Abstract
Background
A variety of cast options are available for the non-surgical treatment of distal radius fractures (DRF) in adults. However, the literature is inconclusive regarding the need to immobilize the elbow joint after reduction in order to prevent rotation of the forearm in order to maintain the reduction of DRF. This study aimed to evaluate the best method of immobilization between above-elbow (AE) and below-elbow (BE) cast groups at the end of six-month follow-up.
Methods
This is a randomized clinical trial with parallel groups and a blinded evaluator. There are two non-surgical interventions: AE and BE. Patients will be randomly assigned. A hundred twenty eight consecutive adult patients with acute (up to 7 days) displaced DRF of type A2, A3, C1, C2 or C3 by the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification will be included. The primary outcome will be the maintenance of reduction by evaluation of radiographic parameters and Disabilities of the Arm, Shoulder and Hand Questionnaire (DASH). Secondary outcomes include function measured by Patient Rated Wrist Evaluation (PRWE), pain measured by the Visual Analogue Scale (VAS), objective functional evaluation (goniometry and dynamometry) and rate of complications. Evaluations will be performed at 1, 2, 3, 4, 6, 8, 12 and 24 weeks. For the Student’s t-test, a difference of 10 points in DASH score, with 95% confidence interval, a statistical power of 95%, and 20% sampling error. We consider an extra 10% for balancing follow up losses results in 64 patients per group.
Discussion
Results from this study protocol will help to define the need for elbow immobilization in maintenance of reduction, as well as functional performance of below elbow cast versus above elbow cast immobilization during the immobilization period.
Trial registration
NCT03126175 (http://clinicaltrials.gov). April 24, 2017.
Similar content being viewed by others
Background
Although distal radius fractures (DRF) are among the most frequent of the upper limb [1], the best method of treatment and outcome of these fractures has not yet been fully defined [2, 3]. Regarding non-surgical treatment, Cochrane review based on randomized controlled trials has concluded there are controversial in terms of the type of casting to be applied after the initial fracture reduction and there is no conclusive evidence of difference in outcome between different positions and methods of plaster and brace management for the common types of DRF [4,5,6].
Below-elbow (BE) splinting is easier to apply, is lower in cost, lighter, provides greater comfort, better function for daily life activities and less articular stiffness of the elbow [7,8,9]. Casts that include the elbow joint, which prevents the rotation of the forearm, may result in greater stability of the fracture and less risk of loss of reduction and need for re-reduction [10,11,12]. Other studies found similar results between immobilization methods in maintaining the initial fracture reduction [13, 14].
This study is based on the hypothesis that above-elbow (AE) splint immobilization in patients with DRF will present better results for loss of reduction and radiographic parameters, but more complication rate and worse functional outcomes when compared to below-elbow (BE) immobilization methods at the end of a six-month follow-up.
Methods/design
Aim
To determine the best method of immobilization in patients with distal radius fractures at the end of a six-months: below-elbow versus above-elbow cast.
Design and setting
Randomized controlled trial developed at Federal University of São Paulo - UNIFESP and Hospital Municipal Dr. Fernando Mauro Pires da Rocha - SP.
Participant characteristics
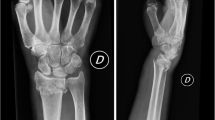
Adults with growth plate closure, both genders, with unilateral and closed acute displaced DRF (up to 1 week), associated or not with the ulnar styloid fractures with no other fractures, which may be closed reduced and meet inclusion criteria (Fig. 1).
Inclusion criteria
Displaced and reducible fractures classified by AO as type A2, A3, C1, C2 and C3 will be included if one of these conditions is present.
The contralateral side is used as a reference.
Exclusion criteria
Patients presenting one or more of the following criteria will be excluded from this study:
-
Open fractures, bilateral fracture or associated with tendon or neurovascular lesions.
-
Associated carpal fractures.
-
Marginal fractures or fractures from shearing mechanism.
-
Fractures with palmar deviation (Smith’s fracture).
-
Irreducible fractures (closed method).
-
Prior history of a degenerative or traumatic disorder of the affected or contralateral wrist joint.
-
Systemic diseases or traumatic lesions associated with fracture that restrict the application of methods or the evaluation of results.
-
Cognitive deficit that does not allow the patient to understand the elements of the functional evaluation.
-
Consent Form Refusal.
Radiological measurements
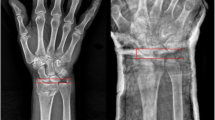
The volar tilt, the radial inclination, the radial height, the ulnar variance and the intra-articular step off or gap were determined on posteroanterior (PA) and lateral (L) radiographs views obtained using a standardised procedure [24].
The standard method of obtaining a PA radiograph is with the shoulder in 90° of abduction, the elbow in 90° of flexion and the wrist in a neutral position. For the lateral view, the shoulder is adducted and the elbow is in 90° of flexion with the hand positioned in the same plane as the humerus [19].
The volar tilt, also called palmar tilt is measured on the lateral view and refers to the distance between a line through the dorsal and palmar boundary points of the radial joint surface and the perpendicular to the longitudinal axis of the radial shaft.
The radial inclination, also know as radial deviation is measured on the PA view and refers to the distance between a line through the radial and ulnar boundaries of the radial joint surface and the perpendicular to the longitudinal axis of the radial shaft.
The radial height, also called radial lenght is measured on the PA view and refers to the difference in axial direction of the radius between the distal tip of the radial styloid and the most distal aspect of the ulnar articular surface.
The ulnar variance, also called the radioulnar index is measured on the PA view and refers to the vertical distance between a line parallel to the medial corner of the articular surface of the radius and a line parallel to the most distal point of the articular surface of the ulnar head, both of which are perpendicular to the long axis of the radius.
The intra-articular step off or gap is measured on PA or lateral view and refers articular incongruity.
The carpal alignment is measured on lateral view. Two lines are drawn, one along the long axis of the capitate and other along the long axis of the radius. The lines do intersect within the carpus.
Initial treatment
All the patients with a distal radius fracture who arrive at the emergency room will undergo a standard protocol with clinical and radiographic examination (bilateral x-rays of the wrist in PA and lateral views). After applying the inclusion and exclusion criteria, eligible individuals will be informed about the nature and purpose of the study, by reading the “Consent Form” and after signing it they will be included. On a pre-scheduled date (up to 7 days), the study participant will be referred to the main operating room to be anesthezied before closed reduction of the fracture under radioscopy control. The reducibility criteria will be evaluated and patients that have reducible fracture will be randomized and treated by one of the two methods of the study (Fig. 2). Patients that do not have closed reducible fracture will be excluded from the study and will receive surgical treatment (open reduction and internal fixation) on a date to be scheduled.
Anesthesia
Intravenous anesthesia will be performed by aseptic technique. A simple bolus injection with Propofol (infusion rate 180 mcg.kg− 1.min− 1) in combination with opioid (fentanyl 5–10 mcg.kg− 1) adjusted to the individual needs of each patient and repeated as many times as necessary according to the anesthesiologist’s criteria [25, 26].
Method for closed reduction and immobilization
The patient will be submitted to the closed reduction of the fracture through a traction and counter-traction technique. Materials needed for application of the two splinting techniques will be available in the operating room. Initially, all patients will receive a short radial splint that will be performed with a 20 cm wide gypsum cut to fit the thumb (Fig. 2a). The splint will be applied to the radial aspect of the wrist covering the volar and dorsal portion of the radius to the elbow. The splint will be moulded with three point fixation as described by Charnley [27]. The three points will be defined after a metal pointer will be placed beside the limb to identify the site of fracture by using the image intensification. Patients randomized to the above-elbow splint will receive a complementation of immobilization with a 15 cm width splint on the ulnar aspect of the forearm that begins at the middle of the forearm and extends into the armpit. The elbow will be immobilized at 90 degrees, and in a neutral position to block pronosupination (Fig. 2b). Cotton tubular mesh, cotton stripes and crepe bandage will be used in both bindings. Regardless of the immobilization adopted, all wrists will be positioned with slight flexion and ulnar deviation. Patients will be encouraged to actively move their fingers and the ipsilateral shoulder.
Patients with above-elbow immobilization will remain for 4 weeks with the splint followed by 2 weeks of below-elbow immobilization. The immobilization will be removed after 6 weeks.
Clinical outcomes
The self-reported functional evaluation DASH and PRWE, visual analogue pain scale (VAS), radiographic measures, objective functional evaluation will be performed by independent evaluators at intervals provided in Table 1. For the outcomes at 8,12 and 24 weeks the evaluators will be blinded to the patient assignment groups. The minimum clinical follow-up will be 24 weeks, with the following parameters being considered to evaluate the results:
Primary outcomes
Radiographic parameters
Maintenance of reduction by evaluation wrist radiographs in PA and lateral x-rays at the following intervals: one, two, three, four, six, eight, twelve and twenty-four weeks after fracture reduction.
The radial height, radial inclination, volar tilt, ulnar variance, intra-articular step off or gap and carpal alignment will be used to determine maintenance of reduction at every follow-up visit. Measurements will be made on the radiographs with a marker, straight edge, and protractor by two researchers independently on different occasions.
We will consider maintenance of reduction if:
-
loss of reduction ≤2 mm in radial height
-
loss of reduction ≤4 degrees in radial inclination
-
dorsal angulation ≤10o
-
≤ 2 mm intra-articular step off
-
positive ulnar variance ≤3 mm
-
any carpal malalignment.
The contralateral side is used as a reference.
Patient-reported functional outcomes
Functional status will be evaluated by means of DASH questionnaire (validated for the Portuguese language) at the following intervals: two, six, eight, twelve and twenty-four weeks after fracture reduction [28]. The DASH was developed as an instrument for patients with upper-extremity injuries. The survey contains 30 questions related to the function of the hand, wrist, elbow, and shoulder based on the conditions to do certain activities in the past week, so the evaluations refer only after the beginning of the immobilization.
Secondary outcomes
Patient Rated Wrist Evaluation – PRWE; [29] Pain (VAS - Visual Analogue Pain Scale); [30, 31] Objective functional evaluation (goniometry and dynamometry); and rate of complications and failures.
The PRWE score (validated for the Portuguese language) will be obtained at eight, twelve and twenty-four weeks. The PRWE contains 15 items that are specific to determining the degree of musculoskeletal disability related to the wrist [29].
Pain in the wrist, elbow and shoulder will be measured separately in all visits at one, two, three, four, six, eight, twelve and twenty-four weeks after fracture reduction by the Visual Analogue Pain Scale (VAS). This is a unidimensional measure of pain intensity, which has been widely used in diverse adult populations [30]. Pain in VAS is a continuous scale comprised of a horizontal line of 10 cm (100 mm) in length, anchored by two verbal descriptors, one for each symptom extreme by “no pain” (score of 0) and “pain as bad as it could be” or “worst imaginable pain” (score of 100). Participants are asked to report pain intensity in the last 24 h. The respondent is asked to place a line perpendicular to the VAS line at the point that represents their pain intensity. Using a ruler, the score is determined by measuring the distance (mm) on the 100 mm line between the “no pain” anchor and the patient’s mark, providing a range of scores from 0 to 100 [31].
Objective functional evaluation
Arcs of motion will be measurement for the wrist, and a goniometer will be employed to measure wrist flexion, extension, ulnar deviation, radial deviation and pronosupination at the six, eight, twelve and twenty-four week follow up visit. The flexion–extension of the elbow will be measurement at six, eight, twelve and twenty-four week follow-up visit.
Palmar grip strength with a digital dynamometer (Jamar Plus - Hand Dynamometer), at the following moments of treatment evolution: eight, twelve and twenty-four week follow-up visit.
Complications
Any clinical situation requiring treatment (clinical or surgical procedure) not provided in the protocol will be considered as a complication. All complications will be recorded for further stratification into major and minor complications.
In cases where there is loss of reduction, patients will be informed and surgical treatment indicated.
Statistical methods
Descriptive data will be exposed as means or proportions followed by standard deviations or 95% confidence intervals. As a method to confirm the effectiveness of the randomization, baseline data will be compared in the two groups of comparison. To ensure the normal distribution of data, we will use visual analysis and Shapiro-wilk test.
For comparison between proportions, we will consider Pearson’s chi-square test. For continuous data, we will use Student T test. Intra-group comparison (1, 2, 3, 4, 6, 8, 12 and 24 weeks) will be analyzed by paired Student T test or Wilcoxon (if data is not normally distributed). We will consider as significant when alpha < 0,05. To analyze the occurrence of complication after treatment, we intend to perform survival analysis associated with Kaplan-Meier curves, if we find greater than 20% complication in any of the comparison groups. All statistical analysis will be performed following intention to treat principle. Statistical advisors will be blinded to the treatment groups as an effort to decrease bias.
Randomization and masking
Patients will be randomly assigned using randomization software (available at: http://www.randomizer.org). The allocation of patients in the AE or BE groups will be performed using opaque envelopes numbered on their outer face with consecutive numbers (concealment). Additionally, the envelope will be opened only in the operating room after verification of fracture reducibility and the procedure will be delegated to a person who is not directly connected to the study.
Sample size calculation
Based on data derived from one recent randomized clinical trial on the subject [32]. We considered as relevant differences on DASH scores (clinically relevant) when scores are greater than 10 points and standard deviation 15 points [33]. To detect this difference (Student T-test) and statistical power of 95% resulted in a 58 patient sample size per group. We considered an extra 10% for balancing follow up losses. Thus, our inclusion target will be 64 patients per group. We considered the test as bicaudal.
Discussion
This publication presents a randomized clinical trial of the non-operative treatment of DRF. Casts may be applied either “above elbow” or “below elbow”, depending on the particular type of injury and physician preference. Often, the plaster may extend above the elbow to help provide additional stability and neutralize the extensive forces that can be generated by natural movements of the arm and forearm. Above-elbow immobilization is the conservative treatment used by most of the Brazilian orthopedic surgeons (74%) [34].
Short arm immobilization has been used by many orthopedic surgeons around the world, who claimed equally beneficial results [8, 13]. Hence, controversy still persists regarding the length of the immobilization for the treatment of DRF [4, 5].
The value of the study includes all participants will be reduced in the main operating room under general intravenous anesthesia and with the aid of radioscopy which will allow better control of the pain and maximum quality in the reduction. All reductions and immobilizations will be performed by a single researcher, specialist in hand surgery. The follow up during the immobilization period will be weekly, with radiographic documentation, which allows the early identification of the reduction loss. This is the only trial to apply DASH questionnaire at the beginning and end of immobilization period (2 and 6 weeks) to compare the groups. Pain in the wrist, elbow and shoulder will be measured separately in all visits to verify the influence of immobilization on the elbow and shoulder joints. Adults of all ages will be evaluated, it is known that the DRF in the elderly has different behavior and prognosis when compared to the young [35,36,37,38]. Randomization will equalize the distribution homogeneously between the groups, allowing the sample to be faithful to the population.
Our study has several strengths and limitations. Weekly assessments increase the chance of follow-up loss, however a strict control will be adopted. The study presents limitations because the database was constructed based on measurements of X-ray films calculated manually with goniometer and pen, which may imply in unmeasured tolerance limits. To minimize this, the measurements were performed by two senior researchers independently at different times. All patients in this study will be users of the public health system, many of them may have difficulty responding to self-reported questionnaires. A trained assistant will be available in these cases. Another important point to consider is the work compensation in some patients who want secondary gains, which can influence the information collected.
The results from this randomized clinical trial study are expected to be published in december of 2019. We hope that the study results will provide an answer as to which is the best conservative treatment method for DRF.
Abbreviations
- AE:
-
Above-elbow
- AO:
-
Arbeitsgemeinschaft für osteosynthesefragen
- BE:
-
Below-elbow
- DASH:
-
Disabilities of the arm, shoulder and hand
- DRF:
-
Distal radius fractures
- Kg:
-
Kilograms
- L:
-
Lateral
- mcg:
-
Micrograms
- min:
-
minutes
- PA:
-
Posteroanterior
- PRWE:
-
Patient rated wrist evaluation
- UNIFESP:
-
Federal University of São Paulo
- VAS:
-
Visual analogue scale
References
Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908–15.
Cui Z, Pan J, Yu B, Zhang K, Xiong X. Internal versus external fixation for unstable distal radius fractures: an up-to-date meta-analysis. Int Orthop. 2011 Sep;35(9):1333–41.
Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824–35.
Handoll H, Madhok R. Conservative interventions for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2003;2(2):CD000314.
Lichtman DM, Bindra RR, Boyer MI, Putnam MD, Ring D, Slutsky DJ, et al. Treatment of distal radius fracture. J Am Acad Orthop Surg. 2010;18:180–9.
Raittio L, Launonen A, Hevonkorpi T, Luokkala T, Kukkonen J, Reito A, Sumrein B, Laitinen M, Mattila VM. Comparison of volar-flexion, ulnar-deviation and functional position cast immobilization in the non-operative treatment of distal radius fracture in elderly patients: a pragmatic randomized controlled trial study protocol. BMC Musculoskelet Disord. 2017;18:401.
Pool C. Colles’s fracture. A prospective study of treatment. J Bone Joint Surg Br. 1973 Aug;55(3):540–4.
Stewart HD, Innes AR, Burke FD. Functional cast-bracing for Colles’ fractures a comparison between cast-bracing and conventional plaster casts. J Bone Joint Surg Br. 1984 Nov;66(5):749–53.
Webb GR, Galpin RD, Armstrongs DG. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children. J Bone Joint Surg Am. 2006;88:9–17.
Wahlstrom O. Treatment of Colles’ fracture a prospective comparison of three different positions of immobilization. Acta Orthop Scand. 1982;53:225–8.
Sarmiento A, Pratt GW, Berry NC, Sinclair WF. Colles’ fractures. Functional bracing in supination. J Bone Joint Surg Am. 1975;57(3):311–7.
Bunger C, Solund K, Rasmussen P. Early results after Colles’ fracture: functional bracing in supination vs dorsal plaster immobilization. Arch Orthop Trauma Surg. 1984;103:251–6.
Bong MR, Egol KA, Leibman M, Koval KJ. A comparison of immediate postreduction splinting constructs for controlling initial displacement of fractures of the distal radius: a prospective randomized study of long- arm versus short-arm splinting. J Hand Surg Am. 2006;31:766–70.
Grafstein E, Stenstrom R, Christenson J, Innes G, MacCormack R, Jackson C, Stothers K, Goetz T. A prospective randomized controlled trial comparing circumferential casting and splinting in displaced Colles fractures. CJEM. 2010;12(3):192–200.
McQueen M, Caspers J. Colles fracture: does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70:649–51.
Jenkins NH, Mintowt-Czyz WJ. Mal-union and dysfunction in Colles’ fracture. J Hand Surg Br. 1988;13:291–3.
Tumia N, Wardlaw D, Hallett J, Deutman R, Mattsson SA, Sandin B. Aberdeen Colles brace as a treatment for Colles fracture. A multicenter, prospective, randomized, controlled trial. J Bone Joint Surg Br. 2003;85(1):78–82.
Wilcke MK, Abbaszadegan H, Adolphson PY. Patient-perceived outcome after displaced distal radius fractures: a comparison between radiological parameters, objective physical variables, and the DASH score. J Hand Ther. 2007;20:290–8.
Ng CY, McQueen MM. What are the radiological predictors of functional outcome following fractures of the distal radius? J Bone Joint Surg Br. 2011;93:145–50.
Altissimi M, Antenucci R, Fiacca C, Mancini GB. Long-term results of conservative treatment of fractures of the distal radius. Clin Orthop Relat Res. 1986;206:202–10.
Grewal R, MacDermid JC. The risk of adverse outcomes in extra-articular distal radius fractures is increased with malalignment in patients of all ages but mitigated in older patients. J Hand Surg Am. 2007;32:962–70.
Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg Am. 1986;68(5):647–59.
Batra S, Gupta A. The effect of fracture-related factors on the functional out- come at 1 year in distal radius fractures. Injury. 2002;33:499–502.
Mann FA, Wilson AJ, Gilula LA. Radiographic evaluation of the wrist: what does the hand surgeon want to know? Radiology. 1992;184(1):15–24.
Bailey PL, Stanley TH. Intravenous opioid anesthetics in: Miller DM - anesthesia. 4th ed. New York: Churchill Livingstone; 1994. p. 291–387.
Shafer SL. Towards optimal intravenous dosing strategies. Semin Anesth. 1993;12:222–34.
Charnley J. The Cooles’ fracture. In: The John Charnley trust editor(s). The treatment of closed fractures. 4th ed. Cambridge: Colt Book Ltd; 1999. p. 146–70.
Orfale AG, Araújo PM, Ferraz MB, Natour J. Translation into Brazilian Portuguese, cultural adaptation and evaluation of the reliability of the disabilities of the arm, shoulder and hand questionnaire. Braz J Med Biol Res. 2005;38(2):293–302.
Rodrigues EKS, Fonseca MCR, MacDermid JC. Brazilian version of yhe patient rated wrist evaluation (PRWE-Br): cross- cultural adaptation, internal consistency, test- retest reliability, and construct validity. J Hand Ther. 2015;28(1):69–76.
Revill SI, Robinson JO, Rosen M, Hogg MIJ. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976;31(9):1191–8.
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short Form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 2011;63:S240–52.
Park MJ, Kim JP, Lee HI, Lim TK, Jung HS, Lee JS. Is a short arm cast appropriate for a stable distal radius fractures in patients older than 55 years? A randomized prospective multicentre study. J Hand Surg Eur. 2017;42(5):487–92.
Gummersson C, Atroshi I, Ekdahl C. The disabilities of arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
Belloti JC, Santos JB, Atallah AN, Albertoni WM, Faloppa F. Fractures of the distal radius (Colles’ fracture). Sao Paulo Med J. 2007;125(3):132–8.
Arora R, Gabl M, Gschwentner M, Deml C, Krappinger D, Lutz M. A comparative study of clinical and radiologic outcomes of unstable colles type distal radius fractures in patients older than 70 years: nonoperative treatment versus volar locking plating. J Orthop Trauma. 2009;23:237–42.
Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with non-operative treatment. J Bone Joint Surg Am. 2010;92:1851–7.
Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur. 2010;35:202–8.
Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93:2146–53.
Acknowledgements
This study was conducted in the Hand, Arm and Shoulder Surgery Unit (Head: Prof. Dr. Carlos Henrique Fernandes) of the Universidade Federal de São Paulo – UNIFESP/EPM, with substantial contribution from Hospital Dr. Fernando Mauro Pires da Rocha (Head of orthopaedic unit: Dr. Jonas Aparecido Borracini).
Funding
No external funding.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
AO and JCB designed the project, reviewed the literature and drafted the text. GMM assisted with manuscript writing, editing and helped to revise the manuscript. JR conceptualized the study methods. VYM performed the sample size calculations and defined the types of statistical analyzes. FF gave their expertise in the field of musculoskeletal disorders and critically revised the manuscript for important intellectual content. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Regional Ethics Committee of UNIFESP and Hospital Municipal Dr. Fernando Mauro Pires da Rocha have approved the trial and additional papers, including consent form and patient information sheet. Approval number: CAAE 57857216.8.0000.5505 and 57857216.8.3001.5452.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Okamura, A., de Mendonça, G.M., Raduan Neto, J. et al. Above-versus below-elbow casting for conservative treatment of distal radius fractures: a randomized controlled trial and study protocol. BMC Musculoskelet Disord 19, 92 (2018). https://doi.org/10.1186/s12891-018-2007-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-018-2007-9