Abstract
Background
Congenital diaphragmatic hernia (CDH) is a congenital malformation associated with life-threatening pulmonary dysfunction and high neonatal mortality. Outcomes are improved with protective ventilation, less severe pulmonary pathology, and the proximity of the treating center to the site of delivery. The major CDH treatment center in Croatia lacks a maternity ward, thus all CDH patients are transferred from local Zagreb hospitals or remote areas (outborns). In 2000 this center adopted protective ventilation for CDH management. In the present study we assess the roles of protective ventilation, transport distance, and severity of pulmonary pathology on survival of neonates with CDH.
Methods
The study was divided into Epoch I, (1990–1999, traditional ventilation to achieve normocapnia), and Epoch II, (2000–2014, protective ventilation with permissive hypercapnia). Patients were categorized by transfer distance (local hospital or remote locations) and by acuity of respiratory distress after delivery (early presentation-occurring at birth, or late presentation, ≥6 h after delivery). Survival between epochs, types of transfers, and acuity of presentation were assessed. An additional analysis was assessed for the potential association between survival and end-capillary blood CO2 (PcCO2), an indirect measure of pulmonary pathology.
Results
There were 83 neonates, 26 in Epoch I, and 57 in Epoch II. In Epoch I 11 patients (42 %) survived, and in Epoch II 38 (67 %) (P = 0.039). Survival with early presentation (N = 63) was 48 % and with late presentation 95 % (P <0.001). Among early presentation, survival was higher in Epoch II vs. Epoch I (57 % vs. 26 %, P = 0.031). From multiple logistic regression analysis restricted to neonates with early presentation and adjusting for severity of disease, survival was improved in Epoch II (OR 4.8, 95%CI 1.3–18.0, P = 0.019). Survival was unrelated to distance of transfer but improved with lower partial pressure of PcCO2 on admission (OR 1.16, 95%CI 1.01–1.33 per 5 mmHg decrease, P = 0.031).
Conclusions
The introduction of protective ventilation was associated with improved survival in neonates with early presentation. Survival did not differ between local and remote transfers, but primarily depended on severity of pulmonary pathology as inferred from admission capillary PcCO2.
Similar content being viewed by others
Background
Congenital diaphragmatic hernia (CDH) is a congenital malformation associated with life-threatening pulmonary dysfunction and high neonatal mortality. In neonates with CDH the presence of respiratory distress after delivery indicates severe pulmonary involvement that requires medical management which includes mechanical ventilatory support prior to undergoing surgical correction [1].
The University Hospital Center (UHC) in Zagreb Croatia is the national treatment center for neonates with CDH. Before 2000, neonates with CDH cared for at UHC underwent intermittent mandatory ventilation (IMV) to achieve hyperventilation, a strategy based on the premise that respiratory alkalosis may help control degree of pulmonary hypertension [1–3]. However, ventilation of hypoplastic lungs with high tidal volumes may induce volutrauma associated with intra-alveolar hemorrhage and interstitial pulmonary edema [4–6]. It was subsequently determined that protective ventilation which uses the minimal pressure and volume settings to achieve acceptable oxygenation while allowing for hypercapnia can reduce lung injury [7–11]. Therefore, in the year 2000 UHC adopted protective ventilation for neonates with CDH.
The UHC lacks a maternity ward, thus all neonates with CDH are either transferred from local Zagreb hospitals or remote areas in Croatia. This practice is concerning because remote transfer of CDH neonates may increase mortality [12]. The primary aim of this study was to estimate the effects of the introduction of protective ventilation on survival. A secondary aim was to examine whether remote transfer, compared to local transfer, impacted survival.
Methods
This study was approved by the Institutional Ethics Committee of the University Hospital Centre (UHC), Zagreb, Croatia. Due to retrospective design of this study the written consent was waived by the UHC Institutional Ethics Committee.
Study design
A retrospective cohort study of neonates with CDH born between January 1, 1990 and December 31, 2014 who were treated at a single institution. The primary aim was to assess whether the survival of neonates with CDH improved after the year 2000 with the introduction of protective ventilatory strategy. Since neonates with early presentation are expected to have lower survival, the effects of protective ventilation both overall and in the subset of neonates with early presentation was assessed. The secondary aim was to explore the association between the type of transfer (local vs. remote) and survival. Because the time period covered by conventional ventilatory strategies was characterized by a national conflict (Croatian War of Independence 1991–1995) and travelling was hampered, this association was explored following the introduction of protective ventilation.
Study setting
The neonatal intensive care unit (NICU) of University Hospital Center, Zagreb Croatia. The UHC is the national treatment center for neonates with CDH. UHC lacks a maternity ward; therefore all subjects in this study are outborns.
Definitions
Epoch I is the period between January 1, 1990 and December 31, 1999, during which time hyperventilation with non-synchronized ventilation was used. Epoch II is the period between January 1, 2000, and December 31, 2014, during which time a permissive hypercapnia using protective ventilation was used. Outborn status refers to infants born in another hospital requiring transport to higher level of care. Patients were defined as “local transfers” if they came from local Zagreb hospitals or “remote transfers” from the rest of the country. Early presentation is when respiratory distress is evident immediately after delivery requiring endotracheal intubation and mechanical ventilation. Late presentation, on the other hand, is when the onset of breathing difficulties is delayed >6 h after delivery.
Management strategies
Epoch I
Neonates were sedated, paralyzed, and ventilated with intermittent mandatory ventilation (IMV) to achieve respiratory alkalosis and postductal oxyhemoglobin saturation above 90 % to ameliorate pulmonary hypertension. This strategy often required higher peak inspiratory pressures (PIP), respiratory rates and oxygen concentrations. In those with available records of PIP, the values were between 30 and 40 cmH2O.(new line and header) Epoch II (new line) Ventilation was protocolized, and all neonates received protective ventilation aimed to minimize volutrauma with the use of minimal pressure and volume settings and inspired oxygen concentration to achieve acceptable preductal oxygenation saturations (≥85 %) while permitting hypercapnia (≤65 mmHg). Only two modes of ventilation were used during this time period: assist-control plus volume limit mode (A/C + VL) and pressure support ventilation with volume guarantee mode (PSV + VG). Both modes fully supported synchronized ventilation aided by controlled “demand flow” circuitry which synchronizes inspiratory gas delivery close to the breathing pattern of the neonate. Ventilatory settings were set per protocol. In the A/C + VL mode the tidal volume limit was 6 mL/kg, PEEP of 2–3 cm H2O, PIP ≤ 25 cmH2O, and the backup respiratory rate 40 per min. If respiratory acidosis (obtained from preductal capillary blood) was present (pH <7.25, PcCO2 >65 mmHg), ventilatory settings were changed by increasing PIP by 2 cmH2O (until maximum PIP of 25 cmH2O was achieved). In patients ventilated with PSV + VG mode the mean VG used was 4.0 mL/kg (range 2.6–5.5 mL/kg), PEEP 3.8 (range 2.5–5) cm H2O, PIP ≤ 25 cmH2O, and backup respiratory rate 40/min. If severe respiratory acidosis was present, VG was increased to a maximum 5.5 mL/kg exceeding the PIP limit if needed. With this strategy sedation and muscle paralysis were infrequently used and only in newborns with patient-ventilator asynchrony. High frequency oscillation ventilation (HFOV) was a rescue treatment for neonates who continued to have hypoxia and hypercarbia (PcCO2 >65 mmHg) despite optimization of either ventilatory mode. During Epoch II inhaled nitric oxide (iNO) became available and was used for neonates with ductal shunting (difference between preductal and postductal oxygen saturation >5 %), refractory preductal hypoxemia (PcO2 <60 mmHg with FiO2 >80 %), and for elevated right ventricular pressures. Surgical repair was typically done following initial optimization of respiratory parameters.
Data collection
Patient variables that were abstracted included demographic information (date of birth, sex, place of birth [local vs. remote transfers]); birth information (gestational age, birth weight, Apgar scores); CDH information (prenatal diagnosis, acuity of presentation, pulmonary hypertension, type of CDH, presence of peritoneal sac and diaphragmatic aplasia), and physiologic variables obtained early during hospitalization (admission preductal capillary blood gases, lowest body temperature and lowest mean blood pressure within 12 h of admission). Probability of survival (POS) was assessed from the equation proposed by the Congenital Diaphragmatic Hernia Study Group [13], to categorize neonates into 3 POS score groups based on birth weight and 5-min Apgar score: low (0 %–33 %), moderate (34 %–66 %), and high (67 %–100 %) predicted survival groups. Variables regarding CDH management abstracted included mechanical ventilation mode; occurrence of preoperative pneumothorax; use of iNO, surfactant, and/or vasoactive support; type of surgical repair (primary vs. non-primary with patch); and time between delivery and surgery. Survival to hospital discharge was noted.
Statistical analysis
Data are presented using mean ± SD or median [25th, 75th percentile] for continuous variables, and frequency percentages for categorical variables. Characteristics were compared between groups using the 2- sample t-test, rank sum test, Chi square test, or Fisher’s exact test. Logistic regression was used to assess whether hospital survival was associated with epoch after adjusting for POS score. In order to assess for trends in survival over time, before and after the introduction of the protective ventilation, logistic regression analyses was performed for each time period with hospital survival as the dependent variable and calendar year as the continuous explanatory variable. To explore the association between local vs. remote transfer on survival we focused on neonates with early presentation of symptoms during Epoch II. Survival was compared using the Chi square test. In all cases 2-tailed P values <0.05 were considered statistically significant. Data were analyzed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
Between January 1, 1990, and December 31, 2014, there were 83 neonates who received formal intensive care treatment, 26 were treated in Epoch I, and 57 in Epoch II. Sixteen additional patients were excluded for various reasons (see Fig. 1). All neonatal transfers to our medical center were accomplished by ground ambulance. Ventilation during transfer was accomplished via hand-held self-inflating bags for all neonates in Epoch I, and for the majority of neonates in Epoch II. In Epoch II, 9 neonates, 1 local (survived) and 8 remote (3 died) transfers, received ventilation through a pressure controlled ventilator integrated in transport incubator.
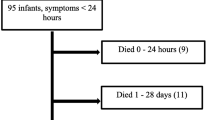
Patients with congenital diaphragmatic hernia. Exclusions: other types of congenital diaphragmal hernia (n = 4): Morgagni hernia, paraesophageal hernia, central hernia, severe diaphragmatic eventration. Lethal anomaly (n = 1) Edwards syndrome (trisomy 18) *Early presentation is defined as respiratory distress immediately after birth requiring endotracheal intubation; †Late presentation is defined as respiratory distress either absent or present >6 h after delivery
Table 1 summarizes demographic and disease characteristics of neonates with CDH. Table 2 summarizes admission capillary blood gases and vital signs within 12 h of admission. Table 3 summarizes medical and surgical interventions. During Epoch I all neonates received IMV, while in Epoch II protective ventilation was utilized, including 8 patients who received rescue HFOV. Surfactant and vasoactive support increased and iNO became available during Epoch II.
Overall cohort survival
In Epoch I 11 of 26 patients (42 %) survived to discharge, compared to 38 of 57 (67 %) in Epoch II (OR = 2.7, 95 % CI 1.1 to 7.1, P = 0.039 for survival during Epoch II vs. Epoch I). The percentage of patients who died after being admitted without surgery was similar between Epochs I and II, 27 % vs. 21 %, respectively (P = 0.815). Among those who were discharged, length of stay did not differ significantly between Epoch I vs. Epoch II (37 [19, 55] vs. 34 [23, 67] days; rank sum test P = 0.615). Calculated POS score negatively correlated with admission end-capillary partial pressure of carbon dioxide, PcCO2 (r = −0.35, P = 0.008). Survival was similar for patients who were local vs. remote transfers, (53 % vs. 67 %, P = 0.216). No temporal trends in survival were observed over calendar time during Epoch I (P = 0.490) or Epoch II (P = 0.373). From an analysis restricted to Epoch II, there was no difference in survival between neonates who were prenatally diagnosed with CDH compared to those without prenatal diagnosis (P = 0.174).
Early vs. late presentation survival
Sixty-three neonates had early presentation and their survival was worse compared to those with late presentation (48 % vs. 95 % survival, P <0.001). Among early presentation neonates, survival was higher in Epoch II vs. Epoch I (25 of 44, 57 % vs. 5 of 19, 26 %, OR 3.7 95 % CI 1.1–12.0, P = 0.031). From multiple logistic regression analysis restricted to early presentation neonates and adjusting for POS score, survival improved in Epoch II compared to Epoch I (OR 4.8, 95 % CI 1.3–18.0, P = 0.019). In an analysis restricted to neonates with early presentation, no temporal trends were observed over calendar time during Epoch I (P = 0.304) or Epoch II (P = 0.777). Figure 2 shows hospital survival in neonates with early presentation of respiratory distress for Epoch I and II according to expected survival based on POS score. Within each expected survival category, observed survival was higher during Epoch II.
Hospital survival in neonates with early presentation of respiratory distress for Epoch I and II according to expected survival (low, moderate and high) based on calculated probability of survival score (see Methods). Within each risk stratification group there was a large increase in survival in Epoch II
Local vs. remote transfer survival
During Epoch II, 44 neonates had early presentation, 31 were local, and 13 were remote transfers. All local transfers, 31 of 31 (100 %), and 11 of 13 (85 %) remote transfers were admitted within 24 h after delivery. Two remote transfers arrived after 24 h from a distant (280 km) hospital via ground ambulance. Of the remote transfers 9 (69 %) survived hospitalization compared to 16 (52 %) of local transfers (P = 0.282). Birth characteristics did not differ between local and remote transfers, including gestational age (38.2 ± 2.7 vs. 38.4 ± 3.0 weeks, P = 0.900), birth weight (3.1 ± 0.7 vs. 2.9 ± 0.6 kg, P = 0.356), Apgar scores at 5 min (6.0 ± 2.2 vs. 4.9 ± 2.7, P = 0.193), and POS score (0.60 ± 0.23 vs. 0.46 ± 0.30, P = 0.113). However, on admission, local transfers had lower pH (7.01 ± 0.19 vs. 7.26 ± 0.12, P <0.001), lower base deficit (−9.8 ± 7.9 mq/L vs. -1.6 ± 4.1 mq/L, P = 0.001), and higher PcCO2 (87.5 ± 30.1 mmHg vs. 59.8 ± 16.4 mmHg, P = 0.003) compared to remote transfers. Also lowest mean blood pressure during the first 12 h after admission was significantly lower in local transfers (34.4 ± 7.2 mmHg vs. 41.2 ± 5.7 mmHg, P = 0.005) as was the lowest body temperature (35.8 ± 0.7 °C vs. 36.5 ± 0.5 °C, P = 0.002). From a multivariable logistic regression analysis that adjusted for PcCO2, survival was not found to be significantly associated with the type of transfer (local vs. remote, P = 0.997). However, survival significantly improved with lower admission PcCO2 (OR 1.16, 95 % CI 1.01–1.33 per 5 mmHg decrease, P = 0.031).
Discussion
The main finding of this study is that protective ventilation for newborns with CDH was associated with improvement in hospital survival, primarily due to a substantial increase in survival among high-risk neonates. Despite higher acuity of CDH disease in local neonates compared to remote transfers, the survival was comparable, reflecting high level of care they have received. The level of admission capillary PcCO2 was an excellent marker for prognostication of survival.
The two most important management changes between epochs were the introduction of protective ventilation and iNO. While iNO is an effective method to control pulmonary hypertension, its use may not reduce CDH mortality [14]. In contrast, ventilator-induced lung injury may substantially increase mortality in neonates with hypoplastic lungs [3, 6–8, 10, 15] while protective ventilation improves survival, and may minimize the need for extracorporeal membrane oxygenation (ECMO) [11]. However, not all studies reported improvement in survival with ECMO [6, 16], and neonatal ECMO was unavailable in Croatia during the study timeframe. In our study the adoption of protective ventilation was associated with a substantial improvement in survival for high-risk neonates with respiratory distress occurring immediately after delivery. Our overall survival in Epoch II was 67 %, which is within the range reported by others [17–21]. However, the true CDH mortality is likely to be higher, as this report does not include “hidden mortality”, i.e. newborns who died before they reached the neonatal unit [22, 23]. A large study reported that 35 % of live-born infants died before being transported to the higher level of care and the population of infants reaching tertiary surgical centers represents approximately 40 % of the total number of cases with CDH [24].
The effect of transport on survival in children with CDH is difficult to assess because of multiple confounders which may introduce patient selection bias. Some studies report higher mortality for outborns (potential bias: more severe cases were transferred and therefore less likely to survive) [12, 25], while others describe lower mortality of outborns (potential bias: transferred only less severe cases and therefore more likely to survive) [26, 27]. In the present study, there was no difference in survival between local and remote transfers, but these findings also reflect probable referral bias secondary to the availability of resources to transport high acuity neonates and transport distance. We speculate that because of the difficulties in long-distance transfers in Croatia (i.e., use of ground ambulance rather than helicopter) the transfer of CDH newborns from remote locations was reserved for less severe cases, introducing a potential bias towards improved survival. This speculation is supported by our observation that local neonates had more severe derangements in vital signs and had more severe lung hypoplasia as inferred from higher capillary PcCO2. Arterial PaCO2 is a good marker for the degree of hypoplastic lung disease. Salas et al. [28] demonstrated that PaCO2 >88 mmHg on admission (which was the mean PcCO2 of our local transfers) was associated with low survival, while PaCO2 <66 mmHg on admission (which was the mean PcCO2 of our remote transfers) was a marker of improved survival. Our finding of comparable survival between “sicker” local referrals and remote transfers suggests that even the sickest neonates who succeed to reach tertiary care may achieve substantial survival. Since we encountered this imbalance of disease severity between remote and local transfers, our study is limited in drawing definitive conclusions regarding the effect of transport per se on survival in neonates with CDH.
Limitations
The limitation of our retrospective study is possibility for presence of unforeseen confounders. Furthermore, the long time span of the study may hide other unaccounted practice changes that occur over calendar time. In order to examine the effect of calendar time on outcomes additional analyses were done that showed that hospital survival did not increase or decrease over time within either epoch, and the improvement of survival after year 2000 suggests that improved survival can likely be attributed to protective ventilation.
Conclusions
With the introduction of protective ventilation, survival for high-risk neonates with early respiratory distress substantially improved. Better survival was associated with lower admission capillary PcCO2. Admission blood gases and vital signs were substantially better in remote transfers indicating on potential referral bias related to transferring neonates with less severe disease. After adjusting for admission PcCO2, survival did not differ significantly between local and remote transfers. This suggests that being delivered close to a tertiary care facility may be advantageous, especially for neonates with high disease acuity. Therefore, mothers living in remote rural areas with less specialized neonatal care should be considered for prenatal screening, and if CDH is detected they should be referred for delivery close to an institution with specialized neonatal care.
Abbreviations
- A/C + VL:
-
Assist-control ventilation with volume limit mode
- CDH:
-
Congenital diaphragmatic hernia
- ECMO:
-
Extracorporeal membrane oxygenation
- HFOV:
-
High frequency oscillation ventilation
- IMV:
-
Intermittent mandatory ventilation
- iNO:
-
Inhaled nitric oxide
- NICU:
-
Neonatal intensive care unit
- PEEP:
-
Positive end expiratory pressure
- PIP:
-
Peak inspiratory pressure
- POS:
-
Probability of survival score
- PSV + VG:
-
Pressure support ventilation with volume guarantee mode
References
Reynolds M, Luck SR, Lappen R. The “critical” neonate with diaphragmatic hernia: a 21-year perspective. J Pediatr Surg. 1984;19:364–9.
Drummond WH, Gregory GA, Heymann MA, Phibbs RA. The independent effects of hyperventilation, tolazoline, and dopamine on infants with persistent pulmonary hypertension. J Pediatr. 1981;98:603–11.
Wung JT, James LS, Kilchevsky E, James E. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985;76:488–94.
Price MR, Galantowicz ME, Stolar CJ. Congenital diaphragmatic hernia, extracorporeal membrane oxygenation, and death: a spectrum of etiologies. J Pediatr Surg. 1991;26:1023–6.
Sakurai Y, Azarow K, Cutz E, Messineo A, Pearl R, Bohn D. Pulmonary barotrauma in congenital diaphragmatic hernia: a clinicopathological correlation. J Pediatr Surg. 1999;34:1813–7.
Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia--a tale of two cities: the Boston experience. J Pediatr Surg. 1997;32:401–5.
Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–66.
Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–32.
Frenckner B, Ehren H, Granholm T, Linden V, Palmer K. Improved results in patients who have congenital diaphragmatic hernia using preoperative stabilization, extracorporeal membrane oxygenation, and delayed surgery. J Pediatr Surg. 1997;32:1185–9.
Kays DW, Langham Jr MR, Ledbetter DJ, Talbert JL. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg. 1999;230:340–8.
Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ. Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg. 1995;30:406–9.
Nasr A, Langer JC. Influence of location of delivery on outcome in neonates with congenital diaphragmatic hernia. J Pediatr Surg. 2011;46:814–6.
Congenital Diaphragmatic Hernia Study Group. Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001;36:141–5.
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–74.
Lally KP. Congenital diaphragmatic hernia. Curr Opin Pediatr. 2002;14:486–90.
Rozmiarek AJ, Qureshi FG, Cassidy L, Ford HR, Hackam DJ. Factors influencing survival in newborns with congenital diaphragmatic hernia: the relative role of timing of surgery. J Pediatr Surg. 2004;39:821–4.
Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB. Congenital Diaphragmatic Hernia Study G. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 2009;44:1315–21.
Sola JE, Bronson SN, Cheung MC, Ordonez B, Neville HL, Koniaris LG. Survival disparities in newborns with congenital diaphragmatic hernia: a national perspective. J Pediatr Surg. 2010;45:1336–42.
van den Hout L, Reiss I, Felix JF, Hop WC, Lally PA, Lally KP, et al. Risk factors for chronic lung disease and mortality in newborns with congenital diaphragmatic hernia. Neonatology. 2010;98:370–80.
van den Hout L, Schaible T, Cohen-Overbeek TE, Hop W, Siemer J, van de Ven K, et al. Actual outcome in infants with congenital diaphragmatic hernia: the role of a standardized postnatal treatment protocol. Fetal Diagn Ther. 2011;29:55–63.
Wright JC, Budd JL, Field DJ, Draper ES. Epidemiology and outcome of congenital diaphragmatic hernia: a 9-year experience. Paediatr Perinat Ep. 2011;25:144–9.
Harrison MR, Bjordal RI, Langmark F, Knutrud O. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg. 1978;13:227–30.
Brownlee EM, Howatson AG, Davis CF, Sabharwal AJ. The hidden mortality of congenital diaphragmatic hernia: a 20-year review. J Pediatr Surg. 2009;44:317–20.
Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:e356–63.
Aly H, Bianco-Batlles D, Mohamed MA, Hammad TA. Mortality in infants with congenital diaphragmatic hernia: a study of the United States National Database. J Perinatol. 2010;30:553–7.
Khmour AY, Konduri GG, Sato TT, Uhing MR, Basir MA. Role of admission gas exchange measurement in predicting congenital diaphragmatic hernia survival in the era of gentle ventilation. J Pediatr Surg. 2014;49:1197–201.
Migliazza L, Bellan C, Alberti D, Auriemma A, Burgio G, Locatelli G, et al. Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization. J Pediatr Surg. 2007;42:1526–32.
Salas AA, Bhat R, Dabrowska K, Leadford A, Anderson S, Harmon CM, et al. The value of Pa(CO2) in relation to outcome in congenital diaphragmatic hernia. Am J Perinatol. 2014;31:939–46.
Moyer V, Moya F, Tibboel R, Losty P, Nagaya M, Lally KP. Late versus early surgical correction for congenital diaphragmatic hernia in newborn infants. Cochrane Database of Systematic Reviews 2000, Issue 4. Art. No.: CD001695. doi: 10.1002/14651858.CD001695.
Acknowledgements
Supported by the Department of Anesthesiology of Mayo Clinic in Rochester, Minnesota, USA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
Authors declare no conflicts of interests.
Authors’ contributions
KB design, data collection, manuscript preparation, final proofing; JV design, data collection, manuscript preparation; EP data collection, manuscript preparation; TL collected information, manuscript preparation and final proofing; TNW data analysis, manuscript preparation, final proofing; DRS, statistics, manuscript preparation, manuscript proofing; WAC study design, manuscript proofing; JS study design, data collection, manuscript preparation, manuscript writing; RG study design, data collection, manuscript preparation. All authors read and approved the final manuscript.
Authors' information
Not applicable.
Katarina Bojanić and Ruža Grizelj contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bojanić, K., Pritišanac, E., Luetić, T. et al. Survival of outborns with congenital diaphragmatic hernia: the role of protective ventilation, early presentation and transport distance: a retrospective cohort study. BMC Pediatr 15, 155 (2015). https://doi.org/10.1186/s12887-015-0473-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-015-0473-x