Abstract
Background
The objective of the study was to evaluate data on early-onset neonatal invasive infections in western Sweden for the period 1997–2017. To identify changes in incidence, etiology and mortality and compare to previous studies from the same area starting from 1975.
Methods
Observational epidemiological, retrospective study on infants 0–6 days of age with a positive culture in blood and/or cerebrospinal fluid between 1997 and 2017. A comparison was made of the incidence between 2008 and 2017 compared to 1997–2007. Changes in the incidence of infections due to Group B streptococci, Staphylococcus aureus and aerobic Gram-negative rods were assessed from 1975.
Results
The total incidence, including both recognized pathogens and commensals as causative agents, was 1.1/1000 live births. The incidence declined from 1.4/1000 LB in 1997–2007 to 0.9/1000 LB in 2008–2017 but the case-fatality rate remained unchanged, (8/119 vs 7/90), at 7%. Among the 209 patients identified during 1997–2017 with sepsis or meningitis the most common organisms were Group B streptococci (40%, 84/209), S. aureus (16%, 33/209) and E. coli (9%, 18/209). The incidence of Group B streptococci infections went from 0.9/1000 live births 1987–1996 to 0.45/1000 live births 1997–2017 and all cases were within 72 h. The proportion of extremely preterm infants (< 28 weeks gestation) rose steadily during the study period but there was no rise in infections due to Gram-negative organisms. The spectrum of cultured organisms changed after 72 h as commensal organisms started to emerge.
Conclusion
There has been a decrease in the incidence of neonatal early-onset infections compared to previous studies in western Sweden. The incidence of GBS infections was not as low as in other reports. Further studies are needed to assess if screening-based intra partum antimicrobial prophylaxis instead of a risk factor-based approach for identifying candidates for intrapartum antimicrobial prophylaxis would be a better option for this study area.
Key notes
-
This study is one of the longest running follow-ups in the world, a follow-up of 43 years of early-onset neonatal infections.
-
The incidence of early-onset GBS infections is higher in Western Sweden compared to other local reports.
-
No difference in the incidence of early-onset GBS depending on the definition of early-onset being within 72 h or 7 days of life.
Similar content being viewed by others
Background
Early-onset (EO) neonatal infections is a major cause of mortality and morbidity [1]. Studies have shown an increase in EO infections due to Gram-negative bacteria, especially among very-low-birth-weight infants [2]. Group B Streptococcus (GBS) remains as one of the most common pathogen even though targeted interventions like intrapartum antimicrobial prophylaxis (IAP) have shown a significant reduction in the incidence of EO GBS infection [2,3,4,5]. A risk factor-based approach for identifying candidates for IAP was published in Sweden 2008 and is the current policy in Sweden [6]. Some cases, however do not present with any risk factors and in many other countries the strategy for IAP is based on prenatal screening of GBS in late pregnancy instead [3, 7, 8].
Early-onset (EO) infection has been defined in a variety of ways. Many international reports use the definition of EO infection being within 72 h after birth, whereas in studies on EO GBS the definition is often within the first week of life [2, 9,10,11].
Two studies on neonatal sepsis and meningitis – from the same region as the present study - have been performed. Documentation on neonatal invasive infection starting from 1975 makes our data one of the longest running databases on neonatal infections in the world. In the first study, including data from 1975 to 1986, the incidence of EO neonatal infection, within the first week of life, caused by recognized pathogens was 1.9/1000 live births (LB) with 17% case-fatality rate [12]. In the second study from 1987 to 1996, the incidence of EO infection had increased to 2.3/1000 LB but the case-fatality rate had declined to 9% [13]. One of the reasons for the increase in incidence was considered to be a better survival of prematurely born infants with a high susceptibility to infections and better survival due to improved neonatal care [13].
The aim of this study was to document the incidence and etiology of EO neonatal invasive infections and changes in mortality for the period 1997–2017 by making a comparison between the last 10 years (2008–2017) and 1997–2007 and to compare with two previous studies from the same population covering the period 1975–1996 [12, 13]. A second aim was to evaluate if a change in the definition on EO neonatal infections to < 72 h after birth, instead of within the first week of life, would lead to missing cases of EO GBS infections.
With follow-up of 43 years, from 1975 to 2017, we wanted to identify if targeted interventions and improved survival of very-low-birth-weight infants have had an impact on incidence, etiology and/or mortality.
Methods
This was an epidemiological, retrospective study on EO neonatal invasive infections with the definition of EO being 0–6 days after birth. All infants, within a week after birth, from whom a pathogenic organism was isolated from blood or cerebrospinal fluid (CSF) during the years 1997–2017 were included in the study. At the time of birth their mothers had to be living in Gothenburg or five surrounding municipalities in western Sweden. The data was obtained from the Clinical microbiology laboratory at Sahlgrenska University Hospital, Gothenburg. This laboratory unit serves all the maternity wards and the pediatric units at the Sahlgrenska University Hospital, where parturients in Gothenburg and surrounding municipalities are delivered. Data on the patients´ characteristics including gestational age, birthweight, type of delivery, Apgar score, and the performance of a lumbar puncture (LP) as well as outcomes were collected from the patients medical records. Risk factors as maternal fever and premature ruptures of membranes were collected from the mothers´ medical records.
The total number of LB from the study area between 1997 and 2017 was 184,853 children, 95,089 male (51.4%). Neonatal mortality 0–6 days of age was 1.4/1000 LB. Population data was retrieved from “The Swedish Medical Birth Register” (www.socialsyrelsen.se) and Statistics Sweden (www.scb.se/en).
Our primary outcome was the etiology of EO neonatal sepsis and meningitis, as determined from blood and CSF cultures between 1997 and 2017. A comparison was made of the incidence of EO neonatal infections between the last 10 years (2008–2017) compared to 1997–2007. Regarding changes in the incidence due to GBS, S. aureus and aerobic Gram-negative rods, changes were assessed from 1975 – the first year from which data on neonatal infections in the study area were collected.
At the laboratory, culturing, isolating and identifying of bacteria and fungi was performed according to validated methods used in the clinical routine diagnostics.
Blood was drawn when central intravascular lines were put in place or drawn peripherally. As a routine a minimum of 1 ml blood should have been drawn for culture.
Organisms defined as recognized pathogen included the following Gram-positive aerobic or facultative anaerobic bacteria: Staphylococcus aureus, Group A, B and C streptococci, pneumococci, Enterococcus spp., Actinomyces and Listeria monocytogenes, and the following Gram-negative aerobic or facultative anaerobic bacteria: Escherichia coli, Klebsiella spp., Haemophilus influenzae, Pseudomonas aeruginosa, Enterobacter spp., Proteus mirabilis, Bacteroides spp., Serratia marcescens and fungi (Candida spp). The occurrence of these bacteria was in all cases regarded as the cause of infection.
Cultures with commensal species or species of less clear clinical significance like coagulase-negative staphylococci (CoNS), Bacillus spp. and Burkholderia cepacia had to meet both of the following criteria to be considered the cause of an infection: 1. At least two of the following signs and symptoms as a change from baseline: apnea, bradycardia (heart rate < 100/min), temperature < 36.5 or > 38 °C. 2. Appropriate antibiotics prescribed for > 120 h and a central intravascular line in place within 72 h before the culture was drawn.
Cultures identifying commensal bacteria or species of less clear clinical significance without fulfilling the above criteria were excluded from the study.
We chose not to follow the criteria stated by Centers for Disease Control that a minimum of two positive blood cultures are required for a commensal-related sepsis since two cultures are seldom drawn as a routine at our unit and the definition was changed in year 2008 [14].
Multiple positive cultures from a single infant yielding the same pathogen and the same antibiotic susceptibility profile were considered to represent the same infectious episode.
Our secondary outcome was mortality of neonatal infections. Death related to infection was defined as death occurring within 7 days of culturing and clinical signs and symptoms of infection being documented as the direct cause of death.
The study was approved by the Ethics Committee of Gothenburg.
Statistical analysis
Demographic data were presented as mean or median after testing for normality with Kolmogorov-Smirnov test. Correlations between continuous outcomes were analysed using the Spearman test and Fisher’s exact test (two-tailed) was used for comparisons of proportions (http://www.socscistatistics.com/tests/fisher/Default2.aspx). Analyses were conducted in SPSS version 25.0 (IBM Corp). A p value < 0.05 was considered significant.
Results
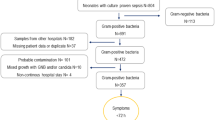
Totally, 558 positive cultures were evaluated and 349 were excluded as contaminants, (Fig. 1). During the period 1997–2017, there were 203 cases of EO sepsis and six cases of meningitis identified in 209 patients. The characteristics of infants according to gestational age are shown in Table 1. No case had missing information. The mean age of EO cases was 1.6 days (95%, CI 1.3–1.9). Most cases, or 160/209 (77%) occurred within 72 h, (Fig. 2). The total incidence of EO infection, including both recognized pathogens and commensals as causative agents, was 1.1/1000 LB. The incidence declined from 1.4/1000 LB in 1997–2007 to 0.9/1000 LB in 2008–2017, (p = 0.004) and the case-fatality rate remained unchanged, (8/119 vs 7/90, (p = 0.8)) at 7%. The three most common organisms isolated from culture were GBS (40%), S. aureus (16%) and E. coli (9%), (Table 2). The proportion of extremely preterm infants (< 28 weeks gestation) among the EO affected group rose steadily during the study period, and was significantly higher 2008–2017 compared to 1997–2007 (25/90 vs 12/119, p = 0.002).
During the years 1997–2017 the incidence of GBS, S. aureus and aerobic Gram negative rods was 154 cases (0.8 cases/1000 LB) compared to 295 cases (1.8 cases/1000 LB) 1975–1996 (p < 0.0001), (Fig. 3).
Thirty-five (35/209, 17%) LPs yielded six cases with meningitis. One infant was born preterm in week 30 and had GBS cultured from CSF. The other five infants were all born at term. There were four cases of GBS, one case of Enterococcus and one case of E. coli. Mean age at diagnosis was 1.3 days. All infants, except the one with Enterococcus meningitis, had blood cultures yielding the same causative agent.
Group B streptococci
The incidence of EO GBS infections 1997–2017 was 0.45/1000 LB and all cases of EO GBS were within 72 h, (Fig. 2). The majority, 52/84 (62%) of the infants were born at term. The mean birth weight was 3038 g (95% CI 2808–3269). The case-fatality rate was 8%, (7/84). Even though the incidence went from 0.5 cases/1000 LB 1997–2007 to 0.4 cases/1000 LB 2008–2017 the reduction in EO GBS infections after the publication of IAP guidelines in 2008 was not significant (p = 0.4). The incidence did not change after 2011 (0.4 cases/1000 LB), when the approach was implemented systematically.
Gram-negative bacteria
The incidence of all EO Gram-negative infections was 0.25/1000 LB and the case-fatality rate was 13% (6/47). The incidence did not change between 1997 and 2007 and 2008–2017 (0.3/1000 LB vs. 0.2/1000 LB (p = 0.2)). The incidence of EO infections caused by aerobic Gram-negative rods during 1997–2017 was 0.2/1000 LB and the case-fatality rate was 11% (4/37). The most common agent was E. coli with an incidence of 0.1/1000 LB. Mean age at time of culture was 2.2 days (95% CI 1.48–2.95) and the mean birth weight was 2497 g (95% CI 2041–2953). Among the infants were 17/37 (46%) born prematurely.
Staphylococcus aureus
Among the infants with EO S. aureus infection 15/33 (45%) were preterm. The mean birth weight was 2484 g (95% CI 2037–2931) and the mean age at time of culture was 2.8 days (95% CI 1.9–3.6). The incidence of S. aureus EO infections was 0.2/1000 LB and the case-fatality rate was 3%. The incidence declined from 0.3/1000 LB 1997–2007 to 0.1/1000 LB 2008–2017 (p = 0.013).
Coagulase negative staphylocci
The incidence of EO CoNS infections was 0.1/1000 LB and it remained almost the same between 1997 and 2007 and 2008–2017 (0.08/1000 LB vs. 0.1/1000LB (p = 0.6)). All infants (18/18) with EO CoNS infection were born prematurely and the mean birth weight was 843 g (95% CI 719–1008). Mean age at time of culture was 5.3 days (95% CI 4.9–5.7). All cases with EO CoNS had a central line or had had a central line removed within 72 h of culture taken.
Other gram-positive bacteria
The mean birth weight among infants with EO infections due to other Gram-positive bacteria was 3205 g (95% CI 2798–3612) and all had a positive culture drawn within 48 h after birth. The incidence of EO Enterococcus infections declined from 0.11/1000 LB during 1997–2007 to 0.02/1000 LB 2008–2017, (p = 0.017) but the incidence of other bacteria within this group remained unchanged.
Fatal cases
Among the fatal cases 13/15 (87%) were born prematurely and their mean birth weight was 1518 g (95% CI 939–2098) compared to a prematurity rate of 84/194 (43%) and a mean birth weight of 2748 g (95% CI 2573–2924) among the infants who survived, (p = 0.002). The pathogens with the highest mortality were Gram-negative bacteria, Klebsiella pneumoniae at 33% (2/6) and E. coli at 11% (2/18). The only case of Proteus mirabilis infection was fatal. Even though the proportion of extremely preterm infants (< 28 weeks gestation) rose from 10% (12/119) 1997–2007 to 28% (25/90) 2008–2017 (p = 0.002) the case-fatality rate remained unchanged, (8/119 vs 7/90, (p = 0.8)).
Discussion
This study shows that there has been a reduction in the incidence of neonatal EO invasive infections, including both recognized pathogens and commensals as causative agents, in the Gothenburg area from 1.4/1000 LB in 1997–2007 to 0.9/1000 LB in 2008–2017, (p = 0.004). Furthermore comparison to previous study from the same area showed a reduction from 2.3 cases/1000 LB in 1987–1996 to 1.1 cases/1000 LB in 1997–2017 (p < 0.0001) [13]. There was no statistical change in the case-fatality rate of 7% in 1997–2017 compared to 9% in 1987–1996 [13]. However when comparing the present study to the study from 1975 to 1986, when the incidence was 1.9 cases/1000 LB, the case-fatality rate was much higher at 17%, (p = 0.007) [12]. The reason for the decreasing incidence is probably due to general advances in neonatal care but the overall neonatal mortality at age 0–6 days in western Sweden went from 1.7/1000 LB in 1997–2007 to 1.2/1000 LB in 2008–2017 (p = 0), see Additional file 1 [15].
Studies from England and the United States have shown similar incidence as in our study. In a multicenter study from England with the definition of EO ≤ 48 h of age the incidence of EO sepsis was 0.9/1000 LB [16] and reports from the United States estimated the incidence, with the definition < 72 h, between 0.77–1/1000 LB [2, 17, 18]. A retrospective cohort from Australia showed an incidence of culture-proven EO infections with the definition < 72 h being 0.69/1000 LB but CoNS and other possible contaminants were not included [19]. A study from Norway reported a lower incidence of culture-confirmed EO infection, 0.5/1000 LB. However, they excluded all cases with single blood cultures yielding possible contaminants and CSF cultures were not considered [20].
The proportional rise of extremely preterm infants (< 28 weeks gestation) from 10 to 28% was probably due to a combination of factors. The percentage of premature neonates among LB infants had increased and they are more susceptibility to infections compared to term infants [1, 15]. At the same time the incidence among infants born closer to term decreased which might have been as a result of IAP.
The majority of EO cases due to recognized EO pathogens were cultured within 72 h. In our study, all EO GBS cases were diagnosed within 72 h after birth, and the spectrum of cultured organisms changed thereafter and organisms like CoNS and Candida albicans emerged as common causes of infection, (Fig. 2). Infections after 72 h are more likely hospital-acquired, due to vascular access or other NICU-procedures.
Reasons for the high contamination rate of 349/558 (63%) is probably multi-factorial. There is a routine at our clinic that blood should be drawn for culture whenever a central line is put in place, whether there is a suspected infection or not. The diagnostic yield of cultures are influenced by the volume taken and from selected patients with reasonable suspicion of bacterial infection and most cases with contamination did not have any clinical suspicion of infection. Phlebotomy for blood culture is carried out by registered nurses, not by dedicated phlebotomy teams and they are often drawn at the same time as other blood tests to to minimize number of punctures of veins. It is important to follow policies and routines regarding blood-culture techniques with correct usage of appropriate antisepsis to avoid accidental inoculation of environmental and skin bacteria.
Six cases of meningitis were identified. All cases but one had the same bacteria cultivated in blood as in CSF. However, a LP was only performed in 35/209 (17%) of cases with a positive blood culture. LP is not recommended in asymptomatic full-term neonates and it is not performed if the neonate has no clinical signs suggesting meningitis [21]. This might be a problem as signs and symptoms of meningitis are often subtle. Even though meningitis in EO infections is quite uncommon a LP should be performed in any infant whose clinical course or laboratory data strongly suggest bacterial infection since it is the only way to rule out meningitis. Confirming the diagnosis of meningitis with cultures can be challenging given that a LP is often delayed and antibiotic treatment may result in negative cultures. Analysis of CSF by PCR for detection of bacterial DNA may be more sensitive in these situations, but the results of such diagnostic tests were not considered in the present study.
Many obstetricians in the region knew about the risk factor-based IAP guidelines and were acting accordingly before the publication of the guidelines in 2008 even though the approach was not implemented systematically until 2011. It is therefore impossible to pinpoint a specific year when the effect of risk factor-based IAP started to have a declining effect on EO GBS infections. EO GBS infections have been declining and the incidence during 1997–2017 at 0.45 cases/1000 LB was significantly lower compared to the previous study, covering the years 1987–1996, with an incidence of 0.9 cases/1000 LB, (p < 0.0001), (Fig. 3).
Even though the incidence of EO GBS continued to decline further to 0.4 cases/1000 LB after the systematically start of risk factor-based IAP after 2011, the reduction is not as low as in other reports. A national population-based Swedish cohort study of EO GBS infections during 2006–2011 reported a decrease from 0.40 to 0.30 cases/1000 LB [5]. There might be a regional difference in the adherence to the guidelines or missed opportunities for IAP and that needs to be studied further.
A study from the United States, where screening-based IAP was implemented in 2002, reported an incidence of EO GBS at 0.23 cases/1000 LB in 2015 [22]. Although the studies are not comparable, the lower incidence in the United States as compared to our study may indicate that a screening-based IAP could have a greater impact on EO GBS infection than a risk factor-based approach. A study from London, United Kingdom, reported a decrease in incidence of EO GBS infection from 0.99/1000 LB with risk factor-based IAP to 0.33/1000 LB with screening-based IAP and after returning back to risk factor-based IAP the incidence went up again [23, 24]. Missed opportunities for prevention occurs both for risk-based IAP and screening-based IAP [5, 22].
Studies have shown an increase in EO Gram-negative sepsis, especially infections caused by E. coli [2]. Since very-low-birth-weight infants are more likely to have invasive infection due to Gram-negative organisms and E. coli in particular, rather than Gram-positive organisms like GBS, the increasing rate could be a reflection of improved survival of very-low-birth-weight infants [25, 26]. In our study we did not see a rise in infections due to Gram-negative organisms even though the number of singleton live born infants in Sweden weighing < 1000 g increased from 0.22% (1975–1987) to 0.38% (2008–2016), (p < 0.0001), (Fig. 3) [15].
One reason for the declining trend observed for S. aureus and aerobic Gram-negative rods as well as GBS might be that IAP affects the outcome of blood cultures. These infants get clinical signs of sepsis but cultures remain negative. During the last decade there has been a lot more focus on hygiene within neonatal units with increased awareness and surveillance of compliance to routines even though they are targeted towards affecting late-onset infections. These factors may have contributed to the overall declining trend in EO infections.
The main strength of this study is the long follow-up time of 43 years. The study includes both patients within a neonatal unit and infants within a community. However, only infants living within Gothenburg and surrounding municipalities are included which effects the study’s generalizability. The study is retrospective and diagnostic/reporting bias is always a concern especially with this long follow-up time and in regards of separating true infections from commensal bacteria. However, since this study only includes EO-infections the risk for this of kind of bias affecting the true incidence is small. In the first two studies from 1975 to 1986 and 1987–1996, CoNS and other commensal bacteria are excluded which could mean that the reduction in incidence of traditional neonatal EO invasive infections compared to previous studies might be underestimated. Regarding culture techniques, the systems used for blood culturing have developed and improved over the years, and this might have increased the ability to detect certain fastidious, “difficult to culture” microorganisms. However, the vast majority of the episodes of early onset neonatal sepsis are caused by bacteria which are easily cultured, detected, and identified in the laboratory, and have been so for many decades, e.g. GBS, S. aureus, CoNS, E. coli and other aerobic gram-negative rods. Therefore we do not believe that changes in culture techniques have had any impact on the incidence. Also, if there had been an impact of culture improvements, that would have resulted in an increased incidence over time, but we see the opposite pattern.
We included cases with a single blood-culture even though they showed commensal bacteria. This might have overestimated the incidence of infections caused by these bacteria. Low sample volume, especially in very-low-birth-weight infants, is a major caveat in clinical practice regarding blood cultures and decreases culture sensitivity. Since we only included culture-positive infections we might have underestimated the true incidence of neonatal infections.
Conclusions
In conclusion the present study showed that the incidence of EO neonatal invasive infections has decreased compared to previous studies within the same population despite a proportional rise of extremely preterm infants (< 28 weeks gestation). The start of a risk factor-based approach for IAP may have contributed but the decrease in the incidence of EO GBS infections was not as significant as in other reports. Further studies are needed to assess if there are missed opportunities for IAP or if screening-based IAP would be a better option.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to that the ethics committee specifically state that no data, which can identify a patient can be publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- CoNS:
-
Coagulase-negative staphylococci
- CSF:
-
Cerebrospinal fluid
- EO:
-
Early onset
- GBS:
-
Group B streptococci
- IAP:
-
Intra partum antimicrobial prophylaxis
- LB:
-
Live births
- LP:
-
Lumbar puncture
References
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal Sepsis. Clin Microbiol Rev. 2014;27(1):21. https://doi.org/10.1128/CMR.00031-13.
Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID, Hale EC et al: Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011, 127(5):817. DOI: https://doi.org/10.1542/peds.2010-2217
Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: Experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31:D20–6. https://doi.org/10.1016/j.vaccine.2012.11.056.
Colbourn TE, Asseburg C, Bojke L, Philips Z, Welton NJ, Claxton K, Ades AE, Gilbert RE: Preventive strategies for group B streptococcal and other bacterial infections in early infancy: cost effectiveness and value of information analyses. (Clinical report). BMJ 2007, 335(7621):655. DOI: https://doi.org/10.1136/bmj.39325.681806.AD
Håkansson S, Lilja M, Jacobsson B, Källén K. Reduced incidence of neonatal early-onset group B streptococcal infection after promulgation of guidelines for risk-based intrapartum antibiotic prophylaxis in Sweden: analysis of a national population-based cohort. Acta Obstet Gynecol Scand. 2017;96(12):1475. https://doi.org/10.1111/aogs.13211.
The Swedish National Board of Health and Welfare: Prevention av tidiga infektioner med grupp B streptokocker (GBS) hos nyfödda [Prevention of early-onset GBS infection in newborns]. In Swedish. Available online at: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/8836/2008-130-7_20081307.pdf ().
Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, Hakansson S, Hod M, Hughes R, Kurtzer M et al: Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Mater Fetal Neonatal Med, 2015, Vol28(7), p766–p782 2015, 28(7):766–782. DOI:https://doi.org/10.3109/14767058.2014.934804.
Doare K, O’driscoll M, Turner K, Seedat F, Russell N, Seale A, Heath P, Lawn J, Baker C, Bartlett L, et al. Intrapartum Antibiotic Chemoprophylaxis Policies for the Prevention of Group B Streptococcal Disease Worldwide: Systematic Review. Clin Infect Dis. 2017;65:S143. https://doi.org/10.1093/cid/cix654.
Bizzarro MJ, Shabanova V, Baltimore RS, Dembry L-M, Ehrenkranz RA, Gallagher PG. Neonatal Sepsis 2004-2013: the rise and fall of coagulase-negative staphylococci. J Pediatr. 2015;166(5):1193–9. https://doi.org/10.1016/j.jpeds.2015.02.009.
Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–80. https://doi.org/10.1016/S0140-6736(17)31002-4.
Schrag SJ, Schuchat A: Easing the burden: characterizing the disease burden of neonatal group B streptococcal disease to motivate prevention. (Editorial Commentary). Clin Infect Dis 2004, 38(9):1209. DOI:https://doi.org/10.1016/j.vaccine.2012.11.056
Tessin I, Trollfors B, Thiringer K. Incidence and etiology of neonatal septicaemia and meningitis in western Sweden 1975-1986. Acta Paediatr Scand. 1990;79(11):1023.
Persson E, Trollfors B, Brandberg LL, Tessin I: Septicaemia and meningitis in neonates and during early infancy in the Göteborg area of Sweden. Acta Paediatr (Oslo, Norway : 1992) 2002, 91(10):1087. DOI: https://doi.org/10.1111/j.1651-2227.2002.tb00104.x
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. https://doi.org/10.1016/j.ajic.2008.03.002.
Socialstyrelsen (The Swedish National Board of Health and Welfare). Statistics on Pregnancies, Deliveries and Newborn Infants 2016. In Swedish. 2018. Available on line at: http://www.socialstyrelsen.se/publikationer2018/2018-1-6:Socialstyrelsen. Accessed 19 June 2018.
Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, Robinson MJ, Collinson A, Heath PT. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F9. https://doi.org/10.1136/adc.2009.178798.
Cohen-Wolkowiez KM, Moran MC, Benjamin HD, Cotten KC, Clark BR, Benjamin BD, Smith BP. Early and late onset Sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28(12):1052–6. https://doi.org/10.1097/INF.0b013e3181acf6bd.
Weston JE, Pondo MT, Lewis IM, Martell-Cleary JP, Morin LC, Jewell JB, Daily JP, Apostol JM, Petit JS, Farley JM, et al. The burden of invasive early-onset neonatal Sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30(11):937–41. https://doi.org/10.1097/INF.0b013e318223bad2.
Braye K, Foureur M, de Waal K, Jones M, Putt E, Ferguson J, East CE. Epidemiology of neonatal early-onset sepsis in a geographically diverse Australian health district 2006-2016. PLoS One. 2019;14(4). https://doi.org/10.1371/journal.pone.0214298.
Fjalstad WJ, Stensvold JH, Bergseng SH, Simonsen EG, Salvesen EB, Rønnestad EA, Klingenberg EC. Early-onset Sepsis and antibiotic exposure in term infants: a Nationwide population-based study in Norway. Pediatr Infect Dis J. 2016;35(1):1–6. https://doi.org/10.1097/INF.0000000000000906.
Johnson CE, Whitwell JK, Pethe K, Saxena K, Super DM. Term newborns who are at risk for sepsis: are lumbar punctures necessary? Pediatrics. 1997;99(4):E10. https://doi.org/10.1542/peds.99.4.e10.
Nanduri S, Petit S, Smelser C, et al. Epidemiology of invasive early-onset and late-onset group b streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 2019. https://doi.org/10.1001/jamapediatrics.2018.4826.
Gopal Rao G, Nartey G, McAree T, O'Reilly A, Hiles S, Lee T, Wallace S, Batura R, Khanna P, Abbas H, et al. Outcome of a screening programme for the prevention of neonatal invasive early-onset group B Streptococcus infection in a UK maternity unit: an observational study. BMJ Open. 2017:7(4). https://doi.org/10.1136/bmjopen-2016-014634.
Gopal Rao G, Townsend J, Stevenson D, Nartey G, Hiles S, Bassett P, Lamagni T, Nicholl R. Early-onset group B (EOGBS) infection subsequent to cessation of screening-based intrapartum prophylaxis: findings of an observational study in West London, UK. BMJ Open. 2017;7(11):e018795. https://doi.org/10.1136/bmjopen-2017-018795.
Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB, Manzoni P, Jacqz-Aigrain E, Kaguelidou F, Cohen-Wolkowiez M. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88:S69–74. https://doi.org/10.1016/S0378-3782(12)70019-1.
Lim WH, Lien R, Huang Y-C, Chiang M-C, Fu R-H, Chu S-M, Hsu J-F, Yang P-H. Prevalence and pathogen distribution of neonatal Sepsis among very-low-birth-weight infants. Pediatr Neonatol. 2012;53(4):228–34. https://doi.org/10.1016/j.pedneo.2012.06.003.
Acknowledgements
We thank Dr. Nils Ekvall and Dr. Jenny Lövbrand for participating in acquisition of clinical data. We also appreciate help with proofreading from Dr. Ólöf Viktorsdóttir.
Funding
The study was financed with grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF- agreement (70150 and 77360). Margrét Johansson Gudjónsdóttir has received funding from The Samariten foundation for paediatric research. These funders had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. For the remaining authors none were declared. Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
MJG had the primary responsibility in acquisition, analysis and interpretation of data. She wrote the major part of the manuscript and performed the statistical analyses. AE participated in the design of the study, acquisition of data and revision of the manuscript. EH was main responsible and first author of the second of the Incidence studies in neonatal invasive infection in the Gothenburg area. She participated in acquisition of data, the design of the study and revision of the manuscript. IA participated in acquisition of data and revision of the manuscript. IT was one of whom initiated the study and participated in its design and coordination. He was main responsible and first author of the first of the Incidence studies in neonatal invasive infection in the Gothenburg area. He participated in acquisition of data and revision of the manuscript. BT was one of whom initiated the study and participated in its design and coordination. He participated in the revision of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Gothenburg. The committee did not require individual consent, because the clinical data were obtained in retrospect, when many patients and/or relatives could not be found.
Consent for publication
Not Applicable.
Competing interests
None of the authors have any financial or non-financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
List of live births within the study area along with the incidence of neonatal (0-6 days) mortality per 1000 live births per year within western Sweden between 1975 - 2017. Numbers were retrieved from the statistical database of The National Board of Health and Welfare, Stockholm, Sweden on 22-10-2019.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Johansson Gudjónsdóttir, M., Elfvin, A., Hentz, E. et al. Changes in incidence and etiology of early-onset neonatal infections 1997–2017 – a retrospective cohort study in western Sweden. BMC Pediatr 19, 490 (2019). https://doi.org/10.1186/s12887-019-1866-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-019-1866-z