Abstract
Background
Fluid in the subcutaneous fat is a common finding anterior to the knee on MRI. This may be caused by chronic low-grade shearing injuries in patients who are overweight. The purpose of this study was to determine if there is a difference in the amount of subcutaneous fat around the knee between patients with these appearances and controls.
Methods
This was a retrospective case-control study. Following a sample size calculation on pilot data, eighteen sequential patients demonstrating hyper-intense subcutaneous signal changes around the knee on fat-saturated T2-weighted MRI were identified from PACS (18 females, mean age 45, range 31–62). Age and gender-matched patients without abnormal T2 MR signal changes were selected. Two observers independently drew regions of interest representing cross-sectional areas of bone and fat. The location of T2 signal hyper-intense lesions was characterized by consensus.
Results
Inter and intra-rater intraclass reproducibility was “excellent” (ICC > 0.8). The mean cross-sectional area of bone for patients with T2 hyper-intense lesions was 31.79cm2 (SD 2.57) and for controls 30.11cm2 (SD 3.20) which was not significantly different (p = 0.09). The median cross-sectional area of fat for the study group was 62.29cm2 (IQR 57.1–66.5) and for controls was 32.77cm2 (IQR 24.8–32.3) which was significantly different (p < 0.0001). Consensus agreement demonstrated all T2 hyper-intense lesions were anterior to the knee extensor mechanism.
Conclusion
Subcutaneous fluid around the knee is associated with an increased amount of subcutaneous fat, anterior to the knee extensor mechanism. This may be caused by shearing injuries in fat with reduced elasticity associated with metabolic syndrome.
Similar content being viewed by others
Background
It is well established that obesity is an important risk factor for structural alterations in the knee joint. Numerous studies have demonstrated the role obesity plays in the development of osteoarthritis [1–3], cartilage defects [4–7], bone marrow lesions [6–8], pes anserine syndromes [9], and patello-femoral osteoarthritis [10]. These changes are largely attributed to a combination of increased load and altered joint biomechanics [11].
Increased body weight in obesity leads to changes in both joint loading and joint biomechanics during normal day-to-day activities. Obese individuals walk more slowly with shorter and broader strides, have a longer stance duration and a greater toe-out angle when compared with normal weight individuals [12–14]. Obesity also adversely affects the biomechanics of fat surrounding joints. Healthy adipose expansion relies on the presence of a well-coordinated process including the presence of endothelial precursor cells and pre-adipocytes. However, adipose is poorly oxygenated in the obese state [15–17] and hypoxia has been shown to stimulate the propagation of pro-inflammatory adipokynes [18–22], resulting in adipose tissue fibrosis [23].
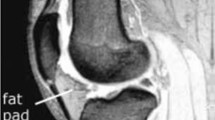
An anecdotal observation on magnetic resonance imaging (MRI) is that in some patients with a large amount of subcutaneous fat around the knee, there are sheet-like collections of fluid in the subcutaneous fat particularly over the extensor mechanism (Fig. 1). It may be that the fibrosis of fat associated with chronic obesity predisposes to this. Repeated low level shear forces through relatively stiff fat might then result in chronic interstitial fluid collections.
Axial T2-weighted fat-saturated image at the most posterior aspect of the femoral condyles (a), and midline sagittal PD-weighted fat-saturated image (b) from an MRI examination of the knee of a 46 year old female. Arrows demonstrate abnormal subcutaneous T2 hyper-intense signal anterior to the knee extensor mechanism
The primary aim of this study was to test for an association between subcutaneous fluid signal changes and the amount of subcutaneous fat around the knee. The secondary aim was to describe the distribution of these fluid signal changes.
Methods
Ethical approval was obtained following proportionate review by the NHS National Research Ethics Service (MICRON reference 15/NW/0486). Informed consent was deemed unnecessary for this retrospective case-control study.
Sample
Mean subcutaneous fat measurements were obtained from a pilot sample of ten patients and used to perform a two tailed sample size calculation for expected mean differences and standard deviations of 0.2, a level of significance of 0.05 and a power of 80%, giving a required sample size of at least 16 patients in each group [24].
A total of 36 MRI examinations were included in the study. Forty-seven examinations exhibiting T2 hyper-intense lesions in the subcutaneous fat around the knee on fat-saturated sequences were identified on our institution’s PACS (examinations between 16 October 2014 and 31 May 2015). Twenty nine were excluded as age and sex-matched controls could not be identified (all female, mean age 53, range 20–66) resulting in eighteen patients included in the study group.
Patients were included if they had undergone an MR examination for chronic knee pain. Patients with a history of acute meniscal, tendon or ligamentous injury or prior knee surgery were excluded, as were those with degenerative or inflammatory arthropathy.
Eighteen age and sex-matched controls without T2 hyper-intense lesions were subsequently identified. The inclusion and exclusion criteria for the control group were the same with the exception that the MR examination had to demonstrate normal fat signal on fat-saturated sequences. The study and control cohorts were selected in a sequential manner until the calculated sample size was exceeded. All cases were independently reviewed by two senior musculoskeletal radiologists (12 and 15 years’ experience) and only included if both agreed that the examinations met the inclusion criteria for each of the cohorts.
MR imaging acquisition
All patients were imaged on a GE HDX, GE 750w or Siemens Avanto 1.5T MRI machine. The MR acquisitions included sagittal proton density (PD) and intermediate-weighted (IW) fat-saturated sequences, coronal intermediate-weighted and axial T2-weighted fat-saturated sequences. Axial T2-weighted fat-saturated imaging parameters included an echo time (TE) of 85ms, repetition time (TR) of 5186ms, slice thickness of 4.0mm, slice spacing of 0.0mm, an echo train of 11, a field of view 16cm, a matrix size of 320 × 384, and a NEX of 2. Sagittal PD-weighted fat-saturated imaging parameters included a TE of 30ms, TR of 5186ms, slice thickness of 3.5mm, slice spacing of 0.0mm, an echo train of 13, a field of view of 16cm, a matrix size of 320 × 320 and a NEX of 2.
MR imaging analysis
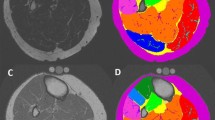
Cross sectional measurement of bone and subcutaneous fat were performed by two independent observers (radiology trainees) using Osirix MD 7.0 on a diagnostic workstation. A single axial slice for each patient at the most posterior aspect of the femoral condyles on T2-weighted fat-saturated sequences was selected. Regions of interest (ROI) were drawn around bone to calculate the cross-sectional area of the femoral condyles. Two further regions of interest were drawn around the outer circumference of the thigh and the deep fascia. The difference between these two latter ROIs was calculated to be the cross sectional area of subcutaneous fat at this level (Fig. 2). Each measurement was performed twice by each observer with at least two weeks between first and second observations [25].
The location of subcutaneous hyper-intense T2 signal was defined by consensus agreement, measured in a cranio-caudal direction relative to the tibial tuberosity, in the sagittal plane.
Statistical analysis
All statistical analyses were performed using the statistics programme R [26]. A Shapiro-Wilk test was used to demonstrate normality of data distribution and therefore tests for data comparison. Paired Student t-tests and Wilcoxon Rank Sum tests were used for hypothesis testing. Reliability was measured using intraclass correlation coefficients (ICC) and 95% levels of agreement from Bland-Altman plots. Descriptive statistics were used to define lesion location.
Results
The study and control groups each comprised 18 females with a mean age of 45, a range of 31–62 years, with 10 left and 8 right knees.
The cross-sectional bone measurements conformed to a parametric distribution (p = 0.53-0.93) (Fig. 3). The mean area in the study group (first measure performed by observer A) was 31.79cm2 (SD 2.57, 95% CI 30.40–32.96 cm2) and 30.11cm2 (SD 3.20, 95% CI 28.63–31.59cm2) in the control group. The difference in means was 1.68cm2 and not statistically significant (p = 0.09) (Fig. 4). The first results for observer B, and results from the second measurements for both observers were similar (Table 1).
Cross-sectional subcutaneous fat measurements were not parametric (p = 0.0008–0.007) (Fig. 3). The median area in the study group (first measure performed by observer A) was 62.29cm2 (IQR 57.09–66.47cm2) and 29.39cm2 (IQR 24.77–32.33cm2) in the control group. The difference in medians was 32.77cm2 and statistically significant (p < 0.0001) (Fig. 4). The first results for observer B, and results from the second measurements for both observers were similar (Table 2).
Inter-rater and intra-rater correlation was “excellent” with coefficients of more than 0.8 for all intraclass correlations [27]. The worst inter-rater reliability was ICC = 0.85 (95% CI 0.64–0.94, p < 0.0001) where the mean difference between observers was 0.28cm2 (95% limits of agreement -3.50–2.93cm2). All other inter-rater ICC scores were better, and can be found in Table 3. The worst intra-rater reliability was ICC = 0.94 (95% CI 0.85–0.98, p < 0.0001) with the mean difference between first and second observations of 0.04cm2 (95% limits of agreement -1.91–1.83cm2). All other intra-rater ICC scores were better, and can be found in Table 4.
There was a non-parametric craniocaudal distribution of T2 hyper-intense signal change, with a median location of 10.4cm (IQR 6.5–16.9cm) superior to the tibial tuberosity (Fig. 5).
Discussion
This study found that patients demonstrating subcutaneous hyper-intense signal on T2-weighted fat-saturated MRI had significantly more subcutaneous fat around the knee when compared to controls. This suggests that there is an association between the amount of subcutaneous fat and subcutaneous oedema. The cross-sectional area of bone was not statistically different between the study and control groups, suggesting they were comparable in measures other than subcutaneous fat. Most of the fluid signal changes were located 10cm proximal to the tibial tuberosity indicating that this is mainly a pre-patella phenomenon.
All of the cases identified in this retrospective study were women with a mean age in the fifth decade. The female preponderance may be accounted for by the fact that excess adipose tissue tends to be peripherally distributed in females and centrally in males [28]. Even so, measures of appendicular subcutaneous fat are considered to be reasonable surrogate measures of body mass index (BMI) [29, 30]. The age distribution might on first inspection suggest that this is a finding most prevalent in middle age patients. However we were unable to find suitable controls—without any subcutaneous T2 hyper-intense signal changes on their MR examination—for some of the older subjects who then had to be excluded from the study. Therefore both increasing age and subcutaneous fat content may be associated with fluid signal changes in subcutaneous fat.
An association between fluid in the subcutaneous fat and obesity does not necessarily imply causation but there is a mechanism that may explain this association. After eight to 10 years of obesity, individuals develop metabolic syndrome in which the blood supply to adipocytes is reduced causing hypoxia leading to the over-expression of pro-inflammatory cytokines [15–21]. The over-expression of these inflammatory mediators causes macrophage infiltration, extracellular matrix protein up-regulation and fibrosis [23]. A positive feedback loop is accelerated where inflammation in fat propagates to a systemic state of inflammation which can further be attributed to the development of other comorbidities related to obesity [18–20, 31].
Acute injuries of normal subcutaneous fat can result in a Morel-Lavallée lesion, which is a closed de-gloving injury that occurs as a result of blunt trauma. Tangential sheer forces between the subcutaneous tissues and underlying fascia cause a disruption in the vascular and lymphatic supply to the overlying tissues, creating a potential space which fills with blood, lymphatic fluid and necrotic fat [32–34]. Subsequently, blood is largely resorbed and replaced with serosanguinous fluid. Eventually, a capsule forms around the collection meaning the lesion persists [35–37]. The Morel-Lavallée lesion most commonly occurs overlying the greater trochanters, owing to their susceptibility to trauma [27] but has also been reported around the knee [38, 39] and lumbar spine [40].
The development of sheets of fluid in subcutaneous fat of obese patients may then be the result of a chronic shearing injury similar to a Morel-Lavallée lesion [32–34, 36, 38]. In contrast the chronic lesions identified in this study comprised fluid signal but were not well defined and not encapsulated.
If this mechanism of injury is valid then the results of this study would suggest that the shearing forces are greatest over the extensor surface anterior to the patella. To our knowledge there are no published measures of normal soft tissue shearing forces around the knee with which to compare our findings. It is possible that these chronic shearing injuries are in themselves a source of chronic knee pain but this has not been addressed by the current study.
The study is limited by the retrospective design and as a result is prone to selection bias. All subjects had each presented for MRI examination with chronic knee pain, and therefore were a subset of a normal population. The confidence intervals for the means and medians of the study and control groups were narrow suggesting that the sample size was sufficient to provide a good representation of the study population. However this does not mean that these findings can necessarily be generalized to an asymptomatic obese population.
Although the primary endpoint measures were dependent on manually derived ROIs, and the inherent variability associated with these, the measures of inter and intra-rater reproducibility were excellent, and the 95% limits of agreement were narrow, indicating that these are reliable results.
Conclusion
In conclusion subcutaneous fluid demonstrated on MRI examination of the knee, in patients with chronic knee pain, is associated with increased volumes of subcutaneous fat and is predominantly anterior to the patella, 10cm proximal to the tibial tuberosity.
Abbreviations
- CI:
-
Confidence interval
- DM:
-
Difference in means
- ICC:
-
Intra-class correlation coefficient
- IQR:
-
Interquartile range
- IW:
-
Intermediate weighted imaging
- LA:
-
Limits of agreement
- MRI:
-
Magnetic resonance imaging
- NEX:
-
Number of excitations
- PD:
-
Proton density weighted imaging
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- TE:
-
Echo Time
- TR:
-
Repetition time
References
Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Frammingham study. Ann Intern Med. 1988;109:18–24.
Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Frammingham study. Ann Intern Med. 1992;116:535–9.
Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20(2):331–5.
Ding C, Cicuttini FM, Scott F, Cooley H, Boon C, Jones G. Natural history of knee structural alteration and body mass index: a cross sectional study. Obes Res. 2004;13:350–61.
Ding C, Cicuttini FM, Scott F, Cooley H, Boon C, Jones G. Natural history of knee cartilage defects and factors affecting change. Arch Intern Med. 2006;166(6):651–8.
Berry A, Wluka AE, Davies-Tuck ML, et al. The relationship between body composition and structural changes at the knee. Rheumatology. 2010;49:2362–9. doi:10.1093/rheumatology/keq255.
Brennan SL, Cicuttini FM, Pasco JA, et al. Does an increase in body mass index over 10 years affect knee structure in a population-based cohort study of adult women? Arthritis Res Ther. 2010;12:R139. doi:10.1186/ar3078.
Zhai G, Stankovich J, Cicuttini F, Ding C, Jones G. Familial, structural and environmental correlates of MRI-defined bone marrow lesions: a silbpair study. Arthritis Res Ther. 2006;8:R137.
Helfenstein M, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50(3):313–27.
Hanna FS, Bell RJ, Davis SR, et al. Factors affecting patella cartilage and bone in middle-aged women. Arthritis Rheum. 2007;57(2):272–8.
Runhaar J, Koes BW, Clockaerts S, Bierma-Zeinstra SMA. A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis. Obes Rev. 2011;12:1071–82. doi:10.1111/j.1467-789×.2011.00916.x.
de Souza SA, Faintuch J, Valezi AC, Sant’Anna AF, Gama-Rodrigues JJ. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005;15(9):1238–42.
Lai PP, Leung AK, Li AN, Zhang M. Three-dimensional gait analysis of obese adults. Clin Biomech. 2008;23(s1):s2–6. doi:10.1016/j.clinbiomech.2008.02.004.
Andrews M, Noyes FR, Hewett TE, Andriacchi TP. Lower limb alignment and foot angle are related to stance phase knee adduction in normal subjects: a critical analysis of the reliability of gait analysis data. J Orthop Res. 1996;14(2):289–95.
Kabon B, Nagele A, Reddy D, et al. Obesity decreases perioperative tissue oxygenation. Anaesthesiology. 2004;100(2):274–80.
Virtanen KA, Lonnroth P, Parkkola R, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in non-obese and obese humans. J Clin Endocrinol Metab. 2002;87(8):3902–10.
Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55.
Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–11.
Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293(4):E1118–28.
Chen B, Lam KS, Wang Y, et al. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341(2):549–56.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91.
Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37(3):753–68. doi:10.1016/j.ecl.2008.07.002.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodelling and obesity. J Clin Invest. 2011;121(6):2094–101. doi:10.1172/JCI45887.
Eng J. Sample size estimation: How many individuals should be studied? Radiology. 2003;227(2):309–13.
Marx RG, Menzez A, Horovitz L, et al. A comparison of two time intervals for test-retest reliability of health status instruments. J Clin Epidemiol. 2003;56(8):730–5.
R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. URL http://www.R-project.org/. ISBN 3-900051-07-0.
Landis J, Koch G. The measure of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Vague J. La différenciation sexuelle; facteur déterminant des formes de l’obésité. Presse Med. 1947;55(30):339.
Khadivzadeh T. Mid upper arm and calf circumferences as indicators of nutritional status in women of reproductive age. East Mediterr Health J. 2002;8(4–5):612–8.
Tsai AC, Chang T-L. The effectiveness of BMI, calf circumference and mid-arm circumference in predicting subsequent mortality risk in elderly Taiwanese. Br J Nutr. 2011;105:275–81. doi:10.1017/S0007114510003429.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–55.
Li H, Zhang F, Lei G. Morel-Lavallee lesion. Chin Med J (Engl). 2014;127(7):1351–6.
Hudson DA, Knottenbelt JD, Krige JEJ. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853–5.
Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallee lesion. J Trauma. 1997;42(6):1046–51.
Parra JA, Fernandez MA, Encinas B, Rico M. Morel-Lavallee effusions in the thigh. Skeletal Radiol. 1997;26(4):239–41.
Mukherjee K, Perrin SM, Hughes PM. Morel-Lavallee lesion in an adolescent with ultrasound and MRI correlation. Skeletal Radiol. 2007;36:43–5.
Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavallee lesion. AJR Am J Roentgenol. 2004;182(5):1347–8.
Van Gennip S, Van Bokhoven SC, Van Den Eede E. Pain at the knee: the Morel-Lavellee lesion, a case series. Clin J Sport Med. 2012;22:163–6. doi:10.1097/JSM.0b013e318246ee33.
Goodman BS, Smith MT, Mallempati S, Nuthakki PA. A comparison of ultrasound and magnetic resonance imaging findings of a Morel-Lavallee lesion of the knee. PM R. 2013;5(1):70–3. doi:10.1016/j.pmrj.2012.08.001.
Garrison M, Westrick RB, Johnson MR. Morel-Lavallee lesion of the lumbar spine. J Orthop Sports Phys Ther. 2014;44(3):223. doi:10.2519/jospt.2014.0404.
Acknowledgements
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
The datasets analyzed during the current study’s results are available from the corresponding author on reasonable request.
Authors’ contributions
TG literature research, concepts and study design, experimental studies, statistical analysis and manuscript preparation and editing. FC experimental studies and manuscript editing. JC experimental studies and manuscript editing. AT concepts and study design, experimental studies, statistical analysis and manuscript preparation and editing. All authors read and approved the final manuscript.
Authors’ information
TG: 3rd year Clinical Radiology Specialist Training Registrar.
FC: 3rd year Clinical Radiology Specialist Training Registrar.
JC: Consultant Musculoskeletal Radiologist.
AT: Consultant Musculoskeletal Radiologist, Honorary Professor University of East Anglia.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was obtained following proportionate review by the NHS National Research Ethics Service (MICRON reference 15/NW/0486). Informed consent was deemed unnecessary for this retrospective case-control study.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1186/s12891-016-1361-8.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gaunt, T., Carey, F., Cahir, J. et al. Fluid signal changes around the knee on MRI are associated with increased volumes of subcutaneous fat: a case-control study. BMC Musculoskelet Disord 17, 487 (2016). https://doi.org/10.1186/s12891-016-1345-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-016-1345-8