Abstract
Background
Previous studies have reported a high prevalence of excessive gestational weight gain (GWG) in women with prepregnancy BMI classified as overweight and obese. However, the joint evidence regarding GWG and prepregnancy BMI in the worldwide population has not been synthesized. Thus, this systematic review and meta-analysis aimed to estimate global and regional mean GWG and the prevalence of GWG above, within and below 2009 Institute of Medicine (IOM) guidelines. Second, we aimed to estimate global and regional prepregnancy BMI and the prevalence of BMI categories according to World Health Organization (WHO) classification.
Methods
We searched Medline, Embase, the Cochrane Library and Web of Science to identify observational studies until 9 May 2018. We included studies published from 2009 that used 2009 IOM guidelines, reporting data from women in general population with singleton pregnancies. The 2009 IOM categories for GWG and the WHO categories for prepregnancy BMI were used. DerSimonian and Laird random effects methods were used to estimate the pooled and their respective 95% confidence intervals (95% CIs) of the mean and by category rates of GWG and prepregnancy BMI, calculated by global and regions.
Results
Sixty-three published studies from 29 countries with a total sample size of 1,416,915 women were included. The global prevalence of GWG above and below the 2009 IOM guidelines, was 27.8% (95% CI; 26.5, 29.1) and 39.4% (95% CI; 37.1, 41.7), respectively. Furthermore, meta-regression analyses showed that the mean GWG and the prevalence of GWG above guidelines have increased. The global prevalence of overweight and obesity, was 23.0% (95% CI; 22.3, 23.7) and 16.3% (95% CI; 15.4, 17.4), respectively. The highest mean GWG and prepregnancy BMI were in North America and the lowest were in Asia.
Conclusions
Considering the high prevalence of GWG above the 2009 IOM guidelines and women with overweight/obesity and their continuously increasing trend in most regions, clinicians should recommend lifestyle interventions to improve women’s weight during reproductive age. Due to regional variability, these interventions should be adapted to each cultural context.
Trial registration
Prospectively registered with PROSPERO (CRD42018093562).
Similar content being viewed by others
Background
In 2009, the Institute of Medicine (IOM) updated the international gestational weight gain (GWG) cut-off points published in 1990 [1] based on the prepregnancy body mass index (BMI) following the BMI classification of the World Health Organization (WHO) [2, 3]. The recommended amount of GWG in the 2009 IOM guidelines was 12.5-18 kg, 11.5–16 kg, 7–11.5 kg, and 5–9 kg for women with prepregnancy BMI classified as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2); overweight (25–29.9 kg/m2) and obese (≥ 30 kg/m2) respectively. The main change in the IOM cut-off points included an updated limitation of the recommended amount of GWG to improve pregnancy outcomes in women with obesity and the change in the classification criteria of prepregnancy BMI according to the WHO classification [3, 4]. This fact modified the GWG categoy prevalence and the advice about GWG that public health practitioners gave to women.
Gestational weight gain above IOM guidelines and prepregnancy overweight/obesity have been continuously increasing during recent decades although data differ across countries [5, 6]. Recent studies have reported high rates of GWG above the IOM guidelines and the prepregnancy overweight/obesity in Europe over 36 and 29%, respectively, while in the USA these figures are 44 and 42% respectively [5, 6].
Several maternal and infant health problems have been related to excessive GWG and prepregnancy BMI [5,6,7], such as: (i) maternal comorbidities during pregnancy including gestational diabetes [8,9,10,11] and preeclampsia [9, 12]; (ii) delivery complications such as instrumental or cesarean delivery [3, 11,12,13]; (iii) being born large for gestational age [14]; and (iv) long-term effects in offspring such as adiposity [15] or lower cognitive skills [3, 16, 17].
Moreover, studies examining the compliance with the 1990 [6] or 2009 IOM GWG guidelines [5] and the WHO classification reported heterogeneous results in Europe and USA. The lack of compliance across countries could be explained by intercountry variability in several factors [18, 19] such as physical activity [20,21,22]. dietary patterns [21, 22], and psychological or social maternal characteristics [23,24,25].
For these reasons, this review aimed to estimate global and regional GWG (in kilograms) and the prevalence of GWG above, within and below the 2009 IOM guidelines. Second, we aimed to estimate prepregnancy BMI (in kg/m2) and the prevalence of BMI categories according to the WHO classification.
Methods
This meta-analysis was registered in PROSPERO (registration number: CRD42018093562), and was reported following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement [26]. The Cochrane Collaboration Handbook guidelines were used to guide this meta-analysis [27].
Eligibility criteria, information sources, and search strategy
This systematic review and meta-analysis aimed to identify the studies reporting GWG published after the 2009 IOM guidelines. Due to the differences between 2009 IOM and 1990 guidelines, we decided to include only papers published following the 2009 IOM guidelines because differences in the classification of GWG categories could affect pooled GWG estimations. Studies were identified in the following databases: Medline (via PubMed), the Web of Science, the Cochrane Library and Embase (via Scopus) from 2009 (including studies published in 2009), when the IOM published the new cut-off points of GWG to 9 May 2018. The search strategy combined the following terms: (1) population (gestational, gestation, pregnancy, maternal) and (2) outcome (weight gain, weight change) (Table S1). The literature search was completed by screening the references included in the articles considered for inclusion in the systematic review.
Study selection
The search was aimed to identify papers that reported GWG using the 2009 IOM cut-off points. Inclusion criteria were as follows: (i) participants: population-based pregnant studies of women with a singleton pregnancy; (ii) study design: observational studies; and (iii) outcomes: included studies had to report GWG mean and GWG classified with the 2009 IOM criteria [3]. Studies were excluded when they were written in languages other than English or Spanish or the target population was: (i) samples of a specific age-range; (ii) women with any specific prepregnancy weight status or BMI category (we excluded data of GWG and prepregnancy BMI categories of studies when they reported various categories combined in a data). (iii) women with any specific GWG category; (iv) women at any specific pregnancy trimester (v) pregnant women with specific diseases (diabetes mellitus, preeclampsia, cardiovascular health problems, anemia, gestational nausea and vomiting); (vi) pregnant women under pharmacological treatment during pregnancy or who underwent prepregnancy bariatric surgery; and (vii) only preterm deliveries. Additionally, studies were excluded when they used BMI classification criteria different from those of the WHO [2].
Data synthesis
The following data were extracted from the original reports: (i) study data (author, the year of publication, country, regions, cohort year of birth, full term rate, sample size); (ii) characteristics of participants (mother’s age at delivery, mean GWG, the percentage of participants meeting the IOM guidelines, mean BMI and the prevalence of WHO BMI categories). When more than one study provided data from the same cohort and they did not overlapped, we included all available studies. However, when various studies provided data for the same cohort and they overlapped, we included only the one presenting the most detailed results or providing data with the largest sample size. However, data regarding sample characteristics could be extracted from all reports to obtain the most complete information.
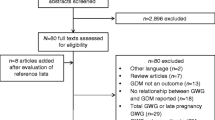
The search results were compiled in the Mendeley reference manager and the results of the systematic search are presented in Fig. 1. The characteristics of the included studies are presented in Table 1. DerSimonian and Laird random effects models [90] were used to compute global, and regional pooled estimates and their respective 95% confidence intervals (95% CIs) for: i) mean GWG, ii) prevalence of GWG categories according to the 2009 IOM guidelines (including below, within and above according to the 2009 IOM guidelines) [3] and iv) the prevalence of prepregnancy BMI according to the WHO BMI categories (including underweight, normal-weight and overweight BMI) [2].
The heterogeneity of results across studies was evaluated by using the I2 statistic and could be considered: not important (0 to 40%), moderate (30 to 60%), substantial (50 to 90%) and considerable (75 to 100%); the corresponding p-values were also considered [91].
Furthermore, subgroup analyses by the method of collecting GWG or prepregnancy BMI data (self-reported by pregnant women, measured the GWG in the follow-up period or used data of medical records) were conducted for global and by regions estimations. In addition, random-effects meta-regressions estimations were conducted to evaluate the study and sample characteristics such as maternal age, year of the recruitment, full-term rate and country income (Tables S2, S3, S4, S5 and S6). In the analysis by country income we take into account the 2018 IMF list (Table S6), in which negative estimates mean that higher country’s income increases the dependent variable [92]. Statistical analyses were performed using StataSE software, version 15 (StataCorp).
Assessment of risk bias
The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to evaluate the risk of bias of the included studies [93]. In the Table S7 each yes added a point to the total score (+); and “no”, “cannot determine” (CD), “not applicable” (NA) or “not reported” (NR) kept the total score the same [93]. Each study could be scored as good (most methodological criteria met, low risk of bias), fair (some criteria met, low risk of bias), or poor (few criteria met, high risk of bias).
The literature search, data extraction and quality assessment were independently performed by two reviewers (JAM-H and IC-R), and inconsistencies were solved by consensus. A third researcher was consulted when consensus could not be reached (CA-B).
Results
Study selection
After screening 8432 articles, 63 studies were included in this systematic review and meta-analysis (Fig. 1). Included studies were published between 2010 and 2018, 21 were from North America [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81], 20 were from Europe [5, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60], 13 were from Asia [29,30,31,32,33,34,35,36,37,38,39,40,41], four were from Oceania [82,83,84,85], four were from South America [86,87,88,89] and one was from Africa [28].
Studies were published from 2009 to 2017 [5, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89]. The population recruitment periods were 1 year in 12 studies [28, 34, 35, 39, 41, 50, 53, 58, 59, 67, 85, 86], 2 years in 15 studies [30, 33, 36, 37, 40, 54,55,56, 60, 74, 83, 84, 87,88,89] and more than 2 years in 36 studies [5, 29, 31, 32, 38, 42,43,44,45,46,47,48,49, 51, 52, 57, 61,62,63,64,65,66, 68,69,70,71,72,73, 75,76,77,78,79,80,81,82].
The number of participants was 1.416.915. The mean maternal age ranged from 23.6 to 33.0 years. Preterm delivery rates were reported by 40 studies ranging from 0.0 to 21.34% (Table 1) [28, 29, 31,32,33,34,35, 37, 39,40,41,42, 46, 47, 49, 51, 52, 54,55,56, 59, 60, 62, 63, 65,66,67, 69, 70, 72, 73, 76, 77, 79,80,81,82, 84, 88, 89]. Twenty studies did not report the mean BMI [30, 31, 33, 35, 36, 38, 41, 42, 55, 58, 61, 70,71,72, 74, 78, 80, 82, 83, 88] and only two studies did not report the prevalence of BMI categories [48, 63]. Furthermore, regarding the methods of collecting GWG: eighteen studies measured the GWG [30, 36, 43, 45,46,47,48, 52, 54, 57, 59, 60, 62, 63, 65, 78, 83, 84], thirty-eight used medical records [5, 28, 29, 31,32,33,34,35, 38,39,40,41,42, 44, 49,50,51, 53, 55, 56, 58, 61, 66, 68,69,70,71,72, 75, 77, 79, 81, 82, 85,86,87,88,89] and seven studies used women’s self-reported data [37, 64, 67, 73, 74, 80]. However, regarding the methods of collecting prepregnancy BMI data: only two studies measured it [52, 60], twelve used medical records [5, 28, 29, 39, 44, 49, 57, 68, 77, 79, 86, 89], two used both medical records and self-reported data [43, 76], and twenty four used self-reported prepregnancy BMI [29, 32, 34, 37, 45,46,47,48, 50, 51, 53, 54, 59, 62,63,64,65,66,67, 73, 75, 84, 85, 87].
Synthesis of results
Gestational weight gain
Figure S1 display the estimations of GWG mean in the global population and by regions. The pooled GWG mean was 13.39 kg (95% CI: 11.97, 13.83) in the global population. Our findings showed that in global population 27.8% (95% CI: 26.5, 29.1) of women had GWG below guidelines and 39.4% (95% CI: 37.1, 41.7) GWG above guidelines.
Data by regions showed the highest pooled GWG mean in North America with 14.74 kg (95% CI: 13.97, 15.51) and the lowest in Asia with 11.36 kg (95% CI: 10.14, 12.58) (Figure S1). The highest pooled prevalence of GWG below the 2009 IOM guidelines was found in Asia with 39.4% (95% CI: 30.1, 49.6%) and the lowest in North America with 19.1% (95% CI: 16.1, 22.5%) (Figure S2). The pooled prevalence of GWG within the 2009 IOM guidelines was similar across regions (ranging from 33.3 to 37.8%), except in Africa and North America, with a prevalence of 29.0% (95% CI: 27.7, 30.3%) and 28.0% (95% CI: 23.9, 32.5%), respectively (Figure S3). Finally, data for pooled prevalence of GWG above the 2009 IOM guidelines showed the highest prevalence in North America and the lowest in Asia, 50.6% (95% CI: 46.2, 55.0%) and 20.2% (95% CI: 12.9, 30.2%), respectively (Figure S4).
Prepregnancy BMI
Figure S5 displays the estimations about the mean prepregnancy BMI in the global population and by regions. The pooled mean prepregnancy BMI was 23.08 kg/m2 (95%CI: 22.87, 23.30) in the global population. The highest mean prepregnancy BMI was found in South America with 25.05 kg (95% CI: 23.39, 26.72) and the lowest was found in Asia with 11.36 kg (95% CI: 10.14, 12.58). Furthermore, our findings showed a low prevalence of prepregnancy BMI classified as underweight with 5.5% (95% CI 5.2, 5.9). However, the prevalence of women with a prepregnancy BMI classified as overweight and obese was high with 23.0% (95% CI: 22.3, 23.7) and 16.3% (95% CI: 15.4, 17.3), respectively.
Data by region are shown in Figures S5, S6, S7, S8 and S9. The pooled prevalence of prepregnancy BMI categories showed that studies conducted in Asia reported the lowest pooled mean prepregnancy BMI with 21.24 kg/m2 (95% CI 20.76, 21.71), the highest prevalence of underweight with 11.1% (95% CI: 9.6, 12.7) and the lowest prevalence of obesity with 5.4% (95% CI: 2.4, 11.6). Finally, the highest prevalence of obesity was in North America with 17.6% (95% CI: 16.5, 18.7).
Subgroup analyses and meta-regressions
There were no differences among subgroups in the global population and across regions (p > 0.05) when subgroup analysis was based on the methods of collecting GWG and prepregnancy BMI (studies that measured them or those that used medical records).
The random-effects meta-regression model showed that GWG decreases in the global population, Europe and North America as maternal age increases. Additionally, mean GWG and the prevalence of GWG above guidelines increased when the year of recruitment was later in the global population, Europe and North America. Finally, GWG increased when there were higher full-term rates (p < 0.05) (Table S2).
The random-effects meta-regression model showed that prepregnancy BMI decreased as maternal age increased in Europe, North America and the global population. Furthermore, the mean prepregnancy BMI decreased in the global population and in Europe with a later year of recruitment. In contrast, prepregnancy BMI increased in North America with a later year of the recruitment (p < 0.05) (Table S3).
Tables S4 and S5 display the results of the meta-regression by the year of recruitment and prevalence of GWG and prepregnancy BMI categories. We found that the prevalence of GWG above guidelines increase in Europe, North America and global population with a more recent the year of recruitment, while the prevalence of GWG below guidelines decreased in Europe. Additionally, we found that the prevalence of underweight and normal weight decreased in all regions, while overweight and obesity increased with a later year of recruitment.
Finally, Table S6 shows that the mean GWG and prepregnancy BMI were higher in wealthy countries, as well as the prevalence of GWG above guidelines, overweight and obesity. Conversely, when countries’ income was lower the prevalence of GWG within and below guidelines, underweight and normal weight prevalence were higher.
Risk of bias of included studies
The methodological quality was good in 95% of the studies and fair in the remaining 5%. Assessors were not blinded to the exposure status of participants in any of the included studies. Furthermore, only 35 studies reported a participation rate of eligible women over 50% and 17 studies reported loss of follow-up rate less than 20% after baseline measures (Table S7).
Discussion
Main findings
Our findings display a global high prevalence of GWG above and below the 2009 IOM guidelines, 27.8 and 39.4%, respectively. Furthermore, the mean GWG and prevalence of GWG above guidelines have increased. Finally, there was a global high prevalence of overweight and obesity, 23.0 and 16.3%, respectively.
Comparison with existing literature
Our pooled estimates of the mean GWG and prepregnancy BMI, as well as the prevalence of GWG above, within and below guidelines, are similar to those previously reported [5, 6, 94]. Moreover, the findings of a previous study [95] were in line with our data because it reported higher rates of GWG above guidelines and obesity in the USA and Europe than in Asia. Finally, previous evidence are in line with our findings because it suggests that the mean and prevalence of GWG above guidelines have increased [5, 6].
The high prevalence of GWG above guidelines may be a consequence of several factors: (i) lifestyle changes, such as lower level of physical activity during pregnancy or inadequate diet [96]; (ii) psychological and social maternal influences, such as low knowledge about the importance of gaining adequate GWG, emotional instability or locus of control [97]; and (iii) the global nutritional transition in recent decades, which has accelerated the consumption of processed food and prepared meals, which could produce higher rates of GWG above guidelines [98].
The differences in GWG and prepregnancy BMI across regions could be influenced by countries’ income because the rates of GWG above guidelines, overweight and obesity are higher in high- or middle-income countries [7]. However, differences between populations in the same countries could be due to by individual socioeconomic status because a larger fraction of the global overweight and obese populations become relatively poor in countries that are economically developed, while in lower−/middle- income countries, the rates of overweight and obesity are higher among wealthier individuals [99]. Moreover, the economic crisis could increase the probability to being obese because it could reduce diet quality among populations with fewer resources [100], and this fact could increase the GWG and prepregnancy BMI [3].
There were differences between the 2009 and 1990 IOM guidelines (Tables S8 and S9); therefore, we decided to use the 2009 IOM guidelines because rates of GWG categories could be affected if we included studies that reported results according both guidelines [1, 3]. As GWG and prepregnancy BMI have a multifactorial origin [3, 101], other factors were studied using subgroup analyses and meta-regressions. The relationship of maternal age and GWG agrees with previous studies supporting that older women have lower GWG in Europe and in the global population [19, 36]. The year of recruitment showed that GWG is rising in all regions, according to previous studies in all regions [5, 6]. Finally, higher full-term rates are positively related with GWG as GWG increases with the number of weeks of gestation, peaking at 37 weeks or more of gestation [3, 102]. Since, preterm birth rates could be higher in sub-Saharan African or South Asian countries than in European countries, this fact could explain why GWG was lower in these regions [103].
The variability in subgroup and meta-regression analyses could be explained by several factors influencing both GWG and prepregnancy BMI: (i) low knowledge about the importance of adequate GWG, including the women’s perception that they can not control their own weight by themselves. (low external locus of control) [25, 97, 104]; (ii) inadequate physical activity rates and dietary patterns [20,21,22]; (iii) low maternal education and professional class, maternal age, multiparity and minority ethnicity [105], and a lack of access to nutrition programs in low-income women [106]. Furthermore, national GWG guidelines and energy-intake guidelines could increase differences among regions because their guidelines varied around the world [107].
Our estimations of global GWG and prepregnancy BMI could help promote health interventions and programs among pregnant women and women of childbearing age because the American Congress of Obstetricians and Gynecologists (ACOG) emphasizes the importance of managing GWG and prepregnancy BMI through diet and physical activity counseling [108]. Thus, public health practitioners and policy-makers should explain to women the optimal GWG according to current guidelines to improve perinatal outcomes [3]. Routine medical visits during the prenatal period could be an opportunity for health-care providers to implement physical activity and diet counseling to ensure adequate GWG and prepregnancy BMI [101, 109, 110], although recent evidence calls into question the efficacy of dietary and lifestyle interventions to prevent the consequences of excessive GWG [111, 112] and the need to assess interventions in order to recommend the best option [113]. Regardless the inconclusive evidence about interventions based on diet and exercise, health-care providers should implement interventions based on diet and exercise due to the low adverse consequences of lifestyle interventions and their potential benefits.
Strengths and limitations
Some limitations of this study that could compromise our results should be stated. First, there is a lack of data from some countries, because we did not retrieve studies that reported data on GWG and prepregnancy BMI; therefore, there is a lack of information from specific world areas. Second, differences in the sample characteristics and geographic locations of the included studies may increase heterogeneity, which could threaten the generalization of our results; for example a study published in 2018 suggested that IOM guidelines could be applicable only in the USA, western Europe and eastern Asia [95]; however, we used the same classification criteria for all regions of the world, in the way that was reported by the different studies. Third, we included studies with different rates of preterm birth, although when they reported in the methodology or limitation section that these rates were higher than those of the reference population or they only included preterm births, we excluded them to limit bias. Fourth, we could not evaluate publication bias due to the design of our study. Fifth, all included studies had observational designs; therefore, the drawing of causal inferences was not possible. Sixth, we included studies with self-reported weights, which produce an underreported prepregnancy and delivery weight or an overestimated GWG, although the magnitude of error could be small and it is a practical measurement approach [114]. Seventh, only studies written in English or Spanish were included and grey literature was not reviewed. Eight, the IOM classification criteria were not be suitable for the Asians population because there is a different cut-off for BMI categories. Ninth, we could not perform subgroup analyses by ethnic characteristics as data were heterogeneously reported by the studies. Finally, most of the literature was from Europe and America and the data from other regions were fewer, thus the small number of studies from Africa, South America and Oceania could limit the validity of our results and findings should be taken with caution.
To improve the strength of this article we decided to include studies published since 2009 (including 2009), because the studies published before this year cannot include the new IOM cut-off points published in 2009. However, we included studies with cohorts of women before 2009 that were classified with 2009 IOM cut-off points. We performed this because we aimed to improve comparability and generalization of our results. Furthermore, we excluded studies with specific samples of GWG or pregestational BMI because they could be overrepresented, and the ability to produce generalizable results could be limited.
Conclusions
In summary, our study showed a global high prevalence of GWG above 2009 IOM guidelines and overweight/obesity, as well as an increase in mean GWG and the prevalence of GWG above guidelines. Thus, health practitioners and policy-makers should encourage a healthy GWG and prepregnancy BMI to improve perinatal outcomes, through novel lifestyle interventions in each clinical context. Notwithstanding, our data highlight the need for additional population-based studies, especially using the 2009 IOM guidelines.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional file.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- GWG:
-
Gestational weight gain
- IOM:
-
Institute of Medicine
- WHO:
-
World Health Organization
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology
- ACOG:
-
The American Congress of Obstetricians and Gynecologists
References
National Academy of Sciences. Nutrition during pregnancy: part I, weight gain: part II, nutrient supplements. Natl Academy Pr. 1990;10. ISBN: 0-309-55558-2.
World Health Organization. Body mass index - BMI. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
Institute of Medicine. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): The National Academies Press; 2009.
Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–25.
Ferrari N, Mallmann P, Brockmeier K, Strüder HK, Graf C. Secular trends in pregnancy weight gain in German women and their influences on foetal outcome: a hospital-based study. BMC Pregnancy Childbirth. 2014;14(1):228.
Johnson JL, Farr SL, Dietz PM, Sharma AJ, Barfield WD, Robbins CL. Trends in gestational weight gain: the Pregnancy Risk Assessment Monitoring System, 2000–2009. Am J Obstet Gynecol. 2015;212(6):806.e1–8.
NCD Risk Factor Collaboration (NCD-RisC), Risk Factor Collaboration N, NCD Risk Factor Collaboration (NCD-RisC), Risk Factor Collaboration N. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96 www.thelancet.com.
Torloni MR, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203.
Ovesen P, Rasmussen S, Kesmodel U. Effect of Prepregnancy Maternal Overweight and Obesity on Pregnancy Outcome. Obstet Gynecol. 2011;118(2, Part 1):305–12.
Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597–604.
Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal Prepregnancy Body Mass Index and Gestational Weight Gain on Pregnancy Outcomes. PLoS One. 2013;8(12):e82310.
Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynecol Obstet. 2006;93(3):269–74.
Dietz PM, Callaghan WM, Morrow B, Cogswell ME. Population-based assessment of the risk of primary cesarean delivery due to excess Prepregnancy weight among nulliparous women delivering term infants. Matern Child Health J. 2005;9(3):237–44.
Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Neonatal Med. 2015;28(14):1679–86.
O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol. 2013;78(1):9–16.
Álvarez-Bueno C, Cavero-Redondo I, Lucas-de la Cruz L, Notario-Pacheco B, Martínez-Vizcaíno V. Association between pre-pregnancy overweight and obesity and children’s neurocognitive development: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2017;46(5):1653–66.
Huang L, Yu X, Keim S, Li L, Zhang L, Zhang J. Maternal prepregnancy obesity and child neurodevelopment in the collaborative perinatal project. Int J Epidemiol. 2014;43(3):783–92.
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet (London, England). 2016;387(10026):1377–96.
Tabet M, Nelson E, Schootman M, Chien L-C, Chang JJ. Geographic variability in gestational weight gain: a multilevel population-based study of women having term births in Florida (2005–2012). Ann Epidemiol. 2017;27(7):421–428.e2.
Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, González-García A, Sánchez-López M, Martínez-Vizcaíno V. Effects of exercise-based interventions on neonatal outcomes: a meta-analysis of randomized controlled trials. Am J Health Promot. 2016;30(4):214–23.
International Weight Management in Pregnancy (i-WIP) Collaborative Group TIWM in P (i-WC). Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119.
Ross R, Dagnone D, Jones PJH, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med. 2000;133(2):92.
Kapadia MZ, Gaston A, Van Blyderveen S, Schmidt L, Beyene J, McDonald H, et al. Psychological factors and trimester-specific gestational weight gain: a systematic review. J Psychosom Obstet Gynaecol. 2015;36(1):15–22.
Abitbol MM. Weight gain in pregnancy; 2012. p. 1–2. Available from: https://thewomens.r.worldssl.net/images/uploads/fact-sheets/Weight-gain-in-pregnancy.pdf.
Ali SM, Lindström M. Socioeconomic, psychosocial, behavioural, and psychological determinants of BMI among young women: differing patterns for underweight and overweight/obesity. Eur J Pub Health. 2006;16(3):324–30.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. In: The Cochrane Collaboration; 2011. Table 7.7.a: Formulae for combining groups.
Asefa F, Nemomsa D. Gestational weight gain and its associated factors in Harari regional state: institution based cross-sectional study, Eastern Ethiopia. Reprod Health. 2016;13(1):101.
Guo L, Liu J, Ye R, Liu J, Zhuang Z, Ren A. Gestational weight gain and overweight in children aged 3&ndash;6 years. J Epidemiol. 2015;25(8):536–43.
Abeysena C, Jayawardana P. Sleep deprivation, physical activity and low income are risk factors for inadequate weight gain during pregnancy: a cohort study. J Obstet Gynaecol Res. 2011;37(7):734–40.
Munim S, Maheen H. Association of gestational weight gain and pre-pregnancy body mass index with adverse pregnancy outcome. J Coll Physicians Surg Pak. 2012;22(11):694–8.
Shi P, Yang W, Yu Q, Zhao Q, Li C, Ma X, et al. Overweight, gestational weight gain and elevated fasting plasma glucose and their association with Macrosomia in Chinese pregnant women. Matern Child Health J. 2014;18(1):10–5.
Radhakrishnan U, Kolar G, Nirmalan PK. Cross-sectional study of gestational weight gain and perinatal outcomes in pregnant women at a tertiary care center in southern India. J Obstet Gynaecol Res. 2014;40(1):25–31.
Soltani H, Lipoeto NI, Fair FJ, Kilner K, Yusrawati Y. Pre-pregnancy body mass index and gestational weight gain and their effects on pregnancy and birth outcomes: a cohort study in West Sumatra, Indonesia. BMC Womens Health. 2017;17(1):102.
Li C, Liu Y, Zhang W. Joint and Independent Associations of Gestational Weight Gain and Pre-Pregnancy Body Mass Index with Outcomes of Pregnancy in Chinese Women: A Retrospective Cohort Study. PLoS One. 2015;10(8):e0136850.
Abbasalizad FM. Gestational weight gain and its related social and demographic factors in health care settings of rural and urban areas in Northwest Iran. Ecol Food Nutr. 2016;55(3):258–65.
Papazian T, Abi Tayeh G, Sibai D, Hout H, Melki I, Rabbaa KL. Impact of maternal body mass index and gestational weight gain on neonatal outcomes among healthy middle-eastern females. PLoS One. 2017;12(7):e0181255.
Kheirouri S, Alizadeh M. The contribution of prenatal maternal factors to maternal gestational weight gain. Health Care Women Int. 2017;38(6):544–55.
Enomoto K, Aoki S, Toma R, Fujiwara K, Sakamaki K, Hirahara F. Pregnancy outcomes based on pre-pregnancy body mass index in Japanese women. PLoS One. 2016;11(6):e0157081.
Liu L, Hong Z, Zhang L. Associations of prepregnancy body mass index and gestational weight gain with pregnancy outcomes in nulliparous women delivering single live babies. Sci Rep. 2015;5:12863.
Thapa M, Paneru R. Gestational weight gain and its relation with birth weight of the newborn. JNMA J Nepal Med Assoc. 2017;56(207):309–13.
Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Alexi CD, Georgoulis M, et al. Association between gestational weight gain and risk of obesity in preadolescence: a longitudinal study (1997-2007) of 5125 children in Greece. J Hum Nutr Diet. 2017;30(1):51–8.
Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised Institute of Medicine Recommendations for maternal weight gain. J Clin Endocrinol Metab. 2012;97(10):3648–54.
Beyerlein A, Nehring I, Rzehak P, Heinrich J, Müller MJ, Plachta-Danielzik S, et al. Gestational Weight Gain and Body Mass Index in Children: Results from Three German Cohort Studies. PLoS One. 2012;7(3):e33205.
Jacota M, Forhan A, Saldanha-Gomes C, Charles MA, Heude B. Maternal weight prior and during pregnancy and offspring’s BMI and adiposity at 5-6 years in the EDEN mother-child cohort. Pediatr Obes. 2017;12(4):320–9.
Henriksson P, Eriksson B, Forsum E, Löf M. Gestational weight gain according to Institute of Medicine recommendations in relation to infant size and body composition. Pediatr Obes. 2015;10(5):388–94.
Walsh JM, McGowan CA, Mahony RM, Foley ME, McAuliffe FM. Obstetric and metabolic implications of excessive gestational weight gain in pregnancy. Obesity. 2014;22(7):1594–600.
Kinnunen TI, Waage CW, Sommer C, Sletner L, Raitanen J, Jenum AK. Ethnic differences in gestational weight gain: a population-based cohort study in Norway. Matern Child Health J. 2016;20(7):1485–96.
Chmitorz A, von Kries R, Rasmussen KM, Nehring I, Ensenauer R. Do trimester-specific cutoffs predict whether women ultimately stay within the Institute of Medicine/National Research Council guidelines for gestational weight gain? Findings of a retrospective cohort study. Am J Clin Nutr. 2012;95(6):1432–7.
Popa AD, Popescu RM, Botnariu GE. Adequate weight gain in pregnancy: an analysis of its determinants in a cross-sectional study. Srp Arh Celok Lek. 2014;142(11–12):695–702.
Przybyłowicz K, Przybyłowicz M, Grzybiak M, Janiszewska K. Effects of physical activity during pregnancy and gestational weight gain on newborn weight and length at birth in Warmińsko-Mazurskie province. Acta Sci Pol Technol Aliment. 2014;13(2):203–11.
Diemert A, Lezius S, Pagenkemper M, Hansen G, Drozdowska A, Hecher K, et al. Maternal nutrition, inadequate gestational weight gain and birth weight: results from a prospective birth cohort. BMC Pregnancy Childbirth. 2016;16(1):224.
Heery E, Kelleher CC, Wall PG, McAuliffe FM. Prediction of gestational weight gain – a biopsychosocial model. Public Health Nutr. 2015;18(08):1488–98.
Vila-Candel R, Soriano-Vidal FJ, Navarro-Illana P, Murillo-Llorente MT, Martín-Moreno JM. Relationship between maternal body mass index, gestational weight gain and birth weight; prospective study in a health department. Nutr Hosp. 2015;31(4):1551–7.
Logan CA, Bornemann R, Koenig W, Reister F, Walter V, Fantuzzi G, et al. Gestational weight gain and fetal-maternal Adiponectin, Leptin, and CRP: results of two birth cohorts studies. Sci Rep. 2017;7:41847.
Özdek S, Kul M, Barış Akcan A, Çekmez F, Aydemir G, Aydınöz S, et al. The effect of the pre-pregnancy weight of the mother and the gestational weight gain on the bilirubin level of term newborn. J Matern Neonatal Med. 2015;29(15):1–4.
Cinelli G, Fabrizi M, Ravà L, Ciofi Degli Atti M, Vernocchi P, Vallone C, et al. Influence of Maternal Obesity and Gestational Weight Gain on Maternal and Foetal Lipid Profile. Nutrients. 2016;8(6):368.
Maier JT, Schalinski E, Gauger U, Hellmeyer L. Antenatal body mass index (BMI) and weight gain in pregnancy - its association with pregnancy and birthing complications. J Perinat Med. 2016;44(4):397–404.
Ramón-Arbués E. Ganancia de peso gestacional y retención de peso posparto en una cohorte de mujeres en Aragón (España). Nutr Hosp. 2017;34(5):142–9.
Tulmaç ÖB, Dağ ZÖ, Erdoğan F, Sayan CD, Sağsöz N. Association of body mass index and weight gain patterns with albumin excretion in pregnancy. J Obstet Gynaecol Res. 2018;44(3):384–9.
Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202(6):574.e1–8.
Widen EM, Factor-Litvak PR, Gallagher D, Paxton A, Pierson RN, Heymsfield SB, et al. The pattern of gestational weight gain is associated with changes in maternal body composition and neonatal size. Matern Child Health J. 2015;19(10):2286–94.
Wander PL, Sitlani CM, Badon SE, Siscovick DS, Williams MA, Enquobahrie DA. Associations of early and late gestational weight gain with infant birth size. Matern Child Health J. 2015;19(11):2462–9.
Larouche M, Ponette V, Correa JA, Krishnamurthy S. The effect of recent immigration to Canada on gestational weight gain. J Obstet Gynaecol Canada. 2010;32(9):829–36.
Badon SE, Dyer AR, Josefson JL. Gestational weight gain and neonatal adiposity in the hyperglycemia and adverse pregnancy outcome study-north American region. Obesity. 2014;22(7):1731–8.
Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr. 2011;158(2):221–6.
Polinski KJ, Liu J, Boghossian NS, McLain AC. Maternal obesity, gestational weight gain, and asthma in offspring. Prev Chronic Dis. 2017;14:170196.
Ferraro ZM, Barrowman N, Prud’homme D, Walker M, Wen SW, Rodger M, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med. 2012;25(5):538–42.
Davis RR, Hofferth SL, Shenassa ED. Gestational weight gain and risk of infant death in the United States. Am J Public Health. 2014;104(Suppl):S90–5.
Fontaine PL, Hellerstedt WL, Dayman CE, Wall MM, Sherwood NE. Evaluating body mass index-specific trimester weight gain recommendations: differences between black and white women. J Midwifery Womens Health. 2012;57(4):327–35.
Park S, Sappenfield WM, Bish C, Salihu H, Goodman D, Bensyl DM. Assessment of the Institute of Medicine Recommendations for weight gain during pregnancy: Florida, 2004–2007. Matern Child Health J. 2011;15(3):289–301.
Fuemmeler BF, Wang L, Iversen ES, Maguire R, Murphy SK, Hoyo C. Association between Prepregnancy body mass index and gestational weight gain with size, tempo, and velocity of infant growth: analysis of the newborn epigenetic study cohort. Child Obes. 2016;12(3):210–8.
Gawade P, Markenson G, Bsat F, Healy A, Pekow P, Plevyak M. Association of gestational weight gain with cesarean delivery rate after labor induction. J Reprod Med. 2011;56(3–4):95–102.
Kowal C, Kuk J, Tamim H. Characteristics of weight gain in pregnancy among Canadian women. Matern Child Health J. 2012;16(3):668–76.
Gallagher K, Migliaccio L, Rogers RG, Leeman L, Hervey E, Qualls C. Impact of nulliparous women’s body mass index or excessive weight gain in pregnancy on genital tract trauma at birth. J Midwifery Womens Health. 2014;59(1):54–9.
Simas TAM, Waring ME, Liao X, Garrison A, Sullivan GMT, Howard AE, et al. Prepregnancy weight, gestational weight gain, and risk of growth affected neonates. J Women’s Health (Larchmt). 2012;21(4):410–7.
Pawlak MT, Alvarez BT, Jones DM, Lezotte DC. The effect of race/ethnicity on gestational weight gain. J Immigr Minor Health. 2015;17(2):325–32.
Ashley-Martin J, Dodds L, Arbuckle T, Morisset A-S, Fisher M, Bouchard M, et al. Maternal and neonatal levels of Perfluoroalkyl substances in relation to gestational weight gain. Int J Environ Res Public Health. 2016;13(1):146.
Kominiarek MA, Crockett A, Covington-Kolb S, Simon M, Grobman WA. Association of Group Prenatal Care with Gestational Weight Gain. Obstet Gynecol. 2017;129(4):663–70.
Subhan FB, Colman I, McCargar L, Bell RC. Higher pre-pregnancy BMI and excessive gestational weight gain are risk factors for rapid weight gain in infants. Matern Child Health J. 2017;21(6):1396–407.
Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the healthy start study. Am J Clin Nutr. 2015;101(2):302–9.
Mamun AA, Callaway LK, O’Callaghan MJ, Williams GM, Najman JM, Alati R, et al. Associations of maternal pre-pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy Childbirth. 2011;11(1):62.
Blumfield ML, Schreurs M, Rollo ME, MacDonald-Wicks LK, Kokavec A, Collins CE. The association between portion size, nutrient intake and gestational weight gain: a secondary analysis in the WATCH study 2006/7. J Hum Nutr Diet. 2016;29(3):271–80.
de Jersey SJ, Nicholson JM, Callaway LK, Daniels LA. A prospective study of pregnancy weight gain in Australian women. Aust N Z J Obstet Gynaecol. 2012;52(6):545–51.
Hartley E, McPhie S, Fuller-Tyszkiewicz M, Hill B, Skouteris H. Psychosocial factors and excessive gestational weight gain: the effect of parity in an Australian cohort. Midwifery. 2016;32:30–7.
Castillo H, Santos IS, Matijasevich A. Maternal pre-pregnancy BMI, gestational weight gain and breastfeeding. Eur J Clin Nutr. 2016;70(4):431–6.
Drehmer M, Camey S, Schmidt MI, Olinto MTA, Giacomello A, Buss C, et al. Socioeconomic, demographic and nutritional factors associated with maternal weight gain in general practices in southern Brazil. Cad Saude Publica. 2010;26(5):1024–34.
de Paulino DSM, Surita FG, Peres GB, do Nascimento SL, Morais SS. Association between parity, pre-pregnancy body mass index and gestational weight gain. J Matern Fetal Neonatal Med. 2016;29(6):880–4.
Garmendia ML, Mondschein S, Matus O, Murrugarra R, Uauy R. Predictors of gestational weight gain among Chilean pregnant women: the Chilean maternal and infant nutrition cohort study. Health Care Women Int. 2017;38(8):892–904.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Report for Selected Countries and Subjects. Available from: https://www.imf.org/external/pubs/ft/weo/2018/01/weodata/weorept.
National Heart, Lung and BI. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, vol. 2015. Bethesda: National Institutes of Health; 2014.
Rogozińska E, Zamora J, Marlin N, Betrán AP, Astrup A, Bogaerts A, et al. Gestational weight gain outside the Institute of Medicine recommendations and adverse pregnancy outcomes: Analysis using individual participant data from randomised trials. BMC Pregnancy Childbirth. 2019;19(1):322 [cited 2020 May 11].
Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16(1):153 [cited 2019 Aug 26].
Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane database Syst Rev. 2015;6:CD007145. https://doi.org/10.1002/14651858.CD007145.pub3.
Kapadia MZ, Gaston A, Van Blyderveen S, Schmidt L, Beyene J, McDonald H, et al. Psychological antecedents of excess gestational weight gain: a systematic review. BMC Pregnancy Childbirth. 2015;15(1):107.
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21 [cited 2019 Sep 2].
Templin T, Hashiguchi TCO, Thomson B, Dieleman J, Bendavid E. The overweight and obesity transition from the wealthy to the poor in low- And middleincome countries: A survey of household data from 103 countries. PLoS Med. 2019;16(11):e1002968.
Norte A, Sospedra I, Ortíz-Moncada R. Influence of economic crisis on dietary quality and obesity rates. Int J Food Sci Nutr. 2019;70(2):232–9.
Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9(2):140–50 [cited 2018 Apr 12].
Pugh SJ, Albert PS, Kim S, Grobman W, Hinkle SN, Newman RB, et al. Patterns of gestational weight gain and birthweight outcomes in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies–Singletons: a prospective study. Am J Obstet Gynecol. 2017;217(3):346.e1–346.e11.
Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth, vol. 52: Best Practice and Research: Clinical Obstetrics and Gynaecology. Elsevier Ltd; 2018. p. 3–12. https://doi.org/10.1016/j.bpobgyn.2018.04.0031521-6934/©2018.
Klohe-Lehman DM, Freeland-Graves J, Anderson ER, McDowell T, Clarke KK, Hanss-Nuss H, et al. Nutrition knowledge is associated with greater weight loss in obese and overweight low-income mothers. J Am Diet Assoc. 2006;106(1):65–75.
Bogaerts A, Van den Bergh B, Nuyts E, Martens E, Witters I, Devlieger R. Socio-demographic and obstetrical correlates of pre-pregnancy body mass index and gestational weight gain. Clin Obes. 2012;2(5–6):150–9.
Rossin-Slater M. WIC in your neighborhood: new evidence on the impacts of geographic access to clinics. J Public Econ. 2013;102:51–69.
Alavi N, Haley S, Chow K, McDonald SD. Comparison of national gestational weight gain guidelines and energy intake recommendations. Obes Rev. 2013;14(1):68–85.
American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013;121(1):213–7.
Smith GN, Pudwell J, Roddy M. The maternal health clinic: a new window of opportunity for early Heart disease risk screening and intervention for women with pregnancy complications. J Obstet Gynaecol Canada. 2013;35(9):831–9.
Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118(3):278–84.
Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5(1):65–76 [cited 2019 Sep 3].
Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. Obstetr Gynecological Survey. 2014;69:1–12.
Scott C, Andersen CT, Valdez N, Mardones F, Nohr EA, Poston L, et al. No global consensus: a cross-sectional survey of maternal weight policies. BMC Pregnancy Childbirth. 2014;14(1):167.
Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18(3):350–69.
Acknowledgements
Not applicable.
Funding
This study did not receive any funding. Miriam Garrido Miguel is supported by a grant from the Spanish Ministry of Education, Culture and Sport (FPU15/03847). Finally, Alba Soriano Cano is supported by a grant from the Spanish Ministry of Economy, Industry, and Competitiveness (FI17/332). The funding bodies did not play any role in the design of the study, collection, analysis and interpretation of the data, nor in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
JAMH and CA-B conceived the idea for the meta-analyses, conducted the database searches, selected and retrieved the relevant papers, designed the data extraction tool, carried out data extraction and, checking of data, and quality assessments, and wrote the article. IC-R contributed to the analysis and interpretation, carried out data extraction, and checking of data and quality assessments. IC-R, MG-M and AS-C reviewed and edited the article. VMV contributed to the study conception, made the final decisions regarding the inclusion/exclusion of all papers, and reviewed and edited the article. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Mean gestational weight gain in kilograms. Figure S2. Prevalence of gestational weight gain below the 2009 IOM guidelines. Figure S3. Prevalence of gestational weight gain within guidelines according to the 2009 IOM guidelines. Figure S4. Prevalence of excessive gestational weight gain according to the 2009 IOM guidelines. Figure S5. Mean prepregnancy BMI in kilograms per meter squared. Figure S6. Prevalence of underweight prepregnancy BMI. Figure S7. Prevalence of normal-weight prepregnancy BMI. Figure S8. Prevalence of overweight prepregnancy BMI. Figure S9. Prevalence of obesity prepregnancy BMI. Table S1. Search strategy for MEDLINE. Table S2. Meta-regression with mean GWG. Table S3. Meta-regression with prepregnancy BMI mean. Table S4. Meta-regression with the prevalence of GWG categories by year of recruitment. Table S5. Meta-regression with prevalence the prepregnancy BMI categories by year of recruitment. Table S6. Meta-regression by economic position according to IMF staff estimates of each country. Table S7. Quality assessment with The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Table S8. The 1990 IOM guidelines for total weight gain during pregnancy. Table S9. The 2009 IOM guidelines for total weight gain during pregnancy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Martínez-Hortelano, J.A., Cavero-Redondo, I., Álvarez-Bueno, C. et al. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: a systematic review and meta-analysis. BMC Pregnancy Childbirth 20, 649 (2020). https://doi.org/10.1186/s12884-020-03335-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-020-03335-7