Abstract
Background
K. pneumoniae become multidrug-resistant (MDR) and commonly poses a serious health threat to patients due to limited therapeutic options. As a result, determining the prevalence and antimicrobial susceptibility patterns of K. pneumoniae isolates from clinical specimens is substantial to patient diagnosis and treatment.
Methods and materials
A retrospective cross-sectional study was conducted from July 2021 to July 2022 at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Sociodemographic and laboratory data were collected from registered books using a data collection sheet. All types of samples were collected and processed using standard procedures. Identification of K. pneumoniae was done using Gram stain, colony characterization on culture media, anda series of biochemical tests. Antimicrobial susceptibility testing was done by the Kirby Bauer disc diffusion technique. The data were entered using Epi-info version 7 and exported to SPSS version 20 for analysis.
Results
Among 2600 clinical specimens, 735 (28.3%) were positive for bacteria, and K. pneumoniae isolates accounted for 147 (20%). Most of them were isolated from neonates and mainly obtained from blood specimens (81.6%). These isolates were 100% resistant to Nalidixic acid, Cefotaxime, and Cefazolin. About 84% and 83.3% of the isolates were also resistant to Ceftriaxone and Tetracycline, respectively. However, they are sensitive to Nitrofurantoin (86.6%), Imipenem (85.7%), Meropenem (79%), and Amikacin (78.3%). The overall proportion of MDR K. pneumoniae isolates accounted for 57.1%.
Conclusion
The magnitude of MDR K. pneumoniae was very alarming. Therefore, strengthening antimicrobial stewardship programs and antimicrobial surveillance practices is strongly recommended in the study area.
Similar content being viewed by others
Background
Klebsiella pneumoniae is a Gram-negative bacterium that belongs to the family Enterobacteriaceae [1]. It is a predominant hospital-acquired opportunistic pathogen, responsible for 30% of all gram-negative bacterial infections, and causes bloodstream infections, pneumonia, urinary tract infections (UTIs), meningitis, pyogenic liver abscesses, and wounds [2, 3]. The globalspread use of antimicrobials in clinical practice has led to the emergence of drug-resistant bacterial pathogens contributing to increased morbidity and mortality [4].
Nowadays, K. pneumoniae strains are recognized as an urgent threat to human health because of the emergence of multi-drug resistant (MDR) strains associated with hospital outbreaks [5]. According to the European antimicrobial resistance surveillance network for 2019, more than one-third (36.6%) of K. pneumoniae isolates were reported to be resistant to at least one of the antimicrobial groups (Fluoroquinolones, third-generation Cephalosporins, Aminoglycosides, and Carbapenems) under regular surveillance [6]. A national sentinel site surveillance reported that the resistance to antibiotics in K. pneumoniae reached 68.3% in South Africa [7]. Mechanisms of acquiring antibiotic resistance in K. pneumoniae related to plasmids and transposons encoding various enzymes and efflux pumps, mutations in different proteins (β-lactamases, efflux proteins, outer membrane proteins, gene replication enzymes, protein synthesis complexes, and transcription enzymes), and biofilm formation [8].
Concerning Ethiopia, factors such as insufficient surveillance, limited diagnostic laboratories, and resource constraints have limited resistance profiling of the etiological agents responsible for leading causes of hospitalization. A systematic review and meta-analysis report in Ethiopia showed an overall Klebsiella resistance of 53.75% with the highest resistance to Ampicillin (90.56%), followed by Amoxicillin (76.01%) and Trimethoprim-sulfamethoxazole (66.91%) [4]. Since an increased MDR K. pneumoniae infections in hospital and community settings, and its burden in the study areanot yet described, this study determined the prevalence and antimicrobial susceptibility pattern of K. pneumoniae in various clinical specimens. This would be beneficial for medical practitioners to avoid empirical treatment and select effective antibioticthat minimizes the emergence of drug-resistant bacterial strains.
Methods and materials
Study setting, period, and design
A retrospective cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital (UoGCSH), Gondar from July 2021 to July 2022. Gondar town is in the northern part of Ethiopia, Amhara National Regional State. Gondar is 747 km from Addis Ababa and 170 km from the regional capital, Bahir Dar. According to the central statistics agency, the town has a total population of 315,856(154,767 females and 161,087 males). The UoGCSHis one of the biggest tertiary-level teaching hospitals in the region with more than 500 beds. The hospital is used as a referral center for more than 10 million people in its catchment population [9].
Study population
All patients came to UoGCSH for health services during the study period with recorded necessary information. Data recorded from July 2021 to July 2022 in the microbiology culture registration book have at least sex, age, and ward type where the specimen come from were included in the study. Data recorded with incomplete age, sex, and ward type was excluded from the study.
Data collection
Data was collected using a retrospective review of one-year (July 2021 to July 2022) laboratory records of various specimen cultures from patients from all departments and units of the UoGCSH. We used a data collection form to collect patients’ sociodemographic and laboratory data such as age, sex, ward type/patient location, specimen type, culture results, the isolated bacteria, and antimicrobial susceptibility pattern results from the laboratory record books.
Laboratory methods
Sample processing and identification of bacterial isolates
Blood samples (10 mL for adults, 3 mL for children, and 0.5-1 mL for neonates) for cultures were obtained from each patient who developed a fever at the time of diagnosis. Blood samples were drawn from two different sites of peripheral veins aseptically (disinfecting with 70% alcohol and 2% tincture of iodine) by experienced nurses before any antibiotic use. The collected blood samples were then transferred into culture bottles of sterile Tryptic Soya Broth (Oxoid Ltd., Basingstoke, UK). Bottles were incubated at 37 °C for 7 days and observed for signs of bacterial growth (turbidity, hemolysis, clot formation) daily for up to 7 days. Bottles that showed signs of growth were gram-stained and sub-cultured on blood agar, chocolate agar, MacConkey agar, and mannitol salt agar (Oxoid Ltd., Basingstoke, UK). These plates were then aerobically incubated for 18–24 h at 37 °C. A blood sample containing broth with no bacterial growth after 7 days was sub-cultured before being reported as a negative result. Specimens from wounds and body fluids were cultured on MacConkey agar, 5% sheep blood agar, and chocolate agar, but urine was only inoculated on Cystine Lactose Electrolyte deficient agar if there was any growth subculture on MacConkey agar and 5% sheep blood agar.
After incubation of all inoculated culture plates at 35–37֯ C for 18–24 h, preliminary identification of bacterial isolates was based on the colony morphology and Gram staining reaction. Thus, the identified isolates underwent biochemical tests (indole, citrate, triple sugar iron agar, oxidative/fermentative, urease test, coagulase test, catalase, and oxidase test for confirmation). The presence of K. pneumoniae was confirmed by various phenotypic tests that included: gram-negative bacilli, catalase positive, oxidase negative, non-fastidious, lactose fermentative, gas positive, hydrogen sulfide negative, hemolysis negative, growth at 37 °C, urease positive, indole negative, citrate positive, motility negative, and utilization of 10% lactose positive.
Antimicrobial susceptibility testing
All K.pneumoniae isolates were investigated for AST against Amikacin (30 µg), Amoxicillin/Clavulanic acid (30/10µg), Cefotaxime (30 µg), Ceftriaxone (30 µg), Gentamicin (10 µg), Meropenem (10 µg), Piperacillin/Tazobactam (100/10µg), Tobramycin (10 µg), Tetracycline (30 µg), Ciprofloxacin(5 µg), Piperacillin (100 µg), Nitrofurantoin (50 µg), Nalidixic acid (30 µg), Imipenem (10 µg), Ertapenem (10 µg), Chloramphenicol (30 µg), Ceftazidime (30 µg), Cefazolin (4 µg), and Trimethoprim-Sulfamethoxazole(1.25 + 23.75 µg) (Abtek Biologicals, Ltd., Liverpool, UK) following the Kirby–Bauer method on Mueller–Hinton agar (Oxoid Ltd., Basingstoke, UK). A suspension of the test organism was prepared in peptone water and matched to 0.5 McFarland standards for AST. With the help of a sterile cotton swab, a lawn culture of the suspension was made on a Mueller–Hinton agar plate. Antibiotic discs were placed, maintaining a 25 mm distance between the two discs, and were incubated at 37֯C for 24 h. After overnight incubation, the zone of inhibition was measured for each antibiotic, and the results were interpreted as sensitive, intermediate, and resistant based on Clinical and Laboratory Standards Institute guidelines, 2022. Isolates not susceptible to at least one agent in three or more antibiotic classes were categorized as multidrug-resistant [10].
Quality control
All media and reagents used in this study were tested and verified for sterility and performance. For AST, the quality was assured using the control strains of E. coli ATCC 25,922 and S. aureus ATCC 25,923. To avoid technical errors that would be encountered during data collection, internal quality control was done. The data collection format of each data collector was checked daily for completeness of missed or other relevant information on meetings and supervision during data collection as well as by the principal investigators.
Data analysis
The collected data was entered into Epi-info version 7 and analyzed using SPSS version 20. Descriptive statistics were performed to correlate the sociodemographic characteristics of the participants and the bacteriological and antimicrobial susceptibility profiles of the isolates. The results were presented by percentage (%) and number using a table and chart.
Results
Sociodemographic characteristics of the study participants
A total of 2600 clinical specimens from patients suspected of bacterial infection were analyzed in the bacteriology laboratory at the UoGCSH. The male-to-female ratio of patients was1.42:1, with 1525/2600 (58.7%) being male. The mean age of the study participants was 25.52 with a standard deviation of 21.72, with a minimum age of 1 day and a maximum age of 96 years. Among age groups, the pediatric age groups were the predominant, which was 943/2600 (36.3%), followed by neonates accounted for 573/2600 (22%). Based on the patient location, NICU and General Ward accounted for the highest number, which was 769/2600 (29.6%) and 761/2600 (29.3%), respectively. Most of the clinical specimens processed were blood and urine, which accounted for 1864/2600 (71.7%) and 358/2600 (13.8%), respectively (Table 1).
Bacterial isolates from various clinical specimens
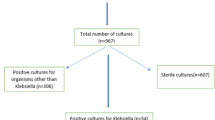
Among various clinical specimens, the culture positivity rate was higher in wounds; 44/84 (52.4%) followed by blood; 608/1864 (32.6%) (Fig. 1). The overall culture positivity rate in this study was 735/2600 (28.3%), and Gram-positive cocci accounted for 413/735 (56.2%), while Gram-negative organisms were 300/735 (40.8%). Yeast cells were also recovered at the rate of 22/735 (3%). Among the isolated Gram-negative organisms, K. pneumoniae was the predominant, which accounted for 147/735 (20%), followed by E. coli 82/735 (11.2%), Acinetobacter spp. 35/735 (4.8%), and Pseudomonas spp. 32/735 (4.4%) (Table 2).
Prevalence of K. pneumoniae by age, specimen, and ward
A high prevalence of K. pneumoniaewas isolated among neonatal age groups followed by pediatric age groups, which were 65/147 (44.2%) and 45/147 (30.6%), respectively. The NICU and General Wards were the predominant isolation areas for K. pneumoniae, which accounted for 84/147 (57.1%) and 29/147 (19.7%). Among processed clinical specimens, blood and urine were predominantly the sources of K. pneumoniae, 120/147 (81.6%) and 19/47 (13%), respectively (Table 3).
Antimicrobial resistance profile of K. pneumoniae
Different routinely used antimicrobial agents were tested against K. pneumoniae and experienced significant resistance to Nalidixic acid, Cefotaxime, and Cefazolin, with the rate of 4/4(100%), 17/17(100%), and 13/13(100%), respectively. A significant level of resistance was also observed by Ceftriaxone79/94(84%), Tetracycline 5/6 (83.3%), Piperacillin 18/23(78.3%), Trimethoprim-Sulfamethoxazole, 51/70 (72.8%). However, antimicrobial agents such as Nitrofurantoin, Imipenem, Meropenem, and Amikacin were effective againstK. Pneumoniae with a percentage of 13/15 (86.6%), 6/7(85.7%), 90/114(79%), and 47/60 (78.3%), respectively, whereas the overall multidrug-resistance among all tested K. pneumoniae isolates was 84/147 (57.1%) (Table 4).
Discussion
K. pneumoniae is one of the most common bacteria thatcauses infections in hospitalized patients [11]. Its resistance to multiple antibiotics poses an urgent threat to human health by the World Health Organization and the Centers for Disease Control and Prevention [12]. It is rapidly becoming untreatable using even last-line antimicrobials, especially in hospital settings [5], suggesting the need for continuous surveillance of antimicrobial resistance patterns. The selection of effective antibiotics is very important for the treatment of nosocomialinfections caused by K. pneumoniae. This study focused on the magnitude and antimicrobial resistance profile of clinical K. pneumoniae isolates in all types of infections.
In this study, the rate of culture positivity among the collected samples was 28.3% and the prevalence of K.pneumoniae was 20.18%. This finding was in line with a study conducted in Iran (20.2%) [13], Iraq (20.8%) [14], and Pakistan (20%) [15]. Our finding was higher than a study conducted in India 18% [16], Indonesia 17.36% [17], Madinah, Saudi Arabia 4% [18], Iran (10.2%) [19], Eastern Ethiopia (15%) [20], and Southwest Ethiopia (14.9%) [21]. However, it was lower than a study conducted in Iran which was (88.7%) [22], Pakistan (40%) [23], and the United Arab Emirates 36% [24]. This discrepancy might be due to variations in geographic location, sample size, study design, study period, anddiagnostic methods.
The proportion of K. pneumoniae was higher in males than in females, which was 55.8% and 44.2%, respectively. This finding was in line with a study conducted in Indonesia [17], India [25], India in 2010 [13], and Saudi [18]. Another study suggested that females had a higher prevalence rate of K. pneumoniae than males [26]. This discrepancy is difficult to explain and could be due to variations in the sample collection, study design, sample population, inclusion criteria of patients, environmental factors, and personal hygiene.
In the present study, the prevalence of K. pneumoniae was high in neonates. This might be because neonates have a chance of getting a risk of infection due to low immune status. According to our findings, the high prevalence of K. pneumoniae was isolated in blood cultures, followed by urine, wounds, and body fluids. This finding was supported by a study conducted in Ethiopia [2] and India [27]. Other studies revealed a high prevalence of K.pneumoniae was observed in wound samples [17, 18, 28, 29]. The variation inthe frequency of isolation of K.pneumoniae from different samples may be due to differences in the sample size, type and severity of infection, and quality of specimen collection.
K. pneumoniae isolates were 100% resistant to Nalidixic acid, Cefotaxime, and Cefazolin, 84% to Ceftriaxone, and 83.3% to Tetracycline. This finding was agreed with a study conducted in Bangladesh [28], India [23], Iraq [30], Iran [31], and Jordan [30]. But another study revealed that K. pneumoniae had 50% resistance to Nalidixic acid, and 62% to Imipenem [32]. Based on our findings, 86.6% of tested K. pneumoniae was sensitive to Nitrofurantoin, 85.7% to Imipenem, 79% toMeropenem, and 78.3%to Amikacin. This finding was consistent witha study conducted in Indonesia [17] and Iran [31]. This increase in the resistance rate might be due to the widespread use of antibiotics in the hospital, community, animal production, agriculture, and the environment. Intensive and prolonged use of antibiotics is very likely the main underlying factor in the widespread transmission of difficult-to-cure, antibiotic-resistant nosocomial infections [33].
The overall proportion of MDR K. pneumoniae isolates in this study was 57.1%. Multidrug-resistant K. pneumoniae is currently one of the most pressing emerging issues in bacterial resistance. Its resistancecan occur via a reduction in the intracellular antibiotic concentration due toreduced uptake or/and active efflux, target site modification, inactivation of antimicrobial agents, acquisition of resistance genes, and beta-lactamase-related mutations [34]. This is due to several factors, such as inappropriate use of antimicrobials, poor adherence to treatment, poor infection control in health care and community settings, and poor hygiene and sanitation [35]. Infection prevention and control strategies, screening of colonized and infected patients, and improvement of antimicrobial stewardship to avoid uncontrolled consumption of antibiotics are fundamental tools to minimize the spread of MDR bacteria and the emergence of new resistant strains [36].
Limitations of the study
Due to a lack of patient details recorded in the laboratory registration book, we could not access the full information regarding the sociodemographic and laboratory-related data. This restricts our intention to associate the possible associated factors with the presence of K. pneumoniae and the antibiotic susceptibility pattern of the isolates.
Conclusion
K. pneumoniae was found to be the most common organism causing various infections. Our results suggest that there is high antibiotic resistance among clinical K. pneumoniae isolates commonly prescribed antibiotics, which is alarming for developing countries like Ethiopia. Continuous monitoring and strict antimicrobial policy will have a great impact on reducing bacterial resistance to antibiotics and the development of proper treatment options against K. pneumoniae infections. As antimicrobial susceptibility varies from time to time and in different geographical areas, more studies with large sample sizes and molecular levels should be conducted at intervals in different geographical areas.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- AMR:
-
Antimicrobial resistance
- AST:
-
Antimicrobial susceptibility test
- ICU:
-
Intensive Care Unit
- MDR:
-
Multi-drug resistance
- NICU:
-
Neonatal Intensive Care Unit
- UoGCSH:
-
University of Gondar Comprehensive Specialized Hospital
References
Li Y, Kumar S, Zhang L, Wu H, Wu H. Characteristics of antibiotic resistance mechanisms and genes of Klebsiella pneumoniae. Open Med. 2023;18(1):20230707.
Awoke T, Teka B, Seman A, Sebre S, Yeshitela B, Aseffa A, et al. High prevalence of Multidrug-resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia. Antibiotics. 2021;10(8):1007.
Kot B, Piechota M, Szweda P, Mitrus J, Wicha J, Grużewska A, et al. Virulence analysis and antibiotic resistance of Klebsiella pneumoniae isolates from hospitalised patients in Poland. Sci Rep. 2023;13(1):4448.
Gebremeskel L, Teklu T, Kasahun GG, Tuem KB. Antimicrobial resistance pattern of Klebsiella isolated from various clinical samples in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):643.
Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proceedings of the National Academy of Sciences. 2015;112(27):E3574-E81.
Prevention, ECfD. Control. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2015. ECDC Stockholm; 2017.
Perovic O, Singh-Moodley A, Dusé A, Bamford C, Elliott G, Swe-Han KS, et al. National sentinel site surveillance for antimicrobial resistance in Klebsiella pneumoniae isolates in South Africa, 2010–2012. South Afr Med J. 2014;104(8):563–8.
Karami-Zarandi M, Rahdar HA, Esmaeili H, Ranjbar R. Klebsiella pneumoniae: an update on antibiotic resistance mechanisms. Future Microbiol. 2023;18(1):65–81.
Taddese AA, Gashaye KT, Dagne H, Andualem Z. Maternal and partner’s level of satisfaction on the delivery room service in University of Gondar Referral Hospital, northwest, Ethiopia: a comparative cross-sectional study. BMC Health Serv Res. 2020;20(1):1–8.
Simner PJ, Hindler JA, Bhowmick T, Das S, Johnson JK, Lubers BV et al. What’s New in Antibiograms? Updating CLSI M39 Guidance with current trends. J Clin Microbiol. 2022:e02210–21.
Saeidynia F, Keihanian F, Saeidynia A. Antibiotic resistance in blood culture samples from patients referred to Razi laboratory of Rasht, 2006–2011. Adv Infect Dis. 2014;2014.
CDC A. Antibiotic resistance threats in the United States. Washington, DC, USA: US Department of Health and Human Services; 2019.
Mehrgan H, Rahbar M, Arab-Halvaii Z. High prevalence of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a tertiary care hospital in Tehran, Iran. J Infect Developing Ctries. 2010;4(03):132–8.
Aljanaby AAJ, Alhasnawi H. Research article phenotypic and molecular characterization of multidrug resistant Klebsiella pneumoniae isolated from different clinical sources in Al-Najaf Province-Iraq. Pak J Biol Sci. 2017;20(5):217–32.
Amin A, Ghumro P, Hussain S, Hameed A. Prevalence of antibiotic resistance among clinical isolates of Klebsiella pneumoniae isolated from a Tertiary Care Hospital in Pakistan. Malaysian J Microbiol. 2009;5(2):81–6.
Aljanaby AAJ, Tuwaij NS, Al-khilkhali HJ. Antimicrobial susceptibility patterns of Klebsiella pneumoniae isolated from older smokers and non-smokers of inpatients in intensive care unit infected with chronic pneumonia in AL-Najaf hospital, Iraq. J Pharm Sci Res. 2018;10(5):1093–7.
Nirwati H, Sinanjung K, Fahrunissa F, Wijaya F, Napitupulu S, Hati VP, et al. editors. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC proceedings; 2019: BioMed Central.
Samah G, Hatem MES, El KAT, Nikhat M. Antimicrobial resistance patterns of Klebsiella isolates from clinical samples in a Saudi hospital. Afr J Microbiol Res. 2017;11(23):965–71.
Babakhani S, Shokri S, Baharvand M. Antibiotic resistance pattern of Klebsiella pneumoniae isolated from nosocomial infections in Aleshtar hospital, Lorestan Province. Rep Health Care. 2015;1(2):55–9.
Seid J, Asrat D. Occurrence of extended spectrum β-lactamase enzymes in clinical isolates of Klebsiella species from Harar region, eastern Ethiopia. Acta Trop. 2005;95(2):143–8.
Abayneh M, Tesfaw G, Abdissa A. Isolation of extended-spectrum β-lactamase-(ESBL-) producing Escherichia coli and Klebsiella pneumoniae from patients with community-onset urinary tract infections in Jimma University Specialized Hospital, Southwest Ethiopia. Canadian Journal of Infectious Diseases and Medical Microbiology. 2018;2018.
Amraie H, Shakib P, Rouhi S, Bakhshandeh N, Zamanzad B. Prevalence assessment of magA gene and antimicrobial susceptibility of Klebsiella pneumoniae isolated from clinical specimens in Shahrekord, Iran. Iran J Microbiol. 2014;6(5):311.
Iqra J, Aizza Z, Muhammad UQ, Hasan E, Junaid A, Abdul W. Multi-drug resistant Klebsiella pneumoniae causing urinary tract infections in children in Pakistan. Afr J Microbiol Res. 2014;8(4):316–9.
Al-Zarouni M, Senok A, Rashid F, Al-Jesmi SM, Panigrahi D. Prevalence and antimicrobial susceptibility pattern of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the United Arab Emirates. Med Principles Pract. 2008;17(1):32–6.
Kaur A, Wasan RK, Kaur C, Sethi P, Kaur V. Antibiotic Resistance Pattern of Klebsiella Pneumoniae A Major Problem for Society. International Journal of Health Sciences. (II):4699 – 712.
Maity SN, Marothi Y, Pradhan R, Waske S, Hemwani K. Antimicrobial susceptibility pattern of Klebsiella pneumoniae isolated from various clinical specimens in Ujjain City, Madhya Pradesh, India. Cent India J Med Res. 2022;1(01).
Singh AK, Jain S, Kumar D, Singh RP, Bhatt H. Antimicrobial susceptibility pattern of extended-spectrum beta-lactamase producing Klebsiella pneumoniae clinical isolates in an Indian tertiary hospital. J Res Pharm Pract. 2015;4(3):153.
Sonia SJ, Afroz S, Rasheduzzaman M, Uddin KH, Shamsuzzaman S. Prevalence and antimicrobial susceptibility pattern of Klebsiella Pneumoniae isolated from various clinical specimens in a tertiary care hospital in Bangladesh. Med Today. 2020;32(2):95–9.
Sarojamma V, Ramakrishna V. Prevalence of ESBL-producing Klebsiella pneumoniae isolates in tertiary care hospital. International Scholarly Research Notices. 2011;2011.
Al Shara MA. Emerging antimicrobial resistance of klebsiella pneumonia strains isolated from pediatric patients in Jordan. Iraqi J Med. 2011;7(2):29–32.
Azar S, Ebadi A. Examining the pattern of susceptibility and antibiotic resistance in Klebsiella pneumoniae strains isolated from urine samples of children with urinary tract infections from the children’s hospital of Tabriz in 2015. Br Biomedical Bull. 2017;5:307.
Karimi K, Zarei O, Sedighi P, Taheri M, Doosti-Irani A, Shokoohizadeh L. Investigation of antibiotic resistance and biofilm formation in clinical isolates of Klebsiella pneumoniae. International Journal of Microbiology. 2021;2021.
Prakash VVD, Agarwal S. Candiduria: its characterization, antifungal susceptibility pattern and biofilm formation. Int J Res Med Sci. 2018;6:4070–6.
Galani I, Karaiskos I, Giamarellou H. Multidrug-resistant Klebsiella pneumoniae: mechanisms of resistance including updated data for novel β-lactam-β-lactamase inhibitor combinations. Expert Rev Anti-infective Therapy. 2021;19(11):1457–68.
Mittal AK, Bhardwaj R, Mishra P, Rajput SK. Antimicrobials misuse/overuse: adverse effect, mechanism, challenges and strategies to combat resistance. Open Biotechnol J. 2020;14(1).
Russo A, Fusco P, Morrone HL, Trecarichi EM, Torti C. New advances in management and treatment of multidrug-resistant Klebsiella pneumoniae. Expert Rev Anti-infective Therapy. 2023;21(1):41–55.
Acknowledgements
We want to acknowledge the University of Gondar Comprehensive Specialized Hospital administrative office for their willingness during the data collection.
Funding
There is no specific fund received for this study.
Author information
Authors and Affiliations
Contributions
MW, TM, and SB have been involved in the conception of the research idea, data collection, and data analysis. MW, MA, and MA have been involved in the conception of the research idea, rationalizing the method, data analysis, interpretation of the result, evaluation of the scientific content of the study, and manuscript preparation. MA also manuscript reviewing and editing. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical review committee of the School of Biomedical and Laboratory Sciences, University of Gondar. Informed consent was obtained from each study participant and their legal guardians after describing the purpose of the study. Information concerning the participants was kept confidential and specimens collected from them were used only for the intended purposes. All procedures in this study were conducted in accordance with the amended Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Worku, M., Belay, S., Molla, T. et al. Prevalence and antimicrobial susceptibility pattern of Klebsiella pneumoniae isolated from various clinical specimens at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Infect Dis 24, 917 (2024). https://doi.org/10.1186/s12879-024-09811-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09811-1