Abstract
Background
Aortic valve infective endocarditis (IE) is associated with significant morbidity and mortality. We aimed to describe the clinical profile, risk factors and predictors of short- and long-term mortality in patients with aortic valve IE treated with aortic valve replacement (AVR) compared with a control group undergoing AVR for non-infectious valvular heart disease.
Methods
Between January 2008 and December 2013, a total of 170 cases with IE treated with AVR (exposed cohort) and 677 randomly selected non-infectious AVR-treated patients with degenerative aortic valve disease (controls) were recruited from three tertiary hospitals with cardiothoracic facilities across Scandinavia. Crude and adjusted hazard ratios (HR) were estimated using Cox regression models.
Results
The mean age of the IE cohort was 58.5 ± 15.1 years (80.0% men). During a mean follow-up of 7.8 years (IQR 5.1-10.8 years), 373 (44.0%) deaths occurred: 81 (47.6%) in the IE group and 292 (43.1%) among controls. Independent risk factors associated with IE were male gender, previous heart surgery, underweight, positive hepatitis C serology, renal failure, previous wound infection and dental treatment (all p < 0.05). IE was associated with an increased risk of both short-term (≤ 30 days) (HR 2.86, [1.36–5.98], p = 0.005) and long-term mortality (HR 2.03, [1.43–2.88], p < 0.001). In patients with IE, chronic obstructive pulmonary disease (HR 2.13), underweight (HR 4.47), renal failure (HR 2.05), concomitant mitral valve involvement (HR 2.37) and mediastinitis (HR 3.98) were independent predictors of long-term mortality. Staphylococcus aureus was the most prevalent microbe (21.8%) and associated with a 5.2-fold increased risk of early mortality, while enterococci were associated with the risk of long-term mortality (HR 1.78).
Conclusions
In this multicenter case-control study, IE was associated with an increased risk of both short- and long-term mortality compared to controls. Efforts should be made to identify, and timely treat modifiable risk factors associated with contracting IE, and mitigate the predictors of poor survival in IE.
Similar content being viewed by others
Introduction
Infective endocarditis (IE) is a potentially severe infection that most commonly affects heart valves. The disease is associated with a poor prognosis despite optimal medical and surgical treatment, with mortality around 20–30% at one year [1]. The epidemiology of IE has changed towards elderly patients and with Staphylococcus aureus (S. aureus) as the predominant causative organism in high-income countries [2]. Complications of IE include septic embolism, heart failure and uncontrolled infection, which all are indications for cardiac surgery [3]. Surgery is potentially lifesaving and is indicated in 50–70% of cases of left-sided IE [3,4,5,6]. Although international guidelines provide recommendations for cardiac surgery in patients with IE, the clinical decision should also consider the age of the patient, comorbidities, and the availability of appropriate surgical expertise [3, 7].
Overall, the rates of IE are increasing with annual incidence rates varying from 7 to 14 per 100,000 person-years in recent studies [4, 8]. This may be explained by an elderly population, advances in medical care including an increasing number of patients receiving prosthetic heart valves, surgery in patients with congenital heart disease and the increasing use of cardiac implantable electronic devices. Few studies, however, have described the incidence or epidemiology of IE in Scandinavia, and we still need data on variables that predict short- and long-term prognosis of IE. The present study is a large multicenter collaboration between tertiary hospitals with cardiothoracic facilities in Scandinavia. The objectives were to: (1) describe incidence, clinical profile and epidemiology of patients with aortic site IE treated with aortic valve (AV)-surgery compared with a control group undergoing AVR due to non-infectious valvular heart disease; and (2) assess predictors of short- and long-term mortality in patients with aortic valve IE treated with AVR.
Materials and methods
Source population and research design
The study was a collaborative project between Karolinska University Hospital, Stockholm, Sweden; University Hospital of North Norway, Tromsø, Norway and Haukeland University Hospital, Bergen, Norway. The study was designed in accordance with the Declaration of Helsinki and conducted in compliance with Norwegian and Swedish legislation with approval obtained by the Regional Committees for Medical and Health Research Ethics in Norway (South-Eastern Norway - REK Helse Sør-Øst C, 2017/768) and Sweden (Swedish Ethical Review Authority 2017/2113-31/2). The need for informed consent was waived for Swedish patients by the Swedish Ethical Review Authority, while in Norway, patients still alive at the time of registry building provided informed consent.
Between January 2008 and December 2013, a source population of 10,347 patients ≥ 18 years undergoing primary aortic valve surgery in all participant centers was considered for the study. A total of 170 underwent aortic valve surgery due to IE (exposed cohort) and remaining 10.177 underwent aortic valve surgery due to non-infectious aortic valve disease. Among the latter group, a total of 677 patients were randomly selected as controls (non-exposed cohort) in a ratio of 1:4. A flow chart of the study design (dual design, a case-control and a retrospective cohort study) is presented in Fig. 1. The diagnosis of IE was based upon the modified Duke criteria for IE [9–10]. Cases were identified from departmental databases and categorized according to the modified Duke criteria and the International Classification of Diseases (ICD) version 10 (I33.0, I38, I33.9). Microbial isolates were cultured from blood and from excised valves.
Most of the included patients were treated with AVR (98.8%), while the remainder were treated with aortic valve repair. Coronary artery bypass grafting (CABG) was concurrently performed in 14.5% of included patients, while none were operated with aortic homograft.
Study endpoints and follow-up
The primary outcome was all-cause mortality at short-term (within 30 days after AVR) and long-term follow-up. Follow-up was complete in all patients and calculated from the date of operation to the date of death or censoring, with February 1st, 2024, as closing date. The predictors of long-term mortality were calculated at 6-year follow-up at which the difference in survival estimates between IE and controls was greatest.
Cardiovascular risk factors and comorbidities
Information regarding smoking history, body mass index (BMI) and other relevant risk factors and comorbidities was obtained from the electronic patient record. Obesity was defined as BMI > 30 kg/m2 and underweight as BMI < 18.5 kg/m2. Left ventricular ejection fraction (LVEF) was measured by Simpson biplane method. Coronary artery disease (CAD) was defined as previous myocardial infarction, CABG or significant coronary obstructions on coronary angiography prior to valve surgery. Cardiovascular disease (CVD) was defined as a composite of CAD as defined above, stroke and/or peripheral artery disease. Renal failure was defined as estimated glomerular filtration rate < 60 mL/min at baseline.
Statistical methods
SPSS version 29.0 (IBM Corporation, Armonk, NY) was used for data management and statistical analyses. Baseline characteristics were compared using chi-square test for categorical variables and t-test for numerical variables. For the case-control study, after univariate analysis, multivariate logistic regression was used to pinpoint independent risk factors of IE by a backward elimination procedure. Association between risk factors and IE were estimated by the odds ratio (OR) and 95% confidence interval (CI). The effects of IE on short- and long-term survival were visualized by survival curves using the Kaplan-Meier method. Univariate predictors of short- and long-term survival were assessed by cox-regression analyses and presented as hazard ratio (HR) and 95% CI. A multivariate cox regression analysis was used to pinpoint independent risk factors of survival by a backward elimination procedure. All significant predictors of survival in the univariate analyses, or those clinically relevant, were entered into the multivariate models. All statistical tests were performed at the 2-sided, α = 0.05 significance level.
Results
Baseline characteristics of IE cases versus controls
The main demographic and clinical characteristics of IE cases versus controls are presented in Table 1. Patients with IE were predominantly men (80.0%) and younger than the controls (59 ± 15 versus 69 ± 12 years, p < 0.001). The IE group included 124 patients (72.9%) with native valve endocarditis (NVE) and 46 patients (27.1%) with prosthetic valve endocarditis (PVE). Patients with PVE were older (mean 62.4 versus 57.1 years), had more severe symptoms (NYHA ≥ 3 74.4% versus 57.8%), and were more likely to have hypercholesterolemia (37.0 vs. 20.2%), atrial fibrillation (AF) at baseline (52.2% versus 34.7%) and AV block (28.3 versus 11.3%) compared to NVE (p < 0.05 for all).
Recent dental procedures (31.2% versus 3.6%), previous wound infections (7.1% versus 0.1%), intravenous drug use (IVDU) (17.0% versus 0.0%), positive serology for viral hepatitis (hepatitis B and C) and renal failure (36.5% versus 17.8%) were all more prevalent in the IE group (p < 0.001 for all; Table 1). A higher prevalence of chronic obstructive pulmonary disease (COPD) (16.7% versus 9.8%, p = 0.013) and smoking history (65.9% versus 53.8%, p = 0.005) was also observed in the IE-cases. AF at baseline was more prevalent in the IE group (39.4% versus 16.7%, p < 0.001), while the prevalence of combined pre- and postoperative AF was comparable (60.7% vs. 57.8%, p = 0.500).
Patients with IE had significantly longer aortic cross clamp time, cardiopulmonary bypass time, postoperative mechanical ventilation duration, as well as greater postoperative drainage volume and need for blood transfusion (p < 0.05 for all).
Microbiology and infection data in the IE group
Lack of infection control was evident in 49.4% and septic emboli in 34.1% of patients with IE preoperatively. Valve vegetations on echocardiography were identified in 141 (82.9%) patients with IE. The prevalence of concomitant infective mitral valve disease (MVD) was 18.2% (n = 31). Isolated microorganisms are presented in Table 2. The most frequently isolated microorganisms were S. aureus (21.8%) (equally represented both in NVE and PVE), streptococci of the viridans group (21.2%) and enterococci (19.4%). Non-viridans streptococci (33.1%) were the most prevalent pathogenic microbes among patients with NVE, while S. aureus (21.7%) was most prevalent in patients with PVE. The proportions of septic embolism to different organs in patients with IE are presented in Fig. 2, with the brain being the target organ for septic emboli in 20% of cases and multi-organs in 6.5% of cases.
Predictors of infective endocarditis
Covariates of IE are presented in Table 3. In a multivariable-adjusted model, younger age (OR 1.03 per year), male gender (OR 2.30), previous heart surgery (OR 6.75), the presence of AF at baseline (OR 2.76), positive hepatitis C serology (OR 20.83), previous wound infection (OR 22.97), previous dental treatment (OR 17.13), underweight (OR 9.91) and renal failure (OR 3.52) were associated with a higher risk of having IE. The presence of hypertension (OR 0.44) and hypercholesterolemia (OR 0.37), which were more prevalent in the elderly control group, were associated with lower odds of IE in the entire study population (all p < 0.01). COPD was associated with a higher likelihood of IE in univariate analysis (OR 1.82, p = 0.014), but did not remain a significant predictor in the multivariable-adjusted model (OR 1.88, p = 0.071). Removing hypertension and hypercholesterolemia from the same primary model did not change our results (data not shown).
Survival data
Entire study population: IE is an independent predictor of total mortality
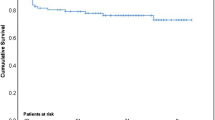
During a mean follow-up of 7.8 years (median 7.6 years, IQR 5.1–10.8 years), a total of 373 (44.0%) deaths occurred: 81 (47.6%) in the IE group and 292 (43.1%) in the control group. The early mortality rate (≤ 30 days) was 7.1% (n = 12) among patients with IE and 2.5% (n = 17) in the control group (p = 0.004). The difference in mortality rates was greatest at 6-year follow-up: 40.0% (68/170 patients) in the IE group and 26.1% (177/677 patients) in the control group. Figure 3 (Kaplan-Meier curve) shows survival probability rates in patients with IE and controls separately for short-term mortality (≤ 30 days) (panel A) and long-term mortality at closing date (panel B). Importantly, this was confirmed by a multivariate Cox regression model, in which IE was a strong and independent predictor of long-term mortality (HR 2.03, [1.43–2.86], p < 0.001), also after adjusting for other variables that predicted total mortality (Table 4). When long-term mortality was assessed at total follow-up of maximum 16 years in the same multivariate Cox regression model, the prognostic impact of IE somehow attenuated, but remained highly significant (HR 1.73, [1.29–2.32], p < 0.001). However, Kaplan-Meier curves showed a cross-over at 12-years with a higher likelihood of death in controls, potentially reflecting that other factors than IE may influence the total mortality in the entire cohort after such a long time.
Short-term mortality in patients with IE
In univariate Cox regression analysis, there was a significant increased risk of short-term (≤ 30 days) all-cause mortality in the IE group compared to controls (HR 2.86, [1.36–5.98], p = 0.005; Table 5). Concomitant infective MVD (HR: 4.85, [1.56–15.03], p = 0.006) and septic embolism (HR 4.01, [1.21–13.31], p = 0.023) were predictors of mortality within 30 days in the IE cohort. There was no significant difference in early mortality between NVE and PVE patients (p = 0.171). In a univariate Cox-regression analysis, IE caused by S. aureus was associated with an increased risk of early mortality (HR 5.24, [1.66–16.50], p = 0.005) compared with IE caused by other microbes (Table 2). Short-term mortality rates in patients with S. aureus were 18.9% compared to 3.8% in IE group caused by other microbes (p = 0.001).
Long-term mortality in patients with IE
Total mortality after 6 years showed the largest differences between IE and controls (Fig. 2B) and this time point was therefore chosen to analyze predictors of long-term mortality in the IE group (Table 6). In univariate Cox regression models, age ≥ 60 years (HR: 1.80, [1.10–2.95], p = 0.020), diabetes mellitus (DM) (HR: 1.98, [1.13–3.47], p = 0.017), COPD (HR: 2.48, [1.46–4.23], p = 0.001), previous wound infections (HR 2.34, [1.20–4.90], p = 0.024), preoperative renal failure (HR: 2.06; [1.28–3.32], p = 0.003), concomitant infective MVD (HR: 1.94, [1.12–3.37], p = 0.018), multi-organ failure (HR: 3.95, [2.24–6.96], p < 0.001) and mediastinitis (HR: 3.65, [1.14–11.72], p = 0.030), were all associated with increased long-term mortality. No difference was found in long-term mortality between NVE and PVE patients (p = 0.526). No association was found between gender and long-term mortality (p = 0.830). Similarly, LVEF < 50% and NYHA functional class ≥ 3 were not associated with increased risk of mortality. IE caused by enterococci had a higher long-term mortality rate than IE caused by other microorganisms (HR 1.78, [1.05–3.03], p = 0.033) (Table 2). In a multivariate Cox regression model, underweight (HR 4.47), mediastinitis (HR 3.98), concomitant infective MVD (HR 2.37), COPD (HR 2.13) and preoperative renal failure (HR 2.05) were identified as independent predictors of all-cause mortality in patients with IE (all p < 0.05). Age, baseline AF and DM were not associated with the risk of all-cause mortality in the same multivariate model (p > 0.1). When IE caused by enterococci was introduced in the same multivariate Cox regression model, it did not retain a significant association with all-cause mortality (HR 1.39, [0.78–2.47], p = 0.263) (data not shown).
Discussion
The key findings of our study are: (1) Patients who underwent AVR due to IE were mostly men, younger and had an almost three-fold increased risk of early mortality and nearly two-fold increased risk of long-term mortality after 6 years compared to patients who underwent AVR due to degenerative valvular heart disease (non-infectious group) in adjusted analyses. However, at maximum follow-up of 16 years, Kaplan-Meier curves showed a cross-over at approximately 12 years, showing a trend towards increasing risk of death in controls in comparison to the younger IE group who were successfully treated; (2) COPD, underweight, renal failure, concomitant infective MVD and mediastinitis were independent risk factors of all-cause long-term mortality in patients with IE; (3) IE caused by S. aureus was associated with an increased risk of early mortality and IE caused by enterococci had a higher long-term mortality rate than IE caused by other microorganisms.
Antibiotic prophylaxis before dental or surgical procedures for prevention of IE has been debated for decades due to limited evidence. However, recent studies showed that antibiotic prophylaxis in high-risk individuals led to a significant reduction of IE after invasive dental procedures (11–12). Consequently, the 2023 ESC guidelines strengthened their recommendation of antibiotic prophylaxis, while updating their risk categories for IE [3]. Our study showed that recent dental treatment was associated with a high risk of IE, supporting the need for preventive action. Conversely, previous studies from our group indicated no association between origin of the IE-causing bacteria and findings during oral infection screening but suggested an association between marginal bone loss – as a marker for reduced oral and/or general health – and mortality [13].
While male gender was identified as a risk factor of acquiring IE, gender per se had no impact on short- or long-term mortality in patients with IE, consistent with previous results [14]. Our finding of 7.1% short-term (≤ 30 days) mortality rates for IE was less than those reported by previous studies (25–40%), with in-hospital mortality of 20% [14,15,16]. Although surgical treatment of IE is associated with an overall better prognosis, this is influenced by the fact that AVR is performed in a selected group of patients, excluding patients with excessive comorbidities, higher surgical risk or poor rehabilitation potentials following surgery. However, the improved short-term prognosis in our study may be explained by the timely and optimal medical treatment before surgery, and the practice of relatively low threshold for surgery in some of our institutions. In general, European guidelines have been followed and differences in practice between institutions have not been investigated in this study.
Long-term mortality was 48% among patients with IE in our study, which is in line with other studies [14, 17]. Notably, the rate of mortality progressively increased and peaked at 6-year follow-up (40%) in patients with IE compared with controls (26%). However, when long-term mortality was assessed at total follow-up of maximum 16 years, Kaplan-Meier curves showed a cross-over at approximately 12 years, showing a trend towards increasing risk of death in controls in comparison to the younger IE group, who remained relatively stable. This may be explained by the fact that the controls undergoing cardiac surgery were older and had a higher burden of cardiovascular risk factors (hypertension, hypercholesterolemia, and obesity) at baseline. Furthermore, they may have developed other age-related comorbid conditions during follow-up, suggesting that other factors than IE were the main risk factors for long-term mortality in the control cohort at this time point.
The ‘obesity paradox’ describes the association between obesity and lower risk of mortality after cardiac surgery compared to patients with normal weight and underweight [18]. However, obesity was not associated with a lower risk of long-term mortality neither in the entire study population nor control group, in whom the prevalence of traditional cardiovascular risk factors was higher. Our study identified underweight, and not obesity, as an independent risk factor of increased long-term mortality in patients with IE. This may suggest a possible interaction between nutritional status, antimicrobial host immune function, bacterial proliferation, and persistent inflammation in patients with IE. Furthermore, underweight may also indicate the severity, duration, and an advanced stage of the disease in patients with IE. Hence, diagnosing IE in an early stage, and appropriate management of IE is essential in terms of better postoperative outcomes. In addition to its correlation with systemic inflammation and metabolic disruption, underweight can also reflect cachexia secondary to malignancies or other advanced systemic diseases, as competing risks.
We demonstrated that most patients with IE had a smoking history (65.9%) and a relatively high prevalence of COPD (16.7%). Although previous studies have not shown COPD to be a risk factor of extrapulmonary infections [19], our study identified COPD as a risk factor of IE. Furthermore, survival data with multivariate Cox regression identified COPD as an independent predictor of long-term all-cause mortality in the entire study population, but this association was specifically stronger within patients with IE, hence being an effect modifier in IE. Interestingly, smoking history, but not COPD was a predictor of long-term mortality in patients operated due to valvular heart disease. With COPD both being a risk factor of IE and an independent predictor of long-term mortality, it is of great importance that clinicians address smoking history and assist their patients in smoking cessation.
In patients with aortic valve IE, the prevalence of concomitant infective MVD was 18.2% and a predictor of both early and long-term all-cause mortality. The mitral valve might be affected in different ways in aortic valve IE, i.e., either due to a kissing vegetation/lesion or local extension to the mitral valve through the aortic root. Furthermore, aortic valve IE can lead to left ventricular remodeling/dilatation, resulting in mitral regurgitation. In primary aortic valve endocarditis, secondary involvement of the mitral valve is well documented and timely surgery may preserve the mitral valve apparatus, favorably affecting long-term prognosis [20]. Moreover, case reports showed that even severe mitral regurgitation resolved rapidly after AVR [21]. Multivalvular IE has a poor prognosis and has been reported in about 15% of patients with IE, which corresponds well to our findings [22]. Early diagnosis and treatment of IE is important and may prevent secondary valve infections and associated complications.
Patients with IE had significantly longer aortic cross-clamp time, cardiopulmonary bypass time, mechanical ventilation time, as well as more mediastinal drainage and increased need for blood transfusion. Furthermore, we found that postoperative complications such as stroke, renal failure and multi-organ failure were more prevalent in the IE group. Interestingly, postoperative pneumonia was more prevalent in the control group, even though patients with IE had a significantly higher prevalence of COPD and smoking history. This may be explained by the aggressive antibiotic treatment for IE preventing pulmonary infection, in addition to the fact that controls were older and had a higher burden of traditional cardiovascular diseases, including obesity which may be a risk factor for postoperative pneumonia [23].
Mediastinitis is a rare, but feared complication after sternotomy, and appears to increase the risk of sudden cardiac death [24]. Our group recently demonstrated that mediastinitis after CABG-surgery was associated with a poor long-term prognosis and a nearly two-fold increased risk of all-cause mortality (24–25). In our current study, mediastinitis was identified as a strong and independent predictor of long-term mortality in the multivariable-adjusted Cox models, both in IE cohort and controls, having an almost four-fold increased risk of all-cause long-term mortality.
DM is a well-established risk factor for infections and CVD [26]. Moreover, previous studies have shown that patients with DM and IE are older and show a higher prevalence of cardiovascular risk factors and an impaired prognosis [27,28,29]. In the present study, however, DM was not associated with a higher prevalence of IE, and although it was associated with an overall worse prognosis in patients with IE, this association was not significant in the multivariable-adjusted model. However, in the entire study population, DM was a significant predictor of all-cause mortality independent of IE. Similarly, previous heart surgery was associated with a higher risk of IE, but was somewhat surprisingly not a predictor of short- or long-term mortality in patients with IE.
S. aureus is the most common microbe in IE and has been shown to be overtaking streptococci as the most frequent causative microorganism [30]. Furthermore, staphylococcal IE occurs more often with healthcare-associated IE. Patients with S. aureus as the pathogenic microbe had a higher risk of early short-term mortality as compared to other microbiology. IE caused by S. aureus is associated with aggressive disease with an increased risk of complications and in-hospital mortality [3, 28]. Interestingly, we found no association between S. aureus and an increased risk of long-term mortality at 6 years. On the contrary, IE caused by enterococci was associated with an increased risk of long-term mortality. International guidelines recommend a combination of aminoglycosides and β-lactam antibiotics in the treatment of enterococcal IE [9]. Combination-therapy with dual ß-lactams (ampicillin in combination with ceftriaxone), a combination with collateral anti-bacterial synergistic effects [31], is considered equally efficient and recommended in case of kidney failure or high-level aminoglycoside resistance (HLAR) [3]. However, the need for long-term dual antimicrobial therapy, rising HLAR and enterococcal IE being more prevalent in elderly patients may partly explain the increased long-term mortality rates compared to IE caused by other microbes [32].
Strength and limitations
The present study benefits from a large sample size from multiple tertiary hospitals with cardiothoracic facilities across Scandinavia, maintaining the same diagnostic criteria and treatment with active and prospective epidemiologic surveillance of IE after aortic valve surgery. A dual case-control study design enabled us to compare the association between risk factors and clinical outcomes between the exposed (IE) and control groups. The limitations are the retrospective nature of the study, the fact that the control group was not matched for age and sex and underwent AVR for valvular heart disease. Information regarding reconstruction of the left ventricular outflow tract or aortic root were not obtained. Furthermore, data concerning different types of MV-lesions or MV-procedures were not collected. We presented information regarding local abscesses and septic emboli, but did not gather information regarding other local complications, such as pseudoaneurysms and fistulas. This is important because these conditions contribute to the complexity of surgery and increase associated surgical risk.
Conclusions
Aortic valve IE is associated with both increased short- and long-term mortality compared to controls undergoing AVR. Male gender, previous heart surgery, baseline atrial fibrillation, underweight, positive hepatitis C serology, previous wound infection and dental procedures, and renal failure were all associated with IE. Staphylococcus aureus was the most prevalent microbe and equally represented both in native valve and prosthetic valve endocarditis. Renal failure, concomitant infective MVD and the presence of Staphylococcus aureus were risk factors of early mortality. COPD, underweight, renal failure, concomitant infective MVD and mediastinitis were independent risk factors of long-term all-cause mortality in patients with IE. Risk reduction through preventive measures is better than cure. Therefore, all efforts should be made to identify and timely treat the modifiable risk factors associated with IE.
Data availability
Data are available by the corresponding author upon reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- AV:
-
Atrioventricular
- AVR:
-
Aortic valve replacement
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass grafting
- COPD:
-
Chronic obstructive pulmonary disease
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- IE:
-
Infective endocarditis
- LVEF:
-
Left ventricular ejection fraction
- LVEDD:
-
Left ventricular end-diastolic dimension
- MVD:
-
Mitral valve disease
- NYHA:
-
New York Heart Association
References
Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, et al. International collaboration on endocarditis-prospective Cohort Study (ICE-PCS) investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169(5):463–73.
Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol. 2017;69(3):325–44.
Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, ESC Scientific Document Group, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. 2023;44(39):3948–4042.
Chu VH, Park LP, Athan E, Delahaye F, Freiberger T, Lamas C, et al. International collaboration on Endocarditis (ICE) Investigators*. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International collaboration on Endocarditis. Circulation. 2015;131(2):131–40.
Iung B, Doco-Lecompte T, Chocron S, Strady C, Delahaye F, Le Moing V, et al. AEPEI Study Group. Cardiac surgery during the acute phase of infective endocarditis: discrepancies between European Society of Cardiology guidelines and practices. Eur Heart J. 2016;37(10):840–8.
Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, et al. EURO-ENDO investigators. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40(39):3222–32.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2021;143(5):e35–71.
Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular surgery and anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: A Scientific Statement for Healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–86.
Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–9.
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128.
Thornhill MH, Crum A, Campbell R, Stone T, Lee EC, Bradburn M, et al. Temporal association between invasive procedures and infective endocarditis. Heart. 2023;109(3):223–31.
Thornhill MH, Gibson TB, Yoon F, Dayer MJ, Prendergast BD, Lockhart PB, et al. Antibiotic Prophylaxis Against Infective endocarditis before Invasive Dental procedures. J Am Coll Cardiol. 2022;80(11):1029–41.
Thoresen T, Jordal S, Lie SA, Wünsche F, Jacobsen MR, Lund B. Infective endocarditis: association between origin of causing bacteria and findings during oral infection screening. BMC Oral Health. 2022;22(1):491.
Abegaz TM, Bhagavathula AS, Gebreyohannes EA, Mekonnen AB, Abebe TB. Short- and long-term outcomes in infective endocarditis patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17(1):291.
Park LP, Chu VH, Peterson G, Skoutelis A, Lejko-Zupa T, Bouza E, et al. International collaboration on Endocarditis (ICE) investigators. Validated risk score for Predicting 6-Month Mortality in Infective Endocarditis. J Am Heart Assoc. 2016;5(4):e003016.
Erdem H, Puca E, Ruch Y, Santos L, Ghanem-Zoubi N, Argemi X, et al. Portraying infective endocarditis: results of multinational ID-IRI study. Eur J Clin Microbiol Infect Dis. 2019;38(9):1753–63.
Tahon J, Geselle PJ, Vandenberk B, Hill EE, Peetermans WE, Herijgers P, et al. Long-term follow-up of patients with infective endocarditis in a tertiary referral center. Int J Cardiol. 2021;331:176–82.
Mariscalco G, Wozniak MJ, Dawson AG, Serraino GF, Porter R, Nath M, et al. Body Mass Index and Mortality among adults undergoing cardiac surgery: a Nationwide Study with a systematic review and Meta-analysis. Circulation. 2017;135(9):850–63.
Lange P. Chronic obstructive pulmonary disease and risk of infection. Pneumonol Alergol Pol. 2009;77(3):284–8.
Piper C, Hetzer R, Körfer R, Bergemann R, Horstkotte D. The importance of secondary mitral valve involvement in primary aortic valve endocarditis; the mitral kissing vegetation. Eur Heart J. 2002;23(1):79–86.
Friess JO, Bruelisauer T, Hurni S, Pasic M, Erdoes G, Eberle B. Resolution of severe secondary mitral valve regurgitation following aortic valve replacement in infective endocarditis. SAGE Open Med Case Rep. 2021;9:2050313X211034377.
Álvarez-Zaballos S, González-Ramallo V, Quintana E, Muñoz P, de la Villa-Martínez S, Fariñas MC, et al. On Behalf of games. Multivalvular endocarditis: a Rare Condition with Poor Prognosis. J Clin Med. 2022;11(16):4736.
Covarrubias J, Grigorian A, Schubl S, Gambhir S, Dolich M, Lekawa M, et al. Obesity associated with increased postoperative pulmonary complications and mortality after trauma laparotomy. Eur J Trauma Emerg Surg. 2021;47(5):1561–8.
Risnes I, Aukrust P, Lundblad R, Ueland T, Rynning SE, Solheim E, et al. Troponin T and N-Terminal Pro-brain Natriuretic peptide are Associated with Long-Term all-cause mortality in patients with Post-sternotomy Mediastinitis following coronary artery bypass grafting: a 15-Year Follow-Up study. Cardiology. 2023;148(6):599–603.
Risnes I, Aukrust P, Lundblad R, Rødevand O, Ueland T, Rynning SE, et al. Increased levels of NT-proBNP and troponin T 2 years after coronary artery bypass grafting complicated by mediastinitis. Front Cardiovasc Med. 2023;10:1008825.
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444–70.
Chirillo F, Bacchion F, Pedrocco A, Scotton P, De Leo A, Rocco F, et al. Infective endocarditis in patients with diabetes mellitus. J Heart Valve Dis. 2010;19(3):312–20.
Kourany WM, Miro JM, Moreno A, Corey GR, Pappas PA, Abrutyn E, et al. Influence of diabetes mellitus on the clinical manifestations and prognosis of infective endocarditis: a report from the International collaboration on endocarditis-merged database. Scand J Infect Dis. 2006;38(8):613–9.
Chu VH, Cabell CH, Benjamin DK Jr, Kuniholm EF, Fowler VG Jr, Engemann J, Sexton DJ, Corey GR, Wang A. Early predictors of in-hospital death in infective endocarditis. Circulation. 2004;109(14):1745–9.
Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, et al. AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54(9):1230–9.
Marino A, Munafò A, Zagami A, Ceccarelli M, Di Mauro R, Cantarella G, et al. Ampicillin Plus Ceftriaxone Regimen against Enterococcus faecalis Endocarditis: A literature review. J Clin Med. 2021;10(19):4594.
Beganovic M, Luther MK, Rice LB, Arias CA, Rybak MJ, LaPlante KL. A review of Combination Antimicrobial Therapy for Enterococcus faecalis Bloodstream infections and Infective endocarditis. Clin Infect Dis. 2018;67(2):303–9.
Acknowledgements
None.
Funding
This study had no financial support.
Author information
Authors and Affiliations
Contributions
IR and PA contributed to the study design, data collection, quality checking and editing the manuscript. SB, RB, ØJ, MD, TU and PS have contributed to data collection and editing the manuscript. RH has contributed to review and editing the manuscript. HD, SJ and SS contributed substantially to analysis, interpretation, literature search, and writing of the manuscript. SS (guarantor) takes the responsibility for the content of the manuscript, including the data and analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Regional Committees for Medical and Health Research Ethics South-Eastern Norway (REK HSØ C, 2017/768) and the Swedish Ethical Review Authority (2017/2113-31/2). The need for informed consent was waived for Swedish patients by the Swedish Ethical Review Authority, while in Norway, patients still alive at the time of registry building provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dingen, H., Jordal, S., Bratt, S. et al. Clinical profile, microbiology and outcomes in infective endocarditis treated with aortic valve replacement: a multicenter case-control study. BMC Infect Dis 24, 913 (2024). https://doi.org/10.1186/s12879-024-09782-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09782-3