Abstract
Background
Acute kidney injury (AKI) is a life-threatening complication of rhabdomyolysis (RM). The aim of the present study was to assess patients at high risk for the occurrence of severe AKI defined as stage II or III of KDIGO classification and in-hospital mortality of AKI following RM.
Methods
We performed a retrospective study of patients with creatine kinase levels > 1000 U/L, who were admitted to the West China Hospital of Sichuan University between January 2011 and March 2019. The sociodemographic, clinical and laboratory data of these patients were obtained from an electronic medical records database, and univariate and multivariate regression analyses were subsequently conducted.
Results
For the 329 patients included in our study, the incidence of AKI was 61.4% and the proportion of stage I, stage II, stage III were 18.8, 14.9 and 66.3%, respectively. The overall mortality rate was 19.8%; furthermore, patients with AKI tended to have higher mortality rates than those without AKI (24.8% vs. 11.8%; P < 0.01). The clinical conditions most frequently associated with RM were trauma (28.3%), sepsis (14.6%), bee sting (12.8%), thoracic and abdominal surgery (11.2%) and exercise (7.0%). Furthermore, patients with RM resulting from sepsis, bee sting and acute alcoholism were more susceptible to severe AKI. The risk factors for the occurrence of stage II-III AKI among RM patients included hypertension (OR = 2.702), high levels of white blood cell count (OR = 1.054), increased triglycerides (OR = 1.260), low level of high-density lipoprotein cholesterol (OR = 0.318), elevated serum phosphorus (OR = 5.727), 5000<CK ≤ 10,000 U/L (OR = 2.617) and CK>10,000 U/L (OR = 8.093). Age ≥ 60 years (OR = 2.946), sepsis (OR = 3.206) and elevated prothrombin time (OR = 1.079) were independent risk factors for in-hospital mortality in RM patients with AKI.
Conclusions
AKI is independently associated with mortality in patients with RM, and several risk factors were found to be associated with the occurrence of severe AKI and in-hospital mortality. These findings suggest that, to improve the quality of medical care, the early prevention of AKI should focus on high-risk patients and more effective management.
Similar content being viewed by others
Background
Rhabdomyolysis (RM) is a syndrome characterized by the disruption of skeletal muscle cell integrity, with subsequent release of intracellular components into the extracellular environment. There are numerous reported causes of RM, and the most frequently associated etiologies are trauma, immobilization, sepsis and surgery [1,2,3]. The clinical manifestations of RM (and their subsequent severity) vary based on the specific cause. These range from isolated elevation of laboratory indices, such as myoglobin and creatine kinase (CK), to life-threatening electrolyte disturbances and organ dysfunction [4, 5]. Acute kidney injury (AKI) is a common complication of RM, and the pathogenesis of RM-associated AKI includes tubular obstruction caused by myoglobin, myoglobin cytotoxicity by lipid peroxidation, and the production of reactive oxygen species. Intracellular components released into the circulation cause capillary damage, which leads to leakage and edema, secondary hypovolemia and decreased renal blood flow, and ultimately reduce renal function [2, 6]. The incidence of AKI is reported to be between 37.8 and 81.4% in patients with RM [7,8,9,10,11], and is independently associated with a 19.2–59.0% increase in mortality [9, 12, 13]. The occurrence of AKI is associated with a worse outcome in patients with RM, which increases medical burden; thus, the prevention and early diagnosis of AKI are critical to improving patient prognosis. Therefore, we conducted a retrospective analysis of 329 patients with RM to characterize the incidence of AKI in these patients, to identify independent risk factors of stage II-III AKI for prevention and early detection of it, and wanted to provide a useful indication of mortality risk in patients with AKI.
Methods
Study population
The present study was a retrospective analysis of data collected from the West China Hospital of Sichuan University, from patients admitted between January 2011 and March 2019. All patients over 18 years old with CK levels > 1000 U/L were eligible. The exclusion criteria were as follows: (i) Patients with pre-existing end-stage renal disease; (ii) patients who had received a kidney transplant; (iii) patients with abnormal CK elevation from acute myocardial infarction; and (iv) patients with incomplete data. To avoid bias among patients who were repeatedly admitted to hospital during the study period, only the first admission was included.
Data collection
Patient sociodemographic, clinical and laboratory data were obtained from an electronic medical records database. Sociodemographic data included age, sex and date of admission. Clinical data included etiology, smoking history, alcoholism and chronic disease history (hypertension, diabetes and hyperlipidemia), the presence of sepsis and the outcomes (length of stay in hospital and mortality). Laboratory data comprised blood levels of CK, baseline biomarkers of renal function (serum creatinine (SCr), blood urea nitrogen (BUN), cystatin C and uric acid), levels of phosphate, calcium, potassium, aminotransferase, albumin, bilirubin, hemoglobin, lipoprotein and prothrombin time, as well as white blood cell (WBC) and platelet counts.
Definition
In the present study, AKI was defined per the Kidney Disease Improving Global Outcomes criteria (KDIGO) [14]: An absolute increase in serum creatinine of ≥26.4 μmol/L within 48 h, or ≥ 50% baseline serum creatinine within 7 days. Due to incomplete urine output data, only the serum creatinine readings were available. For the baseline creatinine levels, we used the lowest value of serum creatinine recorded in the 2 days prior to admission, and if not available, the first serum creatinine reading within 2 days after admission [15]. In this study, the AKI was categorized as AKI I, II, III, respectively, according to increase in serum creatinine ≥26.4 μmol/L or increase to ≥1.5-fold to twofold from baseline, > twofold to threefold from baseline and > threefold from baseline or serum creatinine ≥354 μmol/L with an acute increase of at least 44 μmol/L. Individuals who receive renal replacement therapy were considered to have met the criteria of AKI III regardless of their serum creatinine value. The AKI stage was evaluated weekly, and the maximum degree was regarded as the final AKI stage. By reviewing the medical records of all patients, the etiologies of rhabdomyolysis and outcomes of patients during hospitalization were confirmed.
Statistical analysis
Categorical covariates were recorded as frequency distributions and proportions. According to the distribution pattern, continuous variables were recorded as the mean ± standard deviation (SD). Univariate analysis was employed to predict disease etiology, using a binomial distribution; the Students t-test and Pearson χ2 test were used to estimate baseline characteristics. Independent predictors of AKI incidence and in-hospital mortality were evaluated by univariate and multivariate logistic regression, which were utilized to calculate odds ratios (ORs) and 95% confidence intervals. Only the significant risk factors identified by univariate analysis were considered for multiple regression analysis. The data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and P < 0.05 (two-sided) was considered to indicate a statistically significant difference.
Results
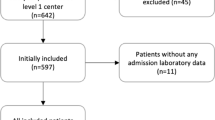
In the present study, we identified 383 hospitalizations of adults with a CK level > 1000 U/L between January 2011 and March 2019. After excluding 6 patients with pre-existing end-stage renal disease or who had received a kidney transplant, 1 case of acute myocardial infraction, 7 patients aged< 18 years and 40 with incomplete data, the final study population included 329 patients (Fig. 1). There were 202 (61.4%) RM-induced AKI patients, among which 18.8% were classified as AKI stage I, 14.9% as AKI stage II and 66.3% as AKI stage III according to the KDIGO criteria. The patient age (mean ± SD) was 45.7 ± 15.9 years, and 74.8% of the patients were male. The overall in-hospital mortality rate was 19.8%, and patients with secondary AKI tended to exhibit higher mortality rates than those without AKI (24.8% vs. 11.8%, P < 0.01). The clinical conditions most frequently associated with RM were trauma (28.3%), sepsis (14.6%), bee sting (12.8%), thoracic and abdominal surgery (11.2%) and exercise (7.0%). Other causes are shown in Table 1. Patients with RM resulting from sepsis, bee sting and acute alcoholism were more susceptible to stage II-III AKI.
The baseline characteristics and their comparison between RM patients with or without stage II-III AKI are displayed in Table 2. Compared with the patients without stage II-III AKI, the proportions of patients aged ≥60 years, chronic alcoholism, hypertension, diabetes, sepsis, CK>10,000 U/L and those that succumbed to the disease were significantly higher in the stage II-III AKI group. Higher WBC counts, serum bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride, biomarkers of baseline renal function (SCr, BUN, cystatin C and uric acid), potassium, calcium and phosphorus levels were detected in patients with stage II-III AKI. The level of high-density lipoprotein cholesterol (HDLC) in patients with stage II-III AKI was lower than that in those without stage II-III AKI. However, in terms of gender, smoking history, hyperlipidemia, hemoglobin, blood platelet count, serum albumin, cholesterol, low-density lipoprotein cholesterol (LDLC), prothrombin time and length of stay, there were no significant differences between the two groups.
We then evaluated the effects of the independent risk factors on the occurrence of stage II-III AKI, using univariate and multivariate logistic regression. Multivariate logistic regression highlighted several distinguishing variables between the two groups, including hypertension (OR = 2.702; 95% CI 1.048–6.968), elevated WBC count (OR = 1.054; 95% CI 1.010–1.100), triglycerides (OR = 1.260; 95% CI 1.090–1.457), HDLC (OR = 0.318; 95% CI 0.139–0.726) and serum phosphorus (OR = 5.727; 95% CI 2.869–11.430), 5000 < CK ≤10,000 U/L (OR = 2.617; 95% CI 1.089–6.289) and CK>10,000 U/L (OR = 8.093; 95% CI 3.679–17.799) (Table 3). Similarly, risk factors for in-hospital mortality in patients with RM-induced AKI were analyzed. Age ≥ 60 years (OR = 2.946; 95% CI 1.072–8.097), sepsis (OR = 3.206; 95% CI 1.351–7.609) and elevated prothrombin time (OR = 1.079; 95% CI 1.004–1.160) were identified as independent risk factors, which may be associated with increased mortality rates among these patients (Table 4).
Discussion
AKI is independently associated with the mortality rates of patients with RM. Our results have identified several independent risk factors for secondary stage II-III AKI, including hypertension, CK>5000 U/L, decreased HDLC, high levels of white blood cell count, triglycerides and serum phosphorus. Age ≥ 60 years, sepsis and elevated prothrombin time were all associated with increased mortality rates. Moreover, patients with RM resulting from sepsis, bee sting and acute alcoholism were at a higher risk of developing stage II-III AKI.
The causes of RM can be classified in a number of ways. According to the mechanisms of skeletal muscle damage, the causes have been categorized into four mechanisms: Hypoxic, physical, chemical and biological [4]. Other classification categories include surgical/medical, acquired/inherited and physical/non-physical [1, 16, 17]. In the present study, the most common cause of RM was trauma (a condition largely associated with RM [1, 2]), followed by sepsis and bee sting. Compared with other causes, patients with RM resulting from sepsis, bee sting and acute alcoholism were observed more frequently in the severe AKI group. In our study, the morbidity rate of AKI was 61.4%, which is almost in accord with previous reports [7,8,9,10,11], although this number varies between different studies.
Patients with RM-related AKI are associated with an increased risk of death and total health-related costs than those who do not develop AKI. Our analysis showed that the total mortality rate of these patients was 19.8%, whereas AKI patients experienced a significantly higher mortality rate than those without it (24.8% vs 11.8%, P < 0.01), which was within the wide range of reported mortality rates for his condition [9, 12, 13].
Different study populations (such as those with RM associated with drug use, trauma, wasp stings, infection and hospitalization) play an important role in the variations in AKI-associated morbidity, and the subsequent mortality rates of RM patients. In addition, different inclusion criteria (such as elevated CK, race and the definition of AKI itself) may also affect these results. A previous study reported that the incidence of AKI was highest according to the KDIGO definition, followed by the Acute Kidney Injury Network criteria and the Acute Dialysis Quality Initiative’s RIFLE criteria [18].
We revealed that hypertension was one of the independent risk factors for stage II-III AKI in RM patients; likewise, a previous study reported that diagnosed hypertension was an independent risk factor for AKI in patients with chronic kidney disease or after emergency department contrast-enhanced computerized tomography [19, 20]. In addition, a retrospective study of 43,611 patients demonstrated that the occurrence of AKI in hospitalized, previously normotensive adults was independently associated with increased blood pressure during the first 2 years after discharge [21]. However, there are also reports that patients with hypotension are at an increased risk of developing renal failure, decreased renal perfusion aggravate renal function [22, 23]. Long lasting hypertension damages renal blood vessels. Hypertensive patients with renin-angiotensin-aldosterone system blockade carry high risk when suffer episode of secondary AKI [24, 25]. Overexpression of reactive oxygen species caused by angiotensin 2 and decreased production of nitric oxide potently participate in the kidney ischemic injury in hypertensive surroundings [26, 27].
Low levels of HDLC have been associated with worse short-term and long-term renal outcomes in patients who suffer AKI caused by sepsis and underwent percutaneous kidney biopsy for histological diagnosis of AKI [28, 29]. In addition, HDLC was observed to have superior predictive ability than routine clinical markers for development of multiorgan dysfunction syndrome [30]. We firstly demonstrated that decreased HDLC was an independent risk factor for RM-associated stage II-III AKI. Apolipoprotein A-I mimetic peptide 4F inhibiting inflammatory responses, strengthening the vascular barrier and protecting kidney in an HDL-dependent manner was observed in animal experiment [31].
Hyperphosphatemia is a common complication of RM with several proposed pathogenic factors, including the release of inorganic phosphorus into the plasma and reduced urinary phosphate excretion [32, 33]. In the present study, increased serum phosphate was determined to be an independent predictor for AKI-associated RM, which is in accordance with previously reported findings [11, 34, 35]. Furthermore, phosphate has also been verified as a potential biomarker of disease severity and prognosis in AKI patients undergoing continuous renal replacement therapy [36]; this phenomenon was not observed in our study, which included patients with AKI of all stages.
The significant correlation between serum CK level and the risk of RM-induced AKI has been demonstrated in previous studies [37,38,39], our analysis confirmed that. Moreover, different classifications (5000 < CK ≤10,000 U/L, CK>10,000 U/L) have different risks for stage II-III AKI in patients with RM. While, elevated serum CK is not associated with the mortality rate of patients with AKI.
Study limitations
The present study includes the following limitations. Firstly, most of the recruited patients were identified by discharge diagnoses in an electronic medical record database; hence, a proportion of the patients with elevated serum CK were missed due to a lack of RM diagnosis. Secondly, where existing urine volume data were not available, AKI diagnosis was based on SCr alone; thus, the incidence of AKI may have been underestimated. Thirdly, some of the possible risk factors for AKI, such as the level of myoglobin, lactic acid, associated drugs, as well as the severity-of-illness scores, were not included due to incomplete data. Finally, no follow-up study was conducted; thus, long-term patient prognoses (including recovery of renal function, risk of recurrent AKI and late mortality) were not determined.
Conclusion
The occurrence of AKI increases the mortality rate of patients with RM. The early evaluation and diagnosis are crucial for the prevention of AKI and improved patient prognosis. Our study demonstrated several risk factors for RM-induced stage II-III AKI and in-hospital mortality in RM patients with AKI. These findings may facilitate the effective prevention and management of RM patients with AKI. However, it also has several limitations. We will conduct further research to improve the study.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.
Abbreviations
- RMf:
-
Rhabdomyolysis
- CK:
-
Creatine kinase
- AKI:
-
Acute kidney injury
- SCr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
- WBC:
-
White blood cell
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- SD:
-
Standard deviation
- OR:
-
Odds ratio
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HDLC:
-
High-density lipoprotein cholesterol
- LDLC:
-
Low-density lipoprotein cholesterol
- PT:
-
Prothrombin time
References
McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in Rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821.
Chavez LO, et al. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20(1):135.
E, E. An observational study on rhabdomyolysis in the intensive care unit. Ann Intensive Care. 2013;3(1):8.
Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058–65.
Chatzizisis YS, et al. The syndrome of rhabdomyolysis: complications and treatment. Eur J Internal Med. 2008;19(8):568–74.
Boutaud O, Roberts LJ. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic Biol Med. 2011;51(5):1062–7.
Koubar SH, et al. Rhabdomyolysis in an HIV cohort: epidemiology, causes and outcomes. BMC Nephrol. 2017;18(1):242.
Grunau BE, et al. Characteristics and thirty-day outcomes of emergency department patients with elevated Creatine kinase. Acad Emerg Med. 2014;21(6):631–6.
Rodríguez E, et al. Risk factors for acute kidney injury in severe Rhabdomyolysis. PLoS One. 2013;8(12):e82992.
Xie C, et al. Clinical features of severe wasp sting patients with dominantly toxic reaction: analysis of 1091 cases. PLoS One. 2013;8(12):e83164.
Nelly C, et al. Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;27(1):682.
Harrois A, et al. Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care. 2018;22(1):344.
Kumar AA, et al. Rhabdomyolysis in community acquired bacterial Sepsis – a retrospective cohort study. PLoS One. 2009;4(9):e7182.
Kellum JA. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204.
Zhou J, et al. A simple risk score for prediction of sepsis associated-acute kidney injury in critically ill patients. J Nephrol. 2019;32(6):947–56.
Petejova N. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(1):224.
Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis -- an overview for clinicians. Crit Care. 2005;9(2):158.
Zeng X, et al. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20.
Traub SJ, et al. Risk factors for Radiocontrast nephropathy after emergency department contrast-enhanced computerized tomography. Acad Emerg Med. 2013;20(1):40–5.
Hsu CY, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–7.
Hsu C, et al. Elevated BP after AKI. J Am Soc Nephrol. 2016;27(3):914–23.
Saito S, et al. Postoperative blood pressure deficit and acute kidney injury progression in vasopressor-dependent cardiovascular surgery patients. Crit Care. 2016;20(1):74.
Badin J. Relation between mean arterial pressure and renal function in the early phase of shock: a prospective, explorative cohort study. Crit Care. 2011;15(3):R135.
Ma M, et al. Renin-angiotensin-aldosterone system blockade is associated with higher risk of contrast-induced acute kidney injury in patients with diabetes. Aging. 2020;12(7):5858.
Scott J, et al. Estimating the risk of acute kidney injury associated with use of diuretics and renin angiotensin aldosterone system inhibitors: a population based cohort study using the clinical practice research datalink. BMC Nephrol. 2019;20(1):481.
Miloradović Z, et al. Angiotensin 2 type 1 receptor blockade different affects postishemic kidney injury in normotensive and hypertensive rats. J Physiol Biochem. 2016;72(4):813–20.
Miloradovic Z, et al. Nitric oxide supplementation in postischemic acute renal failure: normotension versus hypertension. Curr Pharm Biotechnol. 2011;12(9):1364–7.
Konigsfeld HP, et al. Acute kidney injury in hospitalized patients who underwent percutaneous kidney biopsy for histological diagnosis of their renal disease. BMC Nephrol. 2019;20(1):315.
Roveran Genga K, et al. Two-year follow-up of patients with septic shock presenting with low HDL: the effect upon acute kidney injury, death and estimated glomerular filtration rate. J Intern Med. 2017;281(5):518–29.
Cirstea M, et al. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care. 2017;38:289–94.
Moreira RS, et al. Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol Regulatory Integrative Comparative Physiol. 2014;307(5):R514–24.
Leaf DE, Christov M. Dysregulated mineral metabolism in AKI. Semin Nephrol. 2019;39(1):41–56.
Higaki M, et al. Acute kidney injury facilitates Hypocalcemia by exacerbating the Hyperphosphatemic effect of muscle damage in Rhabdomyolysis. Nephron. 2015;131(1):11–6.
Thongprayoon C, et al. Admission hyperphosphatemia increases the risk of acute kidney injury in hospitalized patients. J Nephrol. 2018;31(2):241–7.
Leaf DE, et al. Fibroblast growth factor 23 associates with death in critically ill patients. Clin J Am Soc Nephrol. 2018;13(4):531–41.
Jung S, et al. Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS One. 2018;13(2):e0191290.
Simpson JP, et al. Rhabdomyolysis and acute kidney injury. Eur J Anaesthesiol. 2016;33(12):906–12.
Safari S, et al. The value of serum creatine kinase in predicting the risk of rhabdomyolysis-induced acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. 2016;20(2):153–61.
PINHO FAMO. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int. 2005;67(1):659–67.
Acknowledgements
We thank all of the participants and attending physicians for their contributions.
Funding
The present study was partially supported by grants from the Sichuan Provincial Science and Technology Key R & D Projects [No. 2017SZ0113 and No. 2019YFS0282]. The leaders of these projects were Lichuan Yang and Jiaojiao Zhou, respectively. They played roles in financing, study design, administration, data analysis and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The conception and design of the study was made by LCY, JJZ and JY. Administration was supported by LCY and JJZ. The authors JY, XW, YT and SWW collected and assembled the data. Data analysis and interpretation were performed by JY and JJZ. The article was draft by JY and was revised by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committees of the West China Hospital of Sichuan University. Our retrospective research was in compliance with ethical standards. The Institutional Review Board affirmed our study and informed consent for patients in this retrospective study was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Zhou, J., Wang, X. et al. Risk factors for severe acute kidney injury among patients with rhabdomyolysis. BMC Nephrol 21, 498 (2020). https://doi.org/10.1186/s12882-020-02104-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-020-02104-0