Abstract
Background
Despite the progress in reducing malaria infections and related deaths, the disease remains a major global public health problem. The problem is among the top five leading causes of outpatient visits in Dembia district of the northwest Ethiopia. Therefore, this study aimed to assess the determinants of malaria infections in the district.
Methods
An institution-based case-control study was conducted in Dembia district from October to November 2016. Out of the ten health centers in the district, four were randomly selected for the study in which 370 participants (185 cases and 185 controls) were enrolled. Data were collected using a pretested structured questionnaire. Factors associated with malaria infections were determined using logistic regression analysis. Odds ratio with 95% CI was used as a measure of association, and variables with a p-value of ≤0.05 were considered as statistically significant.
Results
The median age of all participants was 26 years, while that of cases and controls was 22 and 30 with a range of 1 to 80 and 2 to 71, respectively. In the multivariable logistic regression, over 15 years of age adjusted odds ratio(AOR) and confidence interval (CI) of (AOR = 18; 95% CI: 2.1, 161.5), being male (AOR = 2.2; 95% CI: 1.2, 3.9), outdoor activities at night (AOR = 5.7; 95% CI: 2.5, 12.7), bed net sharing (AOR = 3.9; 95% CI: 2.0, 7.7), and proximity to stagnant water sources (AOR = 2.7; 95% CI: 1.3, 5.4) were independent predictors.
Conclusion
Being in over 15 years of age group, male gender, night time activity, bed net sharing and proximity to stagnant water sources were determinant factors of malaria infection in Dembia district. Additional interventions and strategies which focus on men, outdoor work at night, household net utilization, and nearby stagnant water sources are essential to reduce malaria infections in the area.
Similar content being viewed by others
Background
Malaria, which is one of the leading causes of illness and death in the world, is caused by protozoan parasites of the genus plasmodium. About 44% of the world’s population is at risk of malaria infection and over 97 countries are affected by the disease. There are 216 million cases and 445, 000 malaria caused deaths worldwide, and the African Region accounts for about 90% of the cases and deaths. Furthermore, 14 sub-Saharan African countries and India carried 80% of the global malaria burden [1].
Despite the progress gained in reducing malarial morbidity and mortality, the disease remains a major public health problem in many countries of the world. The World Health Organization (WHO) report shows that the number of malaria cases globally fell from the estimated 237 million in 2010 to 216 million in 2016, a decline of 18%. Most of the cases were estimated to have occurred in the African Region (90%), followed by Southeast Asia (7%) and the East Mediterranean Region (2%). Moreover, the number of malarial deaths globally fell from the estimated 446, 000 in 2015 to 445, 000 in 2016 [2].
Malaria is one of the main public health problems in Ethiopia; approximately 75% of the landmass is malaria-endemic, and 65% of the population is at risk of malaria infection [3]. According to the Ethiopian Federal Ministry of Health, malaria accounted for 15% of the reported outpatient visits and nearly 15% of the admissions in the country. Furthermore, malaria is among the ten leading causes of inpatient deaths among children less than 5 years of age [4]. A total of 1,127,241 cases of malaria were reported in Amhara Region; 404,926 and 225,818 of these were in West Gojjam and North Gondar zones, respectively [4].
Dembia is one of the most affected districts in the region. Its proximity to the Lake Tana basin (the largest lake in Ethiopia) and high altitude (2000 m above sea level) are the major factors for the endemicity [5]. In 2016, about 138,842 long lasting insecticidal nets (LLINs) were distributed, and 16 kebeles (lowest administrative units) were sprayed. However, the burden of the disease kept increasing; for instance, in over a 46-week interval, 22,166 malaria cases were reported in 2016 in contrast to 10,415 in 2015 [6].
Malaria prevention and control interventions that included mass distribution of LLINs, the scaled up of indoor residual spraying (IRS), the introduction of rapid diagnostic tests (RDTs) at community levels, and the adoption of artemisinin-based combination therapies (ACTs) have been scaled up since 2005, leading to the reduction of the burden of the disease by about 50%, on average [7].
The national malaria indicator survey demonstrates that in Amhara Region the coverage of LLIN and/or IRS increased from 76.2 in 2007 to 86.3% in 2011 and community knowledge of malaria 26.7% to 48.4% in the same period [8]. Even though written data on malaria cases were limited in the health centers in Dembia district, according to the annual performance report of the FMOH 2017, the total malaria cases treated in the health facilities were 1,820,967 [9].
The burden of malaria remains a major public health problem in Dembia district despite the interventions implemented. Therefore, the aim of this study was to identify the determinants of malaria infections in the district.
Methods
Study design and setting
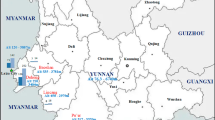
An institution-based matched case-control study was conducted from October to November 2016. The study was conducted in Dembia district, Amhara Region, northwest Ethiopia. It is located in North Gondar zone 729 km north of Addis Ababa at 12°18′30″N and 37°17′30″E. The district has an area of 148,968 sq. km with a total population of 307,967. Out of the total area of the district, plain land accounts for about 87%, mountains and hills 5%, valleys and gorges 4.8%, and water bodies 3.2%. The altitude of the district ranges from 1850 to 2000 m above sea level. The district receives an annual rainfall of 700 to 1160 mm on average [10]. There are 10 health centers and 40 health posts in the district providing healthcare services, such as health education, diagnosis, and treatment (Fig. 1).
The district is divided into 45 sub-districts called Kebeles, the lowest administrative units of Ethiopia. Malaria intervention based on its prevalence is targeted in 16 sub-districts for IRS and the rest for ITN distribution.
Study design and period: An institution-based matched case-control study was conducted from October to November 2016.
Source population: All individuals living in Dembia district were considered as the source population.
Study population: All permanent residents of the districts visiting the health centers.
Sample size and sampling technique
The sample size for case control study was calculated using the following formula:
Alternatively, Epi-Info software (matched case-control) method with a p1 (95%), p2 86.3% of ITN/IRS exposure among controls [11], r(1), OR(3), Zα/2(95%) and Z1-β(80%) was used to calculate the sample size. Accordingly, n1 was 196 malaria-infected patients, whereas n2 was 196, making a total of 392 participants.
Assuming that all of the health centers were likely to be homogeneous and visited by an equal number of cases, we selected 93 participants each from the four health centers of Dembia district in Ethiopia. We collected data from cases and controls in the four selected health centers for an average of 1 month.
Case definition
Cases were confirmed malaria infected individuals confirmed by microscopy or RDT testes (Care Start®, Access Bio, NJ, USA) at the selected health centers from October 1 to November 10, 2016.
Controls were individuals presenting at the same health centers free from malaria infection as diagnosed by microscopy or RDT tests.
Inclusion criteria
We included permanent residents of the district who visited the health centers and screened for malaria infection using microscopy and RDT tests.
Exclusion criteria
Participants unable to provide the required information due to different conditions, like extreme illness and absence of caregivers were excluded.
Study variables
Dependent variable
The dependent variable was malaria infection among health center attendants.
Independent variables
Socio-demographic variables: gender, age, marital status, educational status, ethnicity, occupation, family size.
Materials used for malaria prevention related variables: ITN possession, IRS, number of persons sharing a net.
Environment-related variables: housing condition, surrounding water bodies, water source for domestic use.
Personal activity-related variables: sleeping outdoors at night, travel history to other malaria endemic areas, sleeping sites.
Knowledge: about causes and ways of malaria prevention.
Data collection procedure
An interviewer-administered questionnaire was employed on infected individuals in the selected health centers in the district to get primary data on demographics and potential risk factors, such as socio-demography, knowledge about malaria, environmental factors, and use of malaria prevention measures. The same questionnaire was used to collect data on potential predictors from the control group. Cases were selected based on definitions developed after they completed their examination (exit interview), and patient cards were immediately taken from the examiner to link the results of the diagnosis. Then, they were interviewed for other relevant bits of information. After the recruitment of cases, controls who lived in the same areas as cases were included.
Data quality control
The data collection tool was first prepared in English and translated to the local language, Amharic and back translated to English to cheek for consistency. Pre-test was done on 5% of the respondents prior to the actual data collection in a neighboring district. Training was given forboth data collectors and supervisor on the data collection tools and techniques of interviewing with practical exercises. Daily supervision was done by the supervisor and principal investigator.
Data processing and analysis
Data was cleaned for completeness and consistencies, coded and entered in to Epi info version 7.0 and transported into the Statistical Package for the Social Sciences (SPSS) version 20 for further analysis. The results were organized, summarized and presented using texts, tables, and graphs.
Crude odds ratios (COR) with a 95% confidence interval (CI) were estimated in the bi-variable logistic regression analysis to screen the effect each independent variable on the outcome variable and to select candidate variables for the multi-variable logistic regression analysis. Because of a relatively large number of independent variables considered, we had to screen them using bi-variable analysis to minimize the chance of multicollinearity in the multi-variable regression. Thus, only independent variables with a p-value of 0.20 or less in the bi-variable logistic regression were included in the multi-variable logistic regression to get the adjusted effect of each covariate.
Variables which were significant at p-value 0.05 level and 95% CI in the multivariable logistic regression analysis were considered to be the determinant factors of malaria infections.
Results
Characteristics of the study population
The median age of all participants was 26 years, while that of cases and controls was 22 and 30 with a range of 1 to 80 and 2 to 71, respectively. The cases group contained more men than women, 74% and 26%, respectively. More than one-third of the participants, 175 (37%), were farmers, that is, agriculture was the main source of income for 98% of the cases and 85% of the controls (Table 1).
The majority (85%) of the participants had bed nets, and about one-third of the houses (27%) were treated with IRS in the previous 6 months. Most of the houses (98%) were constructed of mud walls. The sleeping sites of a large proportion (66%) of the respondents were beds without mattresses, and most of (89% cases and 95% controls) the respondents used bed nets consistently (Table 2).
Knowledge of participants about causes and preventions of malaria
Cases and controls had comparable knowledge about the causes and preventions of malaria. Most of the respondents (98% of either group) heard about malaria through various means of communication.
Respondents in each group, that is, 87% of the cases and 94% of the controls at least knew that the mosquito was one of the causes of malaria. On the other hand, 84% of the cases and 78% of the controls wrongly held that malaria was acquired either by getting soaked in rain, eating immature sugarcane or contaminated food or by drinking contaminated water. According to 93% of the cases and 94% of the controls, sleeping under any types of bed net was the most common method of preventing malaria (Table 3).
Determinants of malaria infection
In the bivariable logistic regression, gender, age, outdoor activities at night, travel history in the past 2 weeks to other malarious area, habit of sleeping outdoors at night, self reported bed net use, proximity to stagnant water and a temporary river body, and number of persons sharing a bed net were statistically significantly associated with malaria infection at a p-value of 0.2. All these variables were included in the multivariable logistic regression model, but other variables which had a p-value greater than 0.2 in this model were not included in the multivariable regression model.
When each independent variable was adjusted for other variables, age, gender, sleeping outdoors at night, proximity to stagnant water, and bed net use were found to be statistically significantly associated with malaria infection at a 95% confidence level and a p-value of 0.05.
Age greater than 15 years with an adjusted odds ratio(AOR) and confidence interval (CI) (AOR =18; 95% CI: 2.1–161.5), male (AOR = 2.2; 95%CI: 1.2–3.9), staying outside at night (AOR =5.7; 95% CI: 2.5–12.7), proximity to stagnant water (AOR = 2.7; 95% CI: 1.3–5.4), and three or more people sharing one net had more risk for malaria infection than those sharing one net with two people (AOR = 3.9; 95% CI:2.0–7.7). Variables, such as proximity to temporary river bodies, travel history to another endemic malaria areas, self-reported bed net use, household bed net possession, and household bed net ratio were not statistically significantly associated with malaria infection (Table 4).
Discussion
In this study, being male, 15 and above years of age, staying outdoors at night, bed net sharing with more than 3 persons, and proximity to stagnant water were the determinants of malaria infection. However, ownership and use of ITN were not statistically significant predictors for malaria infection.
In our study, males were more affected by malaria infection than females. This result is in line with those of studies done in Oromia Regional State, Ethiopia, and Eastern Rwanda [12, 13]. This might be so because agriculture is the main occupation and sleeping, and staying outdoors at night is common. Thus, males are more exposed to anopheles mosquito bites. The other possible reason might be that males are particularly less likely to use bed nets even when nets are available [13].
The finding showed that individuals sharing a bed net with more than 3 persons were found to be at more risk for malaria infection than those sharing a net with two other persons. The finding is supported by that of a study done in Laos [14]. In addition, outdoor activity is associated with malarial illness in this study; it is possible that bed nets were not used during outdoors, decreasing their effectiveness in preventing malaria illness. Bed net possession and use is the commonest method of the prevention of malaria infection [9, 15]. In this study, however, it had no statistically significant association with malaria infection contrary to findings of other studies [12, 16]. In this context, these results could derive from a combination of factors, such as the conditions of nets [17], vector resistance issues [18,19,20,21], and sleeping sites [22].
Contrary to the findings of other works, IRS was not significantly associated with malaria protection in our study. This may be due to the fact that few households had IRS in the previous 6 months. In addition, it may be due to vector resistance to spray chemicals [18, 23]. Besides, after 5 months, IRS is insufficient to kill resistant mosquitoes [24].
In this study, malaria infection was more widespread among people aged above 15 years. This result is supported by that of a study done in Machinga district, Malawi and WHO malaria report [12, 25]. This might be due to the fact that productive age groups are usually engaged in outdoor activities at dawn and dusk, increase their exposure [13, 26]. Similarly, it is found that outdoor activity at night is one of the significant risk factors for malaria infection in the area. However, this is contrary to the findings in Zimbabwe [26], where individuals below 5 years of age were more at risk of malaria infections. This may be due to cultural differences in that younger individuals are subject to night time outdoor activities in the study area (in Zimbabwe) with no habit of using repellents.
Stagnant water bodies (marshy areas), which are risk factors for malaria infections, are one of the places where malaria vectors breed [27]. Consequently, people near stagnant water bodies are at higher risk for malarial disease than their counterparts.
Limitation of the study
As a case-control study, our work is prone to selection and recall biases. Besides, LLIN use was reported rather than observed and might have limited the generalizability of the findings.
Conclusion
Despite the high proportion of bed net possession and self-reported utilization, being male, 15 and above years of age, staying outdoors at night, bed net sharing with more than 3 persons and proximity to stagnant water were the determinants of malaria infection. However, the study identified neither household bed net possession nor self-reported bed net use control measures to be independently associated with malaria infection. Therefore, we focused on men, outdoor activities, and the control of the breeding of the malaria vector on stagnant water bodies, and other bed net distributions to family members in this district to prevent malaria infections.
Abbreviations
- ACT:
-
Artemesinin-based combination therapies
- FMOH:
-
Federal Ministry of Health
- IRS:
-
Indoor residual spraying
- LLNs:
-
Long lasting insecticidal nets
- MIS:
-
Malaria indicator survey
- RDT:
-
Rapid diagnostics tests
References
Barber BE, Rajahram GS, Grigg MJ, William T, Anstey NM. World malaria report: time to acknowledge Plasmodium knowlesi malaria. Malar J. 2017;16(1):135.
World Health Organization. World malaria report 2015: World Health Organization; 2016. http://apps.who.int/iris/bitstream/handle/10665/200018/9789241565158_eng.pdf.
Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Ruiz Carrascal D, Pascual M. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science. 2014;343(6175):1154–8.
Alemu A, Fuehrer HP, Getnet G, Kassu A, Getie S, Noedl H. Comparison of Giemsa microscopy with nested PCR for the diagnosis of malaria in North Gondar, north-West Ethiopia. Malar J. 2014;13:174.
Alemu A, Muluye D, Mihret M, Adugna M, Gebeyaw M. Ten year trend analysis of malaria prevalence in kola Diba, North Gondar, Northwest Ethiopia. Parasit Vectors. 2012;5:173.
Toyama Y, Ota M, Molla G, Beyene BB. Sharp decline of malaria cases in the Burie Zuria, Dembia, and Mecha districts, Amhara region, Ethiopia, 2012–2014: descriptive analysis of surveillance data. Malar J. 2016;15(1):104.
Population Census Commission: Summary and statistical report of the 2007 population and housing census: population size by age and sex. 2008.
Jima D, Wondabeku M, Alemu A, Teferra A, Awel N, Deressa W, Adissie A, Tadesse Z, Gebre T, Mosher AW, et al. Analysis of malaria surveillance data in Ethiopia: what can be learned from the integrated disease surveillance and response system? Malar J. 2012;11:330.
Ntonifor NH, Veyufambom S. Assessing the effective use of mosquito nets in the prevention of malaria in some parts of Mezam division, Northwest Region Cameroon. Malar J. 2016;15(1):390.
Weldegebriel ZB, Amphune BE. Livelihood resilience in the face of recurring floods: an empirical evidence from Northwest Ethiopia. Geoenvironmental Disasters. 2017;4(1):10.
Federal demographic repblic of Ethiopia ministry of health National Malaria Indicator Survey 2011 Technical Summary. SEP; 2012. http://www.ephi.gov.et/images/downloads/2011ethiopiamistechsummary.pdf.
Yukich JO, Taylor C, Eisele TP, Reithinger R, Nauhassenay H, Berhane Y, Keating J. Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J. 2013;12:33.
Kateera F, Ingabire CM, Hakizimana E, Rulisa A, Karinda P, Grobusch MP, Mutesa L, van Vugt M, Mens PF. Long-lasting insecticidal net source, ownership and use in the context of universal coverage: a household survey in eastern Rwanda. Malar J. 2015;14:390.
Nonaka D, Laimanivong S, Kobayashi J, Chindavonsa K, Kano S, Vanisaveth V, Yasuoka J, Phompida S, Jimba M. Is staying overnight in a farming hut a risk factor for malaria infection in a setting with insecticide-treated bed nets in rural Laos? Malar J. 2010;9:372.
Kesteman T, Randrianarivelojosia M, Raharimanga V, Randrianasolo L, Piola P, Rogier C. Effectiveness of malaria control interventions in Madagascar: a nationwide case-control survey. Malar J. 2016;15:83.
Mathanga DP, Tembo AK, Mzilahowa T, Bauleni A, Mtimaukenena K, Taylor TE, Valim C, Walker ED, Wilson ML. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar J. 2016;15(1):590.
Atieli FK, Munga SO, Ofulla AV, Vulule JM. The effect of repeated washing of long-lasting insecticide-treated nets (LLINs) on the feeding success and survival rates of Anopheles gambiae. Malar J. 2010;9(1):304.
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122.
Djouaka RJ, Atoyebi SM, Tchigossou GM, Riveron JM, Irving H, Akoton R, Kusimo MO, Bakare AA, Wondji CS. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in south West Nigeria. Malar J. 2016;15(1):565.
Mourou JR, Coffinet T, Jarjaval F, Pradines B, Amalvict R, Rogier C, Kombila M, Pages F. Malaria transmission and insecticide resistance of Anopheles gambiae in Libreville and Port-Gentil, Gabon. Malar J. 2010;9:321.
Quinones ML, Norris DE, Conn JE, Moreno M, Burkot TR, Bugoro H, Keven JB, Cooper R, Yan G, Rosas A, et al. Insecticide resistance in areas under investigation by the international centers of excellence for malaria research: a challenge for malaria control and elimination. Am J Trop Med Hyg. 2015;93(3 Suppl):69–78.
Iwashita H, Dida G, Futami K, Sonye G, Kaneko S, Horio M, Kawada H, Maekawa Y, Aoki Y, Minakawa N. Sleeping arrangement and house structure affect bed net use in villages along Lake Victoria. Malar J. 2010;9:176.
Asale A, Getachew Y, Hailesilassie W, Speybroeck N, Duchateau L, Yewhalaw D. Evaluation of the efficacy of DDT indoor residual spraying and long-lasting insecticidal nets against insecticide resistant populations of Anopheles arabiensis Patton (Diptera: Culicidae) from Ethiopia using experimental huts. Parasites Vectors. 2014;7(1):131.
Protopopoff N, Matowo J, Malima R, Kavishe R, Kaaya R, Wright A, West PA, Kleinschmidt I, Kisinza W, Mosha FW, et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in North-Western Tanzania. Malar J. 2013;12:149.
World Health Organization: World malaria report 2014: summary. 2015.
Mugwagwa N, Mberikunashe J, Gombe NT, Tshimanga M, Bangure D, Mungati M. Factors associated with malaria infection in Honde valley, Mutasa district, Zimbabwe, 2014: a case control study. BMC Res Notes. 2015;8:829.
Deribew A, Birhanu Z, Sena L, Dejene T, Reda AA, Sudhakar M, Alemseged F, Tessema F, Zeynudin A, Biadgilign S, et al. The effect of household heads training about the use of treated bed nets on the burden of malaria and anaemia in under-five children: a cluster randomized trial in Ethiopia. Malar J. 2012;11:8.
Acknowledgments
Authors would like to thank the University of Gondar for the ethical clearance. We would also like to thank the Amhara Regional State Health Bureau, North Gondar Zone Health Department, Dembia Woreda Health Office, and the health centers for the permission, and participants for their cooperation.
Funding
The authors declare that there is no funding source.
Availability of data and materials
Full data set and materials pertaining to this study can be obtained from the correspondence author on reasonable request.
Author information
Authors and Affiliations
Contributions
AS, MT, and FB designed the proposal. FA acquired the data, performed the analysis and drafted the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages. All authors contributed extensively to the work presented in this paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after ethical clearance was given by the Research and Ethics Committee of the University of Gondar. Informed written consent was obtained from all adults and parents, or guardians for enrollment in the study. The confidentiality of the information collected from was maintained by not taking participants’ names.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Agegnehu, F., Shimeka, A., Berihun, F. et al. Determinants of malaria infection in Dembia district, Northwest Ethiopia: a case-control study. BMC Public Health 18, 480 (2018). https://doi.org/10.1186/s12889-018-5370-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-018-5370-4