Abstract
Several studies have shown association of single nucleotide polymorphisms (SNPs) of hepcidin regulatory pathways genes with impaired iron status. The most common is in the TMPRSS6 gene. In Africa, very few studies have been reported. We aimed to investigate the correlation between the common SNPs in the transmembrane protease, serine 6 (TMPRSS6) gene and iron indicators in a sample of Egyptian children for identifying the suitable candidate for iron supplementation.
Patients and methods One hundred and sixty children aged 5–13 years were included & classified into iron deficient, iron deficient anemia and normal healthy controls. All were subjected to assessment of serum iron, serum ferritin, total iron binding capacity, complete blood count, reticulocyte count, serum soluble transferrin receptor and serum hepcidin. Molecular study of TMPRSS6 genotyping polymorphisms (rs4820268, rs855791 and rs11704654) were also evaluated.
Results There was an association of iron deficiency with AG of rs855791 SNP, (P = 0.01). The minor allele frequency for included children were 0.43, 0.45 & 0.17 for rs4820268, rs855791 & rs11704654 respectively. Genotype GG of rs4820268 expressed the highest hepcidin gene expression fold, the lowest serum ferroportin & iron store compared to AA and AG genotypes (p = 0.05, p = 0.05, p = 0.03 respectively). GG of rs855791 had lower serum ferritin than AA (p = 0.04), lowest iron store & highest serum hepcidin compared to AA and AG genotypes (p = 0.04, p = 0.01 respectively). Children having CC of rs11704654 had lower level of hemoglobin, serum ferritin and serum hepcidin compared with CT genotype (p = 0.01, p = 0.01, p = 0.02) respectively.
Conclusion Possible contribution of SNPs (rs855791, rs4820268 and rs11704654) to low iron status.
Similar content being viewed by others
Introduction
The worldwide prevalence of iron deficiency anemia (IDA) is about 24.8% of the whole population, with the highest prevalence (47.4%) among children less than five-years of age [1]. Iron deficiency anemia had been known previously, to be combined with environmental and or dietary factors like infections, however, higher prevalence of IDA is still there despite the intensive iron supplementation programs for high-risk groups (children and women of childbearing age) [2]. Also, iron therapy is ineffective in all cases of IDA [3]. This raised the queries about other factors controlling iron status in the human body. The molecular mechanisms modulating iron metabolism and discovery of hepcidin have raised new insights into iron regulation pathways in the human body [4]. Various studies have indicated that 20–30% variability in iron concentration is attributed to genetic factors [5].
Hepcidin induces ferroportin (Fpn) internalization and degradation by binding it on the plasma membrane of cells in various tissues, thus blocking the flux of iron into the blood stream [6]. Many genome-wide association (GWA) studies have shown that single nucleotide polymorphisms (SNPs) in genes implicated in pathways of hepcidin regulation are combined with compromised iron status [7,8,9]. The commonest SNPs associated with decreased iron status are in the transmembrane protease serine 6 (TMPRSS6) gene, which encodes the matriptase-2 protein [10]. Transmembrane protease serine6 gene inhibits hepcidin formation, and its compromised function has been combined with improperly elevated hepcidin, that inhibits absorption of iron by the duodenum and iron mobilization from storage areas [8, 11, 12].
Above 50 SNPs among the TMPRSS6 gene have been identified to be combined with defective iron status. The most described SNPS are rs855791 followed by rs4820268, rs2235321, rs11704654 and rs2235324. All are associated with low iron status [13,14,15]. Single nucleotide polymorphisms of rs855791, rs4820268 and rs11704654 have the robust effect on indices of red blood cell and iron parameters specifically in the Asian population [8]. In Africa, very few studies identifying genetic risk factors for anemia in children have been reported [16, 17]. Identification of the major drivers of iron deficiency is mandatory for better management strategies. So, we aimed in this work to investigate the correlation between the common SNPs in the TMPRSS6 gene and iron parameters in a sample of Egyptian children as a genetic supportive approach for identifying the suitable candidate for iron supplementation.
Patients and methods
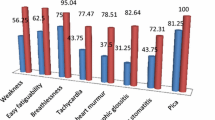
Cross sectional study was conducted in the pediatrics clinic, Centre of excellence, National Research Centre, Egypt as a part of the project titled “Hematological and non-hematological consequences of iron deficiency in children: Genetic study and response to treatment”, ID: 11010150. One hundred and sixty children aged 5–13 years were included, they were subjected to full history taking with stress on dietetic history, manifestation of iron deficiency (fatigue, leg cramps on climbing stairs, poor school performance, cold intolerance, & reduced resistance to infection) and iron supplementation during last 6 months. Full clinical examination was carried out including anthropometric measurements (height, weight, and BMI) and stress on signs of iron deficiency (as pallor of the mucous membranes, koilonychia, glossy tongue, atrophy of the lingual papillae and angular stomatitis). Laboratory investigations in the form of complete blood count (CBC), serum iron, total iron binding capacity, serum ferritin, reticulocyte count %, HB electrophoresis, C reactive protein (CRP) and stool analysis were performed. Exclusion criteria were concurrent infection (C reactive protein (CRP) > 5), chronic inflammatory diseases (rheumatic disease, inflammatory bowel disease), chronic disease, parasitic infestation, thalassemia traits α & β (ruled out by reticulocyte count < 2% and hemoglobin (HB) electrophoresis (A2 < 4%), iron supplementation and blood transfusion during the last 6 months. The included children were classified according to the iron status criteria of World Health Organization (WHO), 2001 [18] into:
-
Iron deficiency (ID) (serum ferritin ≤ 30 mg/l, transferrin saturation ≤ 16%, mean corpuscular volume (MCV) ≤ 73 fl, mean corpuscular hemoglobin concentration (MCHC) ≤ 32 g/dl).
-
Iron deficiency anemia (IDA), had iron deficiency (ID) plus (hemoglobin level ≤ 11 g/dl (adopted by Egypt Demographic and Health Survey [19]).
-
Normal iron status as a control group.
Laboratory investigations were done as follow
Eight ml of venous blood were withdrawn under aseptic conditions from 10 to 11am (to avoid diurnal variation), 5 ml were collected into EDTA vacutainer tubes (one ml for CBC & reticulocyte count, 1 ml for hepcidin gene expression and 3 ml for genotyping), the other 3 ml were collected into plain tubes, centrifuged and serum samples were stored at -80 °C for the rest of laboratory investigations.
Complete blood count was done using automated hematology analyzer Sysmx XN100 (Sysmex America Inc), reticulocyte count (using automated hematology analyzer Sysmx XN100 (Sysmex America Inc), C-reactive protein (CRP) (using IMMUNOSPEC REFE 29–056) (Bioquote Ltd, UK), serum iron & total iron binding capacity (TIBC) (using Olympus AU400 (Autoanalyzer, Japan), serum ferritin (SF) (Biocheck, Inc, Cat. No. EC-1025), serum soluble transferrin receptor (sTfR) by ELISA (Cat. No: E0281Hu) (BioVendorInc), serum hepcidin by ELISA (Cat No: E1019 Hu) (Bioassay Technology Lab, China) and serum Ferroportin (Fpn) by ELIZA (Cat. No: E4820Hu).
Transferrin saturation % was calculated using the following formula (WHO, 2001) [18]:
Body iron store was assessed using the methodology developed by Cook and coworkers as expressed by the following formula (Cook et al.,) [20]:
Molecular study of hepcidin gene expression using reverse transcriptase polymerase chain reaction
Ribonucleic acid (RNA) was extracted using QIAamp RNA blood mini kits (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Samples were extracted on the same day. The final RNA concentration was determined using a spectrophotometer (Nanodrop 2000, Therom Fisher, Walthm, USA) and RNA purity was verified by an average A260/ A280 ratio of 1.98 (range, 1.97–2.01). RNA was reverse transcribed to complementary deoxyribonucleic acid (cDNA) using a high-capacity cDNA reverse transcription kit (Applied Biosystems®, Branchburg, New Jersey, USA) in a final volume of 20 µl. Negative control samples were included in each set of reactions. Reactions were incubated at 25 °C for 10 min, followed by 37 °C for 120 min and final denaturation at 85 °C for 5 min. The reaction was carried out in the Bio-Rad thermal cycler (Life Science Research). cDNA was stored at − 20 °C.
Real-time polymerase chain reaction
Gene expression of hepcidin was measured using TaqMan® amplification system (Applied Biosystems®, Branchburg, New Jersey, USA). All samples were run in a final reaction volume of 20 μl. The reaction mix was combined using 10 µl TaqMan® Universal PCR Master Mix, 3 µl of cDNA, 6 µl of DNase free water and 1 µl of specific primers and probes 20 × 20 (Applied Biosystems®, Branchburg, New Jersey, USA). Expression of hepcidin gene was normalized using the Glyceraldehyde 3-phosphate dehydrogenase (GADPH) housekeeping gene. The PCR run was carried out using the thermal profile 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min on the Rotor -Gene Q – Qiagen -Germany (Ayatollahi et.,) [21].
Genotyping procedure
Genomic DNA was extracted from 3 ml whole blood on EDTA by a commercial DNA extraction kit according to manufacturer’s protocol (QIAamp DNA Blood Mini kit, QIAGEN, USA) CAT No.51104. DNA integrity was determined by 1% agarose gel electrophoresis, stained with ethidium bromide, and visualized through GEL documentation (E-Gel- Imager System with UV Light Base, Thermo Scientific). DNA concentration was determined by Nano Drop 2000 Spectrophotometer (Thermo scientific). TMPRSS6 genotyping polymorphisms
rs4820268: CCTACCTTCCTGGCACTGCTCTTC [A/G] TCGCTGCCGTTGAGACAATCAGGCT, rs855791: GCGTGGCGTCACCTGGTAGCGATAG [A/G] CCTCGCTGCACAGGTCCTGTGGGAT rs11704654: CCTCACAGGCCTTGAACATCCCCTC[C/T] GGCTCCGCTTCCTCGCCATCACCTC |
were performed using the TaqMan genotyping protocol (Applied Biosystems, Foster City, CA, USA). PCR reactions were set up in 20 μl reaction volume including 20–30 ng DNA, 10 μl TaqMan genotyping PCR Master Mix and 1 μl TaqMan SNP genotyping assay. The PCR assay was carried out according to manufacturer's instructions including one step of 10 min at 95 °C followed by 40 cycles of DNA denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min using the Rotor Gene Q real-time PCR (QIAGEN, Germany). Final products were analyzed by Rotor Gene software (Shinta et al.,) [22].
Statistical analysis
Data were collected, revised, coded, and statistical package for social science (SPSS) version 16, Inc., Chicago, IL, USA was used for analysis. The data was tested for normal distribution. Quantitative data were presented as mean ± SD. Polymorphism of the TMPRSS6 genotypes were presented as frequency and percentage. The prevalence of genotypes in the studied groups were tested for deviation from the assumptions of Hardy–Weinberg Equilibrium by using the exact Chi-Square test. Genotype and allele frequencies were compared between patients and control group using Chi-Square tests. Odds ratio (OR) with 95% confidence intervals was calculated. Differences in iron status across the genotype classes were assessed by using ANOVA test to determine the effects of individual SNPs on iron biomarkers. Linear regression analyses were conducted between SNPs rs855791, rs4820268, rs11704654, iron status and hematological parameters. P-values ≤ 0.05 were considered statistically significant.
Results
Based upon the criteria of iron deficiency (ID) and iron deficiency anemia (IDA), 76 (47.5%) of the included children had iron deficiency anemia, 44 (27.5%) had iron deficiency and 40 (25%) were healthy children with normal iron status who served as a control group. Demographic and laboratory data of the three groups are presented in Table 1.
The genotype AG of rs4820268, AG of rs855791 and CC of rs11704654 had the highest percentage among all the studied groups as presented in Table 2.
All the SNPs investigated were in Hardy–Weinberg Equilibrium (P > 0.05) Mayo, [23].
Increasing frequency of heterozygote allele genotype AG of rs855791 in iron deficiency and iron deficiency anemia compared to healthy controls (p = 0.001 & p = 0.02 respectively) was detected as presented in Fig. 1A, while the frequency distribution of allele genotypes of rs4820268 and rs11704654 showed no significant difference (Fig. 1B and C).
By using the odds ratio (OR), an association of iron deficiency with AG genotype of rs855791 was found, Odds Ratio with 95% confidence interval (OR (95% CI) = 3.33 (1.298–8.558), P = 0.01 (Table 3).
The minor alleles frequency for all children (patients and controls) was found to be 0.43, 0.45 and 0.17 for rs4820268, rs855791 and rs11704654 respectively (Table 4).
Children having minor homozygote allele GG genotype of rs4820268 had the highest hepcidin gene expression fold, the lowest serum ferroportin and iron store compared to those having AA and AG genotypes (p = 0.05, p = 0.05, p = 0.03 respectively). Children having minor homozygote allele GG genotypes of rs855791 had lower serum ferritin level compared to AA (p = 0.04). They had the highest level of serum hepcidin and lowest iron store compared to AA and AG genotypes (p = 0.04, p = 0.01 respectively). Children having major homozygote allele CC genotype of rs11704654 had lower level of hemoglobin, serum ferritin and serum hepcidin compared with CT genotype (p = 0.01, p = 0.01, p = 0.01, p = 0.01, p = 0.02 respectively), (Table 5).
By linear regression analysis, and after adjustment of age and BMI, SNPs of rs4820268 explained 4% of the change in HB level (r2 = 0.04, P = 0.04), 5% of the variation in serum ferroportin concentration (r2 = 0.05, P = 0.04). Single nucleotide polymorphisms of rs855791 explained 8.5% of the variation in iron store (r2 =0.085, P = 0.04) and 8.2% of the change in serum hepcidin (r2 = 0.082, P = 0.009). Single nucleotide polymorphisms of rs11704654 explained 4.5% of the change in HB level (r2 =0.045, P = 0.01), and 6.5% of the change in serum hepcidin (r2 = 0.065, P = 0.02).
The impact of gender on genes associations was not detectable, so sex was not considered in the study (data was represented as Supplementary table).
Discussion
Single nucleotide polymorphisms of rs855791 of TMPRSS6 gene has been widely described to affect iron indices and to be correlated with the risk of IDA in Europeans [15] and Asians [14, 25]. Our study showed that iron deficiency and iron deficiency anemia have significantly higher heterozygote allele’s genotype AG of rs855791 compared to control that agreed with Elmahdy et al., [26]. Shinta et al., [22] found that both iron deficiency and iron deficiency anemia were associated with minor homozygote allele of rs855791 genotype. On the other hand, Momodou et al., [27] didn’t find any association with iron status biomarkers.
Among the Asian, African, and European populations there is a significant divergence in the minor allele frequency distribution of SNPs [8, 17, 28]. The minor allele frequency (MAF) of SNP rs855791, rs4820268 and rs11704654 found to be less in African than Asian and European populations [17]. The difference may be attributed to the selective role of certain environmental conditions that alter the frequency of the genetic variants among populations [29]. Our study showed that MAF(G) of rs855791, (G) of rs4820268 and (T) of rs11704654 were 0.43, 0.45 and 0.17 respectively that were comparable to the European ancestry population but not to the African. This similarity may be due to mix of genes between Egyptians and Europeans by marriage in the past times where Europeans existed in our country for different purposes like trading, working or wars. Theoretically these differences in MAFs among populations could result in divergencies in the incidence of iron deficiency outcome [30]. However, the minor alleles for SNPs of certain population may be the major alleles in other populations [17]. For example, Shinta et al., [22] found that the minor allele of rs855791 genotype was [A] while it is the major allele in our work and [G] is our minor allele.
Disparities in the frequencies of minor risk alleles and linkage disequilibrium forms might explain the limited association of results between European, Asian, and African populations. So, investigating population-specific genetic variants, is recommended [27]. Wanjiku et al. [17], called for population-specific genome studies rather than inferring genetic data across populations.
Our study revealed that the minor homozygote allele genotype (GG) of rs4820268 is associated with the highest hepcidin gene expression fold, the lowest serum ferroprotein and iron store. Also, the minor homozygote allele genotype (GG) of rs855791 is associated with the lowest iron store, highest level of serum hepcidin and low serum ferritin. Higher hepcidin level reduces ferroprotein expression, inhibits iron absorption by the duodenum and iron mobilization from storage (Benyamin et al., Chambers et al.) [7, 8]. Thus, minor homozygote allele genotype (GG) of rs4820268 and rs855791 might be associated with Iron-refractory iron deficiency anemia (IRIDA) that might not respond to oral iron supplement. Higher levels of serum hepcidin were found to confirm the diagnosis of (IRIDA) [31, 32]. However, hepcidin assessment has no inter-laboratory comparison of assays and no standardization of units and reference ranges, that could facilitate the clinical use of a hepcidin [33, 34]. So, genetic assessment could be more supportive.
Shinta et al., [22] reported a lower serum ferritin in minor homozygote allele genotype of both rs855791 and rs4820268 even after controlling iron intake. They added that, they are naturally diminished by up to 10% of the total body iron store when compared to the major homozygote. A lower hemoglobin level, serum ferritin and serum hepcidin were detected in our studied children with homozygote major allele CC genotype of rs11704654 that agreed with results of Delbini, et al., [15]. Iron deficiency diminishes hepcidin expression and permits FPN1 to be available for transporting iron from intestine and tissues through the blood stream [35, 36]). Accordingly, this group may benefit from oral iron supplementation.
In a linear regression model, SNPs of rs4820268 explained 4% of the change in HB level, 5% of the variation in serum ferroprotein concentrations while SNPs of rs855791 explained 8.5% of the change in iron store and 8.2% of the change in serum hepcidin. Our results agreed with Tanaka et al., and Shinta et al. [5, 22], who found significant association of SNPs rs855791 and rs4820268 with iron status. Wanjiku et al., [17] could not discover any effect of these two SNPs on iron- status.
The relation of the three SNPs of our results with iron status and hepcidin were comparable to studies by others [7, 8, 15, 25, 28, 37]. On other hand, Momodou et al., [27] did not identify any TMPRSS6 SNP association with hepcidin concentration or iron status. Wanjiku et al., [17] attributed this to heterogeneity in genetic makeup and environmental disparities between populations that alter the frequency genetic variants.
Our study, urges for sub-population specific strategies to address iron deficiency & who can benefit from iron therapy to maintain normal erythropoiesis. Also, validation and discovery of further genetic variants correlated with iron status to clarify new processes and pathways that modify iron status, propounding better understanding into ID/IDA.
Limitation of this study
The children were recruited from a single center; it could be better to be a multicenter study which could not be fulfilled due to budget limitation.
Conclusion
Our work highlights the possible contribution of TMPRSS6 gene SNPs to low iron status in Egyptian children & who can benefit from oral iron therapy. A longitudinal study of the interaction between genetic variants and iron biomarkers on a larger sample size from different environmental sectors is recommended that will be arranged in near future study.
Availability of data and materials
All generated data is present in the manuscript.
Abbreviations
- GWAS :
-
Genome-wide association studies
- SNPs :
-
Single nucleotide polymorphisms
- IRIDA :
-
Iron-refractory iron deficiency anemia
- TMPRSS6 :
-
Transmembrane protease serine 6
- TF :
-
Transferrin
- MAF:
-
Minor Allele Frequency
- MCV:
-
Mean corpuscular volume
- CBC :
-
Complete blood count
- RBC :
-
Red blood cells
- TSAT:
-
Transferrin saturation
- sTfR:
-
Soluble transferrin receptor
- TIBC :
-
Total iron binding capacity
- BMI :
-
Body mass index
- CRP :
-
C reactive protein,
- HCT :
-
Hematocrit
References
WHO Global Database on Anemia. Worldwide Prevalence of Anemia, 1993–2005. Geneva, Switzerland: World Health Organization; 2008.
Teshome EM, Andang PEA, Osoti V, Terwel SR, Otieno W, Demir Y, et al. Daily home fortification with iron as ferrous fumarate versus NaFeEDTA: a randomized Kenyan children. BMC Med. 2017;15:89. https://doi.org/10.1186/s12916-017-0839-z.
Heeney MM, Finberg KE. Iron-Refractory Iron Deficiency Anemia (IRIDA). Hematol Oncol Clin North Am. 2014;28:637–52. https://doi.org/10.1016/j.hoc.2014.04.009.
Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–41.
Tanaka T, Roy CN, Yao W, Matteini A, Semba RD, Arking D, Walston JD, Fried LP, Singleton A, Guralnik J, et al. A genome-wide association analysis of serum iron concentrations. Blood J. 2010;115:94–6 Crossref.
Mleczko-Sanecka K, Roche F, Rita A, da Silva D, Call F D’Alessio, Ragab A, et al. Unbiased RNAi screen for hepcidin regulators links hepcidin suppression to proliferative Ras/RAF and nutrient-dependent mTOR signaling. Blood. 2014;10:1574–85.
Benyamin B, Ferreira MA, Willemsen G, Gordon S, Middelberg RP, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41:1173–5.
Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, Hoggart C, Bayele H, McCarthy MI, Peltonen L, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41:1170–2 CrossRef. PubMed.
Jin Li, Glessner JT, Zhang H, Hou C, Wei Z, Bradfield JP. GWAS of blood cell traits identifies novel associated loci and epistatic interactions in Caucasian and African American children. Hum Mol Genet. 2013;22(7):1457–64.
Finberg EK, Matthew MH, Dean RC, Yeşim A, Howard AP, Kip RH. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–71.
De Falco L, Mayka S, Laura S, Caroline K, Martina UM, Achille I, Laurent G, et al. Iron refractory iron deficiency anemia. Hematologica. 2013;6:845–53. https://doi.org/10.3324/haematol.2012.075515.
Ramsay AJ, Hooper JD, Folgueras AR, Velasco G, Lopez-Otin C. Matriptase-2 (TMPRSS6): A proteolytic regulator of iron homeostasis. Haematologica. 2009;6:840–9.
Pei S-N, Ma M-C, You H-L, Hung-Chun Fu, Kuo C-Y, Rau K-M, Wang M-C, Lee C-T. TMPRSS6 rs855791 polymorphism influences the susceptibility to iron deficiency anemia in women at reproductive age. Int J Med Sci. 2014;11(6):614–9.
Bhatia P, Singh A, Hegde A, Jain R, Bansal D. Systematic evaluation of pediatric cohort with iron refractory iron deficiency anemia (IRIDA) phenotype reveals multiple TMPRSS6 gene variations. Br J Haematol. 2017;177(2):311–8.
Delbini P, Vaja V, Graziadei G, Duca L, Nava I, Refaldi C, Cappellini MD. Genetic variability of TMPRSS6 and its association with iron deficiency anemia. Br J Haematol. 2010;151(3):281–4. https://doi.org/10.1111/j.1365-2141.2010.08349.x.
Momodou WJ, Cerami C, Clark TG, Prentice AM, Campino S. Differences in the frequency of genetic variants associated with iron imbalance among global populations. PLoS ONE. 2020;15(7):e0235141.
Gichohi-Wainaina WN, Melse-Boonstra A, Swinkels DW, Zimmermann MB, Feskens EJ, Towers GW. Common Variants and Haplotypes in the TF, TNF-a, and TMPRSS6 genes are associated with iron status in a female black south African population. J Nutr. 2015;145:945–53.
WHO. Iron deficiency: assessment, prevention, and control. A guide for program managers. Geneva: World Health Organization; 2001.
Egypt Demographic and Health Survey 2014. Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], and ICF International. Cairo, Egypt and Rockville, Maryland, USA: Ministry of Health and Population and ICF International; 2015.
Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–63.
Ayatollahi H, Mousavi Nezhad SF, Talebpour A, Badiei Z, Nezami H. Relation of hepcidin gene expression in blood mononuclear cells with iron overload severity among β-thalassemia major patients. Mol Biol Rep. 2020;47(12):9353–9. https://doi.org/10.1007/s11033-020-06012-2.
Shinta D, Adhiyanto C, Htet MK, Fahmida U. The Association of TMPRSS6 gene polymorphism and iron intake with iron status among under-two-year-old children in Lombok, Indonesia. Nutrients. 2019;11(4):878. https://doi.org/10.3390/nu11040878. PMID:31010126;PMCID:PMC652125.
Mayo O. A century of Hardy-Weinberg Equilibrium. Twin Res Hum Genet. 2008;11:249–56 CrossRef.
1000 Genomes.org. 1000 Genomes project. Cambridge (United Kingdom): European Bioinformatics Institute (EMBL-EBI); c2012. Available from: http://browser.1000genomes.org/index.htm. Cited 2014 Feb 1.
An P, Wu Q, Wang H, Guan Y, Mu M, Liao Y, Zhou D, Song P, Wang C, Meng L, et al. TMPRSS6 but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency anemia. Hum Mol Genet. 2012;21(9):2124–31. https://doi.org/10.1093/hmg/dds028. PMID: 22323359.
Elmahdy M, Mourad F, Elhakeem H, Gaber F. TMPRSS6 Gene Polymorphism and Serum Hepcidin in Iron Deficiency Anemia. Egypt J Hospital Med. 2018;73(7):7090–103.
Momodou W J, Susana Campino, Andrew M P, Carla C. Association of common TMPRSS6 and TF gene variants with hepcidin and iron status in healthy rural Gambians. Sci Rep. 2021;11(1). https://doi.org/10.1038/s41598-021-87565-5.
Gan W, Guan Y, Wu Q, An P, Zhu J, Lu L, Jing L, Yu Y, Ruan S, Xie D, et al. Association of TMPRSS6 polymorphisms with ferritin, hemoglobin, and type 2 diabetes risk in a Chinese Han population. Am J Clin Nutr. 2012;95:626–32.
Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admettla A, Pattini L, Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 2011;7:e1002355 CrossRef.
Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic non communicable diseases in low, middle, and high-income countries. Am Heart J. 2009;158:1–7.
Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–33.
Girelli D, Nemeth A, Swinkels DW. Hepcidin in diagnosis of iron disorders. Blood. 2016;127:2809–13.
Kroot JJ, Kemna EH, Bansal SS, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–52.
Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117:e218–25.
De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18(7):2569–78.
Hentze MW, Muckentheler MU, Galy B, Camaschella C. Regulation of mammalian iron metabolism. Cell. 2010;142:24–38.
Kullo IJ, Ding K, Jouni H, Smith CY, Chute CG. A genome-wide association study of red blood cell traits using electronic medical records. PLoS ONE. 2010;5:e130115.
World Medical Association, WMA Declaration of Helsinki. Ethical principles for medical research involving human subjects, (available at https://www.wma.net/policiespost/wma-declaration-of-helsinki-ethical-principles-formedical-research-involving-human-subjects/2013/).
Acknowledgements
We thank all the participants who shared in this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was part of a project entitled (Hematological and non-hematological consequences of iron deficiency in children: Genetic study and response to treatment) funded by National Research Centre, Cairo, Egypt (Grant Number: 11010150).
Author information
Authors and Affiliations
Contributions
Hanan M Hamed, Ayat A Motawie and Amany M Abd Al-Aziz: Conceptualization, methodology, project administration, funding acquisition and writing the manuscript. Eman El Bostany, Abbass A Mourad, Hassan M. Salama: Help and support in the clinical part. Hanan M. Hamed: statistical analysis. Solaf Kamel, Eman Mahmoud Hassan, Neveen A. Helmy, Gamila S. M. El-saeed, Eman A Elghoroury: visualization and investigation of the laboratory part of the study. All authors made contributions in the analysis of data, revising the manuscript and approved the final version before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the medical research ethics committee, National Research Centre (reference No. 16304).
Written informed consent was taken from all patients’ parents or legal guardians before enrolment in the study and after full explanation of the research & their role in it according to the World Medical Association Declaration of Helsinki (2013) [38]. Data was documented in the patients’ files and on special excel sheets. Confidentiality on handling the database was guaranteed and privacy of participants was ensured.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary table.
Genotype stratified by sex of all studied children.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hamed, H.M., Bostany, E.E., Motawie, A.A. et al. The association of TMPRSS6 gene polymorphism with iron status in Egyptian children (a pilot study). BMC Pediatr 24, 105 (2024). https://doi.org/10.1186/s12887-024-04573-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04573-w