Abstract
Background
This matched-pair study was initiated to validate the results of a retrospective study of 186 patients published in 2007 that compared whole-brain irradiation (WBI) alone and radiosurgery (RS) alone for up to three brain metastases.
Methods
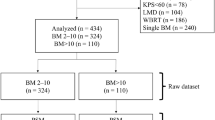
One-hundred-fifty-two patients receiving WBI alone for up to three brain metastases were matched with 152 patients treated with RS of fractionated stereotactic radiotherapy (FSRT) alone 1:1 for each of eight factors (age, gender, Eastern Oncology Cooperative Group (ECOG)-performance score, nature of tumor, brain metastases number, extra-cerebral spread, period from cancer detection to irradiation of brain metastases, and recursive partitioning analysis (RPA)-class. Groups were analyzed regarding intracerebral control (IC) and overall survival (OS).
Results
On univariate analysis of IC, type of irradiation did not significantly affect outcomes (p = 0.84). On Cox regression, brain metastases number (p < 0.001), nature of tumor (p < 0.001) and period from cancer detection to irradiation of brain metastases (p = 0.013) were significantly associated with IC. On univariate analysis of OS, type of irradiation showed no significant association with outcomes (p = 0.63). On multivariate analyses, OS was significantly associated with ECOG performance score (p = 0.011), nature of tumor (p = 0.035), brain metastases number (p = 0.048), extra-cerebral spread (p = 0.002) and RPA-class (p < 0.001).
Conclusion
In this matched-pair study, RS/FSRT alone was not superior to WBI alone regarding IC and OS. These results can be considered a revision of the findings from our retrospective previous study without matched-pair design, where RS alone resulted in significantly better IC than WBI alone on multivariate analysis.
Similar content being viewed by others
Background
Since the prognosis in case of up to three brain metastases is better than in case of four or more lesions, many patients with one to three intracerebral lesions receive stereotactic irradiation or neurosurgery either alone or combined with whole-brain irradiation (WBI) rather than WBI alone [1]. However, since devices for stereotactic irradiation are not available in many centers worldwide, WBI alone is still used also for three or less brain metastases.
Several randomized trials did compare radiosurgery (RS) alone to RS plus WBI [2–5]. These trials demonstrated that RS supplemented with WBI resulted in improved intracerebral control (IC) when compared to RS alone. The benefit not led to improved overall survival (OS). Furthermore, two randomized trials did compare WBI plus a RS boost to WBI alone [6, 7]. One trial was stopped prematurely after inclusion of only 27 patients [6]. According to the second trial, addition of a RS improved control of the irradiated metastatic sites. An OS benefit was seen only for subjects with only one metastasis to the brain.
Until now, only two studies are available that compared RS/FSRT alone to WBI alone. In 2000, a randomized study (n = 67) was reported at an international meeting [8]. However, this study has still not been published as a full paper yet. The second study was a retrospective analysis (n = 186) from our group [9]. However, this was a retrospective study and distributions of patient characteristics varied up to 7%, which likely caused relevant selection biases. The present study was performed to re-examine our previous results with a new study design that would decrease selection bias. The design of a matched-pair study requiring 1:1 matching of eight factors or each pair of patients was chosen in order to considerably lower the possibility of hidden biases. Furthermore, the current study was conducted in a larger patient cohort than the previous retrospective study (304 vs 186 patients).
Methods
Three-hundred-and-four patients irradiated for up to three brain metastases (1998 to 2014) were included in this matched-pair analysis. The data were obtained from an existing anonymized database. Ninety patients (39%) were already included in our previous retrospective study [9]. One-hundred-fifty-two patients received WBI alone and were matched regarding eight factors 1:1 to 152 patients treated with RS alone or fractionated stereotactic radiation therapy (FSRT) alone. These were patient age (≤60 vs ≥61 years, median 60 years), gender, Eastern Oncology Cooperative Group (ECOG)-performance score (0–1 vs 2), nature of tumor (breast cancer vs non-small-cell lung cancer (NSCLC) vs small-cell lung cancer (SCLC) vs kidney cancer vs melanoma vs cancer of unknown primary (CUP) vs gastro-intestinal cancers vs gynecological cancers), brain metastases number (1 vs 2–3), extra-cerebral spread (no vs yes), period from cancer detection to irradiation of brain metastases (<15 vs ≥15 months, median period: 14.5 months), and recursive partitioning analysis (RPA)-class (1 vs 2 [10]) (Table 1). These criteria were chosen in accordance with previous studies that identified significant predictors of survival in patients with brain metastases [10–16]. Of those 77 patients of the WBI alone group with extra-cerebral spread at the time they presented with brain metastases, 37 patients (48%) had involvement of one extra-cerebral site (organ), 27 patients (35%) involvement of two sites and 13 patients (17%) involvement of more than two sites, respectively. In the RS/FSRT group, the corresponding numbers of patients were 34 (44%), 27 (35%) and 16 (21%), respectively. The difference between both treatment groups was not significant (p = 0.90, chi-square test)
RPA class three patients were not included, since they are generally considered unsuitable for RS or neurosurgery. Data regarding systemic treatment prior to irradiation were not available, since the database used for this study was anonymized and did not include this information. This applied also to systemic treatment following irradiation.
The patients of this study had up to three metastases to the brain (size ≤4 cm), no previous irradiation or neurosurgery to brain, and confirming of metastatic brain lesions with magnetic resonance imaging. WBI was administered with 6–10 MV photons from a linear accelerator. Fractionation regimens of WBI included 4Gy × 5 (n = 122), 3Gy × 10 (n = 89) and 2Gy × 20 (n = 41). RS was performed with a conventional linear accelerator (n = 114), a Cyberknife (n = 17) or a GammaKnife (n = 23). Of the patients treated with a conventional linear accelerator, 19 patients received FSRT with three fractions of 7 to 12 Gy or five fractions of 5 to 8 Gy. In the patients treated with RS, doses ranged from 15 to 25 Gy (median 20 Gy), which were prescribed to the outer margins of the metastases (75–90% isodose line with linac-based or Cyberknife radiosurgery/FSRT and 50% isodose line with GammaKnife radiosurgery). These doses corresponded to equivalent doses in 2 Gy fractions (EQD2) between 31.3 and 72.9 Gy (median 50.0 Gy) with respect to tumor cell kill (α/β-ratio = 10 Gy). In those patients receiving FSRT, the EQD2 ranged from 29.8 to 66.0 Gy (median 39.4 Gy).
Both treatment groups were retrospectively analyzed with respect to IC (freedom from progression of the irradiated lesions and development of new lesions within the brain) and OS. IC was chosen instead of local control of the treated lesions and freedom from distant metastases, since for many patients of the WBI alone group data regarding the latter two endpoints were not available.
IC and OS were referenced from the last day of irradiation. Intracerebral failure was diagnosed with magnetic resonance imaging (MRI). The exact frequency and number of MRIs following irradiation were not available, since the anonymized database used did not include these data. In general, the follow-up schedule after RS/FSRT included MRI every 3 to 6 months, whereas in most patients receiving WBI MRI was performed only in case of new or progressive symptoms. The univariate analyses of IC and OS were performed with Kaplan-Meier-method supplemented by the log-rank test [17]. After Bonferroni correction (nine tests), p-values <0.0056 were regarded significant (alpha-level <0.05). The factors that gained significance or showed a trend (p ≤ 0.06) were analyzed multivariate with a Cox-regression-model. Since the RPA-class included age, performance status and extra-cerebral spread, a second analysis was performed if RPA-class and at least one other factor were having significant associations with outcomes on univariate analysis.
Results
In the univariate analysis of IC (Table 2), the type of irradiation did not affect outcome (p = 0.84, Fig. 1). In contrast, improved IC was significantly affected by single brain metastasis (p < 0.001). A trend towards better outcomes was found for a better performance status (ECOG 0–1, p = 0.05), favorable nature of tumor (p = 0.014) and period from cancer detection to irradiation of the brain metastases of >15 months (p = 0.05). The four factors were implemented in a Cox-regression. On multivariate analysis, brain metastases number (risk ratio 1.84; 95%-confidence-interval 1.33–2.56; p < 0.001), nature of tumor (1.16; 1.07–1.24; p < 0.001) as well as period from cancer detection to irradiation of brain metastases (1.52; 1.09–2:13; p = 0.013) were significant, in contrast to performance status (1.28; 0.88–1.82; p = 0.19).
Of the 74 patients developing an intracerebral recurrence after RS/FSRT, 39 patients (53%) had an out-field recurrence, 15 patients (20%) and in-field recurrence and 20 patients (27%) a concurrent out- and in-field recurrence, respectively. In this group, intracerebral recurrences were treated with best supportive care alone in 13 patients (18%), WBI alone in 37 patients (50%) and another course of RS/FSRT in 24 patients (32%), respectively. In the 76 patients of WBI alone group developing an intracerebral recurrence, treatment consisted of best supportive care alone in 37 patients (49%), another course of WBI in 30 patients (39%) and RS/FSRT in 9 patients (12%), respectively.
Median survival times following irradiation were 10 months in the entire cohort, 9 months after WBI alone and 11 months after RS alone, respectively. On univariate analysis of OS (Table 3), the type of irradiation was not significantly related to outcomes (p = 0.63, Fig. 2). In contrast, significantly positive association with OS was shown for ECOG-score 0–1 (p < 0.001), favorable nature of tumor (p < 0.001), no extra-cerebral spread (p < 0.001) and RPA-class 1 (p < 0.001). Trends towards improved OS were seen regarding age ≤60 years (p = 0.019) or a single intracerebral lesion (p = 0.06). On Cox-regression, OS was significantly related to performance status (1.49; 1.10–2.00; p = 0.011), nature of tumor (1.07; 1.00–1.13; p = 0.035), brain metastases number (1.34; 1.00–1.79; p = 0.048), extra-cerebral spread (1.58; 1.19–2.11; p = 0.002) and RPA-class (1.84; 1.37–2.50; p < 0.001). Age did not show such a relation (1.31; 0.96–1.78; p = 0.09).
Discussion
Since patients with one to three brain metastases do much better than those with more than three intracerebral lesions, they could benefit from local radiation therapies alone or combined with WBI when compared to WBI alone [1]. However, local therapies such as RS and FSRT are not available in many institutions worldwide, and WBI alone remains the only reasonable non-surgical option. RS and FSRT alone have become more popular during the last years because randomized trials revealed that WBI given prior to RS does not increase the OS in case of one to three brain metastases [2–5]. In these trials, WBI led increased IC-rates but also the risk of radiation-related decline in neuro-cognitive function. Therefore, radiation oncologists are often hesitant to add WBI to RS/FSRT and use RS/FSRT alone instead.
Another issue regarding the irradiation of one to three brain metastases has not yet been answered properly. In case if RS and FSRT are not available for these patients, is WBI alone inferior to RS/FSRT alone regarding IC or OS? Two reports are available that did compare WBI alone and RS alone [8, 9]. A randomized study (n = 67) with one to three metastases to the brain, 1-year IC was 62% with WBI alone and 87% with RS alone, respectively (p-value not mentioned) [8]. Another study comparing WBI alone to RS alone was a retrospective study of 186 patients [9]. According to its results, better 1-year OS (52% vs 33%, p = 0.045) or IC (49% vs 23%, p = 0.005) were achieved with RS alone on univariate analyses. On multivariate analyses, IC was significantly different (p = 0.003), whereas OS was not (p = 0.89). Taking into account the available data for the comparison of WBI alone and RS alone, it becomes obvious that more studies are required that compare these types of irradiation for one to three metastases to the brain. Therefore, it was decided to run a matched-pair analysis including strict matching criteria. The 152 pairs of patients were required to match 1:1 for all eight factors. This design decreased the possibility of selection biases. However, our data are still retrospective and some remaining hidden selection bias cannot be completely excluded. Furthermore, no sufficient data regarding systemic treatment following irradiation were available. A difference between the two treatment groups regarding post-irradiation systemic treatments may have had an impact on OS. In addition, an unknown difference between both groups regarding the frequency and number of post-treatment MRIs might have influenced the IC rates. Since MRIs were generally performed every 3 to 6 months in the RS/FSRT group and only in case of new or progressive symptoms in the WBI group, intracerebral recurrences likely would have been detected earlier in the RS/FSRT group than in the WBI resulting in a false shorter time to an intracerebral failure in the RS/FSRT group. These aspects should be kept in mind when interpreting the results of this study.
RS/FSRT alone did not increase IC or OS rates in comparison to WBI alone up to three years following irradiation. These results are partly contradictory to the findings of our previous retrospective study that did not include a matched-pair design [9]. In that previous study, RS was associated with significantly better IC on multivariate analysis. In a randomized cohort, RS alone provided better IC than WBI alone [8]. However, it is not clear whether the difference was significant, since the corresponding p-value was not mentioned in the abstract, and the study is not available as a peer reviewed manuscript. Furthermore, the criteria for inclusion in that randomized study are unknown, which makes it difficult to compare their results to ours. When summarizing the data of the three available studies, it is not clear whether RS/FSRT alone does result in significantly better IC than WBI alone [8, 9]. When considering the largest study, i.e. the present matched-pair study, it appears questionable that a benefit with respect to IC exists for RS/FSRT alone. All three studies agree that RS or FSRT alone do not improve OS when compared to WBI alone. Therefore, if RS and FSRT are not available, WBI alone appears reasonable also for one to three metastases to the brain.
However, neuro-cognitive decline is known to be greater after WBI than after RS/FSRT, mainly due to better hippocampal sparing [3, 5]. In a randomized trial of 58 patients that compared RS alone to RS plus WBI, neuro-cognitive deficits at 4 months following irradiation occurred significantly more common after RS plus WBI than after RS alone (96% vs. 24%, p < 0.001) [3]. These results were confirmed in another recent randomized trial of 213 patients [5]. Cognitive deterioration at 3 months following irradiation was observed in 91.7% of patients after RS plus WBI and 63.5% of patients after RS alone, respectively (p < 0.001). However, when using modern radiation techniques for WBI such as volumetric modulated arc therapy (VMAT), considerable hippocampal sparing is possible. According to the RTOG 0933 study, hippocampal sparing led to a reduction of neuro-cognitive decline at 3 months following WBI from 30% (historical control) to 7% (p < 0.001) [18]. Thus, a randomized trial comparing RS alone to RS plus hippocampal sparing WBI for one to three brain metastases is warranted.
Conclusion
According to this matched-pair analysis, RS/FSRT alone did not result in significantly better IC and OS rates when compared to WBI alone. These results can be considered a revision of the findings from our previous retrospective study without a matched-pair design, where RS alone resulted in significantly better IC than WBI alone on multivariate analysis.
Abbreviations
- CUP:
-
Cancer of unknown primary
- ECOG:
-
Eastern cooperative oncology group
- FSRT:
-
Fractionated stereotactic radiation therapy
- Gy:
-
Gray
- IC:
-
Intracerebral control
- OS:
-
Overall survival
- RPA:
-
Recursive partitioning analysis
- RS:
-
Radiosurgery
References
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–25.
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. A randomized controlled trial. JAMA. 2006;295:2483–91.
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44.
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–41.
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker 2nd FG, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316:401–9.
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–34.
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran Jr WJ. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–72.
Chougule PB, Burton-Williams M, Saris S, Zheng Z, Ponte B, Noren G, Alderson L, Friehs G, Wazer D, Epstein M. Randomized treatment of brain metastases with gamma knife radiosurgery, whole brain radiotherapy, or both [Abstract]. Int J Radiat Oncol Biol Phys. 2000;48 Suppl 1:114.
Rades D, Pluemer A, Veninga T, Hanssens P, Dunst J, Schild SE. Whole-brain radiotherapy versus stereotactic radiosurgery for patients in recursive partitioning analysis classes 1 and 2 with 1 to 3 brain metastases. Cancer. 2007;110:2285–92.
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51.
Dziggel L, Segedin B, Podvrsnik NH, Oblak I, Schild SE, Rades D. Validation of a survival score for patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2013;189:364–6.
Rades D, Dziggel L, Nagy V, Segedin B, Lohynska R, Veninga T, Khoa MT, Trang NT, Schild SE. A new survival score for patients with brain metastases who received whole-brain radiotherapy (WBRT) alone. Radiother Oncol. 2013;108:123–7.
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4.
Rades D, Dziggel L, Segedin B, Oblak I, Nagy V, Marita A, Schild SE, Trang NT, Khoa MT. A new survival score for patients with brain metastases from non-small cell lung cancer. Strahlenther Onkol. 2013;189:777–81.
Rades D, Dziggel L, Segedin B, Oblak I, Nagy V, Marita A, Schild SE. The first survival score for patients with brain metastases from small cell lung cancer (SCLC). Clin Neurol Neurosurg. 2013;115:2029–32.
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–61.
Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–6.
Funding
The study was not funded.
Availability of data and materials
Data analyzed for this paper cannot be shared on a publicly available repository due to data protection regulations. According to the local ethics committee, only the evaluation of anonymized data is allowed for this study.
Authors’ contributions
DR, SJ and SES participated in the design and methodology of the study. DR, SJ, LD, OB, AB and TV provided study material. DR and SES were involved in the analyses of the data and their interpretation. The manuscript was drafted by DR and SES and reviewed by the authors, who also approved the final version of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the local ethics committee (University of Lübeck, reference number AZ:16-239). Individual informed consent was not required, since this is a retrospective study solely including anonymized data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rades, D., Janssen, S., Dziggel, L. et al. A matched-pair study comparing whole-brain irradiation alone to radiosurgery or fractionated stereotactic radiotherapy alone in patients irradiated for up to three brain metastases. BMC Cancer 17, 30 (2017). https://doi.org/10.1186/s12885-016-2989-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2989-3