parameters.
Conclusion
The findings suggest that consumption of M. oleifera powder leaves could be beneficial in the diabetes mellitus rat model over glucose values and enterobacterias enumeration. However further research will be needed to evaluate the mechanisms of action over lipids and intestinal microbiota in diabetes mellitus to increase possible uses of M. oleifera in functional foods as a nutraceutical.
Therefore, the study about leaf powder consumption can bring new knowledge about it safety and as an option of plant preservation without loss the nutrient, especially when leaf of this plant can be use as vegetable in soup preparation, cooked and mixed with grounded groundnut cake or in powder pills.
References
Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, Bagyánszki M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192–8.
Olausson EA, Alpsten M, Larsson A, Mattsson H, Andersson H, Attvall S. Small particle size of a solid meal increases gastric emptying and late postprandial glycemic response in diabetic subject with gastroparesis. Diabetes Res Clin Pract. 2008;8:231–7.
Misrha G, Singh P, Verma R, Kumar S, Srivastav S, Jha KK, Khosa RL. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: an overview. Der Pharm Lett. 2011;3(2):141–64.
Olson ME, Fahey JW. Moringa oleiferea: a multipurpose tree for the dry tropics. Rev Mex Biodivers. 2001;82:1071–82.
Asare GA, Gyan B, Bugyei K, Adjei S, Mahama R, Addo P, Otu-Nyarko L, Wiredu EK, Nyarko A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J Ethnopharmacol. 2012;139:265–72.
Gasi S, Nwodobo E, Ofili JO. Hypocholesterolemic effect of crude extract of leaf of Moringa oleifera lam in high-fat diet fed wistar rats. J Ethnopharmacol. 2000;69:21–5.
Robinson S, Delongeas JL, Donald E, Dreher D, Festag M, Kervyn S, Lampo A, Nahas K, Nogues V, Ockert D, Quinn K, Old S, Pickersgill N, Somers K, Stark C, Stei P, Waterson L, Chapman K. A european pharmaceutical company initiative challenging the regulatory requirement for acute toxicity studies in pharmaceutical drug development. Regul Toxicol Pharmacol. 2008;50(3):345–52.
Nikkon F, Hasan S, Salam KA, Mosaddik MA, Khondkar P, Haque ME, Rahman M. Benzylcarbamothioethionate from root bark of Moringa oleifera lam. And its toxicological evaluation. BLACPMA. 2009;8(2):130–8.
Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015;29:796–804.
Yassa HD, Tohamy AD. Extract of Moringa oleifera leaves ameliorates streptoxotocin-induced diabetes mellitus in adult rats. Acta Histochem. 2014;116:844–54.
Etuk EU. Animals models for studying diabetes mellitus. Agricul Biol J NA. 2010;1(2):130–4.
Rodríguez-Nunez I, Caluag T, Kirby K, Rudick CN, Dziarski R, Gupta D. Nod2 and Nod2-regulated microbiota protect BALB/c mice from diet-induced obesity and metabolic dysfunction. Sci Rep 2017;7(1),548. 10.1038/s41598-017-00484-2.
O'Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. IRLAR J. 2014;54(3):259–72.
Lebel M, de Souza-Pinto N, Bohr VA. Metabolism, genomics, and DNA repair in the mouse aging liver. Curr Gerontol Geriatr Res. 2011; https://doi.org/10.1155/2011/859415.
Farroq F, Rai M, Tiwari A, Khan AA, Farooq S. Medicinal properties of Moringa oleifera: an overview of promising healer. J Med Plant Res. 2012;6(27):4368–74.
Anwar J, Latif S, Ashraff M, Gilani AH. Moringa oleifera: a food plan with multiple medicinal uses. Phytother Res. 2007;21:17–25.
Arora DS, Onsare JG, Kaur H. Bioprospecting of Moringa (Moringaceae): microbiological perspective. J Pharmacogn Phytochemistry. 2013;1(6):193–215.
Jaiswal D, Rai PS, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera lam. Leaves aqueous extract therapy on hyperglycemic rats. J Etnopharmacol. 2009;123:392–6.
Al- Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. Seeds on streptozotocin induced diabetes and diabetic nephropaty in male rats. BioMed Res Int. 2015. https://doi.org/10.1155/2015/381040.
Divi SM, Bellamkonda R, Dasireddy SK. Evaluation of antidiabetic and antihyperlipedemic potential of aqueous extract of Moringa oleifera in fructose feed insulin resistant and STZ induced diabetic wistar rats: a comparative study. Asian J Pharm Clin Res. 2012;5(1):67–72.
Anudeep S, Prasanna VK, Adya SM, CH R. Characterization of soluble dietary fiber from Moringa oleifera seeds and its immunomodulatory effects. Int J Biol Macromol. 2016;91:656–62.
Gopalakrisnan L, Doriya K, Kumar DS. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sc Human Welln. 2016;5:49–56.
Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4:164–71.
Helmy SA, Morsy NFS, Elaby SM, Ghaly MAA. Hypolipidemi effect of Moringa oleifera LAM leaf powder and its extract in diet induced hypercholesterolemic rats. J Med Food. 2017;20(8):755–62.
Mehta LK, Balaraman R, Amin AH, Bafna PA, Gulati OD. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol. 2003;86:191–5.
Tété-Bénissan A, Quashie MLA, Lawson-Evi K, Gnandi K, Kokou K, Gbéassor M. Influence of Moringa oleifera levaes on atherogenic lipids and glycaemia evolution in HIV-infected and uninfected malnourished patients. J Appl Biosci. 2013;62:4610–9.
El-Moez SIA, El-Badawi AY, Omer HAA. Assessment of antimicrobial effect of moringa: in vitro and in vivo evaluation. Afr J Microbiol Res. 2014;8(42):3630–8.
Chinedu E, Arome D, Ameh FS. A new method for determining acute toxicity in animal models. Toxicol Internat. 2013;20(3):224–6.
Zúñiga-González G, Gómez-Meda BC, Zamora-Pérez A, Ramos-Ibarra ML, Batista-González CM, Espinoza-Jiménez S, Gallegos-Arreola MP, Álvarez- Moya C, Torres-Bugarín O. Induction of micronuclei in proestrus vaginal cells from colchicine- and cyclophosphamide-treated rats. Environ Mol Mutagen. 2003;42(4):306–10.
Zalacain M, Sierrasesúmaga L, Patiño A. The cytogenetic assay as a measure o genetic instability induced by genotoxic agents. An Sist Sanit Nav. 2005;28(2):227–37.
Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetol. 2008;51:216–26.
Saravan G, Pari L. Effect of an herbal drug, cogent db on plasma and tissue glycoproteins in alloxan-induced diabetic rats. Res J Med Plant. 2007;1:83–91.
Rengpipat S, Rueangruklkhit T, Piyatiratitivorakul S. Evaluations of lactic acid bacteria as protiotics for juvenile seabass Lates calcarifer. Aquacul Res. 2008;39:134–43.
Akinlolu AA, Ghazali OK, Ameen OM, Oyebanji SC, Omotoso GO, Enaibe BU, et al. Moringa oleifera impairs the morphology and functions of the Kidney in adult Wistar rats. Intl J Morphol. 2014;32(2):469–74.
Ambi AA, Abdurahman EM, Katsayal UA, Sule MI, Pateh UU, Ibrahim ND. Toxicity evaluation of Moringa oleifera leaves. Int J Pharm Res Inn. 2011;4:22–4.
Asomugha AL, Ezejindu DN, Asomugha RN, Anyabolu AE, Ojukwu PC. Evaluation of toxicity effect of graded doses of Moringa oleifera leaf extract on blood indices using 20 adult Wistar rats. Int J Biom Adv Res. 2015;6(2):98–102.
Awodele O, Oreagba IA, Odoma S, da Silva JA, Osunkalu VO. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J Ethnopharmacol. 2012;139(2):330–6.
Sidney JS, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015;29:796–804.
Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crop Prod. 2013;44:566–71.
Mohsen M, Gholamreza A, Diana T, Mozhgan S, Salar N. Anti-inflammatory effect of Moringa oleifera lam. Seeds on acetic acid-induced acute colitis in rats. Av J Phytomed. 2014;4(2):127–36.
Georgewill OA, Georgewill UO, Nwankwoala RNP. Anti-inflammatory effects of Moringa oleifera lam extract in rats. Asian Pac J Trop Med. 2010;3(2):133–5.
Alcántar-Díaz BE, Gómez-Meda BC, Zúñiga-González GM, Zamora-Perez AL, González-Cuevas J, Alvarez-Rodríguez BA, Sánchez-Parada MG, García-Bañuelos JJ, Armendáriz-Borunda J. Genotoxic evaluation of pirfenidone using erythrocyte rodent micronucleus assay. Food Chem Toxicol. 2012;50(8):2760–5.
Olayemi AT, Olanrewaju MJ, Oloruntoba AC. Toxicological evaluation of Moringa oleifera lam seeds and leaves in Wistar rats. Pharmacogn Comm. 2016;6(2):100–11.
Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. Seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res Int. 2015:1–13. https://doi.org/10.1155/2015/381040.
Ramachandran V, Baojun X. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab(Lond). 2015;12(60)
Olayaki LA, Irekpita JE, Yakubu MT, Ojo OO. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J Basic Clin Physiol Pharmacol. 2015;26(6):585–93.
Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera lam. Bioresour Technol. 2007;98(1):232–6.
Ndong M, Uehara M, Katsumate S, Suzuki K. Effects of oral administration of Moringa oleifera lam on glucose tolerance in Goto-Kakizaki and Wistar rats. J Clin Biochem Nutr. 2007;40:229–33.
Tshingani K, Donnen P, Mukumbi H, Duez P, Dramaix-Wilmet M. Impact of Moringa oleifera lam. Leaf powder supplementation versus nutritional counseling on the body mass index and immune response of HIV patients on antiretroviral therapy: a single-blind randomized control trial. BMC Complem Altern Med. 2017;17:2–13.
Acknowledgements
The authors thank Rubén Piña Cruz (Nutrition student) for technical support and Universidad Autónoma de Sinaloa.
Funding
This study was supported by the Professional Teacher Development Program, Secretary of Public Education, México (Project number UDG-PTC-1165).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
VLA and TVMR performed microbiological experiments, analyzed data; LMD conducted toxicity experiments and wrote the manuscript; PMAG performed and analyzed the histopathological experiments; VPO contributed to study design, biological assay, and statistical analysis; GQLA cultivated, identified and analyzed Moringa oleifera composition, NK contributed to study design, conducted the animal experiments, analyzed results and manuscript writing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving animal models were in accordance with the ethical standards of the institutional ethics committee of Centro Universitario de Tonalá and the Mexican Official Standard NOM-062-ZOO-1999, which were approved by the same committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Villarruel-López, A., López-de la Mora, D.A., Vázquez-Paulino, O.D. et al. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement Altern Med 18, 127 (2018). https://doi.org/10.1186/s12906-018-2180-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-018-2180-2