Abstract
Background
During the pandemic period, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mutated, leading to changes in the disease’s severity and the therapeutic effect of drugs accordingly. This study aimed to present the actual use of therapeutics and clinical outcomes based on the prevalence of each variant using real-world data.
Methods
We analyzed the electronic medical records of adult patients admitted to Busan Medical Center after confirming coronavirus disease 2019 (COVID-19) from February 1, 2020, to June 30, 2022. Patients with mild-to-moderate COVID-19 who were at a high risk of disease progression were selected as study subjects, and the time period was classified according to the variants as ancestral strain, Delta variant, or Omicron variant. We compared drug use status and clinical outcomes by time period.
Results
Among all 3,091 patients, corticosteroids were the most commonly used therapy (56.0%), being used most frequently in the Delta variant (93.0%), followed by the Omicron variant (42.9%) and ancestral strain (21.2%). Regdanvimab accounted for the majority of therapeutic use in the Delta variant (82.9%) and ancestral strain (76.8%), whereas remdesivir was most frequently used during the Omicron variant period (68.9%). The composite outcomes of death or disease aggravation were ranked in the order of the Delta variant, Omicron variant, and ancestral strain (14.5, 11.9, and 6.0%, respectively, P < 0.001).
Conclusion
Regdanvimab was primarily used during the ancestral strain period, regdanvimab plus corticosteroids during the Delta variant period, and remdesivir during the Omicron variant period. The rate of death or disease aggravation was highest in the Delta variant, followed by the Omicron variant and the ancestral strain.
Similar content being viewed by others
Background
Since the initial report of coronavirus disease 2019 (COVID-19) in Wuhan, Hubei Province, China, in late December 2019 [1], the world has witnessed nearly 775 million cumulative confirmed cases and over 7 million deaths by the end of February 2024 [2]. In South Korea, the first confirmed case was reported on January 20, 2020, and by February 25, 2024, the country has recorded a total of 34.57 million confirmed cases [2, 3]. Of these, 35,934 individuals have died, yielding a fatality rate of 0.10% [2]. Throughout the four-year span of the pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has undergone numerous mutations, leading to variations in viral transmission and disease severity [4, 5]. Additionally, advancements in treatments and vaccines have played a significant role in shaping the pattern of the disease [6,7,8].
According to the recently revised COVID-19 treatment guidelines, ritonavir-boosted nirmatrelvir, molnupiravir, and remdesivir are recommended for patients with mild-to-moderate COVID-19 at high risk of progressing to severe disease [7, 9]. Previously, anti-SARS-CoV-2 monoclonal antibodies (mAbs) were recommended for these patients [10,11,12], but since it was reported that their neutralizing ability was lost or decreased for Omicron variants [13, 14], the recommended pharmacotherapies for patients with mild-to-moderate COVID-19 have changed significantly [7].
In South Korea, remdesivir became the first COVID-19 treatment to be conditionally approved on July 24, 2020, for patients with severe conditions requiring hospitalization [15]. On January 20, 2022, its use was expanded to include patients with mild-to-moderate symptoms [16]. Regdanvimab received conditional approval as the initial treatment for mild-to-moderate COVID-19 on February 5, 2021 [17], and was officially approved on September 17, 2021, following the results of Phase 2/3 clinical trials [18]. The effectiveness and safety of regdanvimab have been corroborated by several real-world studies [19,20,21,22]. Nevertheless, its distribution was suspended as of February 18, 2022, due to diminished efficacy against SARS-CoV-2 mutations [23]. On December 27, 2021, ritonavir-boosted nirmatrelvir and, on March 23, 2022, molnupiravir were authorized for emergency use as oral treatments for patients with mild-to-moderate COVID-19 [24, 25]. Tixagevimab/cilgavimab received emergency use authorization on June 30, 2022, as pre-exposure prophylaxis for immunocompromised patients, and remains the only mAb treatment currently used in South Korea [26].
Vaccination against SARS-CoV-2 commenced in several countries starting mid-December 2020, and as of March 23, 2024, 70.6% of the global population has received at least one dose of a COVID-19 vaccine [6]. In South Korea, vaccination began on February 26, 2021, 44.78 million individuals having received a total of 129.65 million doses [6]. Although the bivalent vaccine did not exhibit a significant difference in immune response to the Omicron subvariants BA.4/BA.5 compared to the monovalent vaccine [27, 28], vaccinations have been reported to prevent severe disease progression regardless of the variant type [29, 30].
The collected data provide insights into variables such as prevalence, vaccination rates, and mortality [2, 3, 6]; however, there is a lack of information on the current status of actual drug usage and clinical outcomes relative to the time periods dominated by each mutant strain and the disease’s severity. While several prior studies have explored treatment patterns and clinical outcomes, they primarily cover the era before the development of COVID-19-specific treatments or focus on periods dominated by certain mutant strains [31, 32]. Therefore, in this study, utilizing real-world data, we aimed to comprehensively analyze the actual use of drugs and their effectiveness over time during periods when specific variants were dominant.
Methods
Study design and participants
In this observational study, we retrospectively analyzed the electronic medical records (EMRs) of adult patients aged 18 years or older who were admitted to Busan Medical Center (BMC) after a confirmed COVID-19 diagnosis through reverse transcriptase-polymerase chain reaction (RT-PCR) from February 1, 2020, to June 30, 2022. Pregnant and lactating women were excluded from the study. Additionally, patients were categorized based on disease severity as mild, moderate, or severe. Severe cases were those presenting with oxygen saturation ≤ 94% at admission, requiring supplemental oxygen, or showing a respiratory rate ≥ 30/minute [33, 34]. Among those not identified as severe, patients with pneumonia were classified as moderate, while all others were classified as mild [33, 34].
Patients with mild-to-moderate COVID-19 at high risk of disease progression were selected as study subjects, comparing their medication use and clinical outcomes across different time periods. Risk factors for disease progression included age > 50 years, body mass index (BMI) > 25 kg/m2, immunosuppressed state (anticancer treatment, bone marrow or organ transplant status, autoimmune disease, or human immunodeficiency virus [HIV] infection), and underlying comorbidities (cardiovascular disease, chronic lung disease, diabetes mellitus, hypertension, chronic kidney disease, or chronic liver disease) [35, 36].
Classification of time periods by SARS-CoV-2 variants
The classification of time period according to the dominance of SARS-CoV-2 variants was determined by the intervals in which a dominant variant accounted for ≥ 90% of the variant proportions monitored biweekly [3]. Figure 1 shows the distribution of variants in South Korea by period. Supplementary Table 1 provides the specific data figures. The period before the Delta variant became dominant was referred to as the ancestral strain period. The period after February 19, 2021, when regdanvimab was approved and began its actual use as the first COVID-19 treatment in Korea, was designated as the comparative analysis period. Consequently, the time periods were classified as follows: from February 19, 2021 to May 31, 2021, as the ancestral strain period; from September 1, 2021 to December 31, 2021, as the Delta variant period; and from February 1, 2022 to June 30, 2022, as the Omicron variant period. This method of period classification is similar to that used in previous studies [31, 37], but we allowed for longer intervals to further minimize errors in variant classification.
Data collection
Baseline characteristics, including age, sex, height, body weight, comorbidities, co-medications, vaccination details (doses and dates), and diagnosis dates, were collected from EMRs. Comorbidities comprised hypertension, coronary artery disease, congestive heart failure, cerebrovascular disease, diabetes mellitus, chronic lung disease, asthma, chronic kidney disease, dialysis, chronic liver disease, an immunosuppressed state, and pneumonia. Co-medications encompassed those previously presumed to affect COVID-19 treatment, as well as those administered to treat comorbidities. These medications were categorized as angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) [38, 39], statins [40, 41], oral anticoagulants [42, 43], aspirin [44, 45], and immunomodulators [46, 47], with immunomodulators including immunosuppressants and corticosteroids.
Regarding the use of COVID-19 therapeutics, we investigated the administration status and duration of regdanvimab, remdesivir, ritonavir-boosted nirmatrelvir, corticosteroids (dexamethasone, prednisolone, and methylprednisolone), hydroxychloroquine, and azithromycin.
Clinical outcomes
The primary outcome was defined as a composite of in-hospital death or disease aggravation. Indicators of disease aggravation included the need for oxygen therapy (either low- or high-flow oxygen therapy, or mechanical ventilation) in BMC, or transfer to a tertiary hospital for further invasive treatment. The secondary outcome focused on the length of hospital stay, measured in days.
All study participants were followed up until discharge or death, with the study period concluding on July 4, 2022, which was the last discharge date for all participants.
Statistical analysis
Descriptive statistics are presented as medians with interquartile ranges (IQRs) for continuous variables and numbers with percentages for categorical variables. For differences among the three groups, analysis of variance (ANOVA) and chi-square tests were used for continuous and categorical variables, respectively. Normality was assessed using the Shapiro–Wilk Test; if normality assumptions were not met, the Kruskal–Wallis test was utilized in place of ANOVA. When cells with an expected frequency of less than 5 exceeded 20%, Fisher’s exact test was used instead of chi-square tests.
All statistical analyses were performed using R (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria), and the significance level was set at P < 0.05.
Results
Characteristics of study participants
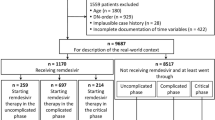
A total of 7,738 adult patients were admitted to BMC with COVID-19 between February 1, 2020, and June 30, 2022. Among these, 13 pregnant and lactating women were excluded. After excluding severe cases and mild cases without risk factors, 5,658 patients were identified as being at high risk of progressing to severe disease. Following the refinement of the analysis to the specified periods, the total number of study subjects included was 3,091. Patients corresponding to each time period were distributed as follows: the ancestral strain, Delta variant, and Omicron variant accounted for 799 (25.8%), 1,155 (37.4%), and 1,137 (36.8%) of the patients, respectively (Fig. 2). Patients in the intervals between these periods were excluded from the analysis. Supplementary Tables S2, S3, and S4 present detailed breakdowns of patient numbers by period and severity, outlining each step of the study subject extraction process.
Table 1 shows the demographic and clinical characteristics of the 3,091 patients. Patients in the Omicron variant period were older (median age 70 [IQR, 61–81] years, P < 0.001), had a higher proportion of women (60.5%, P < 0.001), and had a lower BMI (23.2 [20.7–25.7] kg/m2, P < 0.001) compared to patients in the other two periods. Moreover, the proportion of patients with moderate COVID-19 (with pneumonia) was highest in the Delta variant (60.9%), followed by the Omicron variant (41.4%) and ancestral strain (32.2%). The prevalence of comorbidities, excluding chronic liver disease, and the use of co-medications, with the exception of ACEIs or ARBs, were highest in the Omicron variant. No significant difference was observed in the prevalence of chronic liver disease among the three groups; however, the use of ACEIs/ARBs was most prevalent in the ancestral strain (10.8%, P = 0.049). The proportion of vaccinated patients was highest in the Omicron variant (79.3%, P < 0.001), and most had completed the second vaccination. The time from the last vaccination to the COVID-19 diagnosis was also significantly longer in the Omicron variant (median 158 [112–212] days, P < 0.001).
In calculating vaccination rates, individuals with missing vaccination status were categorized as unvaccinated. Additionally, cases with missing vaccination dates were omitted from the analysis of the period between vaccination and COVID-19 diagnosis. Details of missing values are provided in Supplemental Table S5.
Therapeutics use and clinical outcomes
The therapeutic uses and clinical outcomes of study participants are shown in Table 2. The most commonly administered therapy for all patients was corticosteroids (56.0%), with the highest usage in the Delta variant (93.0%), followed by the Omicron variant (42.9%) and ancestral strain (21.2%). Regdanvimab was the predominant therapy for the Delta variant (82.9%) and the ancestral strain (76.8%) but it constituted only a small fraction of treatments for the Omicron variant (9.5%). Remdesivir was most frequently used in treating the Omicron variant (68.9%), followed by the Delta variant (14.8%) and ancestral strain (4.0%). Finally, ritonavir-boosted nirmatrelvir was administered only for Omicron variants (13.1%).
The composite outcomes, including death or disease aggravation, occurred in the following order: Delta variant, Omicron variant, and ancestral strain (14.5, 11.9, and 6.0%, respectively, P < 0.001). Disease aggravation in BMC was most pronounced in the Delta variant (13.9%), followed by the Omicron variant (10.3%) and the ancestral strain (6.0%). The rates of transfer to a tertiary hospital and mortality were highest in patients with the Omicron variant, followed by those with the Delta and ancestral strains. The length of hospital stay was the shortest for the Omicron variant, at 6 [5–7] days (P < 0.001).
Discussion
In this retrospective observational study, we investigated the actual drug use and clinical outcomes over time in adult in-patients with mild-to-moderate COVID-19 at a single institution. During the ancestral strain, Delta variant, and Omicron variant periods, regdanvimab, regdanvimab plus corticosteroids, and remdesivir were primarily used, respectively. Clinical outcomes showed that the death or disease aggravation rate was the highest during the Delta variant period, followed by the Omicron variant and the ancestral strain periods.
Our study's time-based classification approach aligns with similar methodologies in the literature. Noh et al. and Ryu et al. used time periods to classify patients and evaluate the clinical outcomes associated with different SARS-CoV-2 variants [31, 37]. These studies also reported increased severity and worse clinical outcomes during the Delta variant period compared to earlier strains, but did not include the Omicron variant period.
According to the COVID-19 Treatment Guidelines, the recommended therapeutics have continued to change depending on the epidemic period and the development of new therapeutics [7, 48]. Specifically, anti-SARS-CoV-2 mAbs were recommended for patients with mild-to-moderate COVID-19 who were at high risk of disease progression during the ancestral strain and Delta variant epidemics, but antiviral agents were recommended as preferred therapies during the Omicron epidemic [7, 48]. Furthermore, the mAbs recommended by the NIH guidelines differed between the ancestral strain and Delta variant epidemic period. Bamlanivimab and casirivimab plus imdevimab were recommended for the ancestral strain, and bamlanivimab plus etesevimab, casirivimab plus imdevimab, and sotrovimab were recommended for the Delta variant [49].
However, in South Korea, regdanvimab was the only available mAb in both periods. Notably, it has been reported that the neutralizing ability of regdanvimab against the Delta variant was reduced in cells, but the efficacy of symptom remission remained in vivo [50]. Nonetheless, as evidenced by the drug usage data in this study, the real-world effectiveness of regdanvimab on the Delta variant appeared to be significantly diminished. Consequently, corticosteroids had to be administered to most patients.
During the Omicron outbreak, the antiviral agents ritonavir-boosted nirmatrelvir and remdesivir were recommended as the preferred therapies, in that order [7]. However, since ritonavir-boosted nirmatrelvir, which started to be introduced sequentially from January 13, 2022, was not available in sufficient quantities in South Korea, remdesivir played a significant role as an accessible recommended treatment [51,52,53]. This significantly differs from the findings of a retrospective cohort study conducted in Wales, UK, around the same time. According to the study by Andrew Evans et al., the treatments administered to higher-risk COVID-19 patients were sotrovimab (52.9%), ritonavir-boosted nirmatrelvir (29.5%), and molnupiravir (17.6%) [54]. Unlike in Wales, sotrovimab was not introduced in Korea, and molnupiravir was approved for emergency use on March 23, 2022 [24]. Furthermore, it took additional time for molnupiravir to be supplied in sufficient quantities. Additionally, our study focused solely on hospitalized patients, leading to a presumption that the calculated usage rate of oral antivirals is lower than the actual usage rate in South Korea. This discrepancy is due to the availability of the oral drugs ritonavir-boosted nirmatrelvir and molnupiravir for outpatients.
In South Korea, after the first COVID-19 vaccination began on February 26, 2021 [55], 70% of the population was fully vaccinated by October 23, 2021 [56]. It was found that repeated vaccinations were less effective in preventing infections with the Omicron variant and the duration of effectiveness was shorter. However, they were effective in preventing hospitalization or death due to disease worsening [57]. The results of this study also indicated that although the vaccination rate was significantly higher among patients infected during the period of Omicron dominance, the time elapsed since vaccination was also significantly longer. This suggests that the vaccine’s duration of effectiveness was shorter and its efficacy in preventing infection was reduced. However, due to a significant amount of missing data on the date of the last vaccination, further research is needed on the treatment outcomes according to the time elapsed since vaccination.
The finding that the Delta variant, Omicron variant, and ancestral strain follow a specific order in terms of death or disease aggravation rates is partially consistent with previous studies. Xue et al. and Choe et al., found the Omicron variant to be less fatal than the Delta variant [58, 59], a finding supported by Lin et al., who showed the Delta variant had a higher risk than other variants [60]. However, while the study by Xue et al. and Choe et al. indicated a lower risk associated with the Omicron variant compared to all other variants, our study revealed a higher risk than that of the ancestral strain. Understanding this discrepancy requires considering the hospitalization policies for COVID-19 patients in Korea. Specifically, in the early stages of the COVID-19 pandemic in South Korea, all confirmed cases were hospitalized or admitted to care centers regardless of the severity of the disease. However, as the number of patients surged, the policy shifted on November 26, 2021, to primarily home treatment for all confirmed cases, with hospitalization reserved for those with specific reasons, such as the presence of factors necessitating inpatient care [61]. Consequently, inpatients during the Omicron variant epidemic are likely to have a significantly elevated risk of disease exacerbation compared to those during the ancestral strain period, rendering a straightforward comparison between the two notably challenging. In the same context, the fact that the mortality of inpatients was the highest in the Omicron variant in our study does not mean that the Omicron variant has the highest severity and should be interpreted considering that the patient’s age and comorbidity rate were the highest in the Omicron variant. The fact that the mortality of the Omicron variant was higher than that of the ancestral strain in relation to the hospitalization policy in South Korea showed the same result as the official data collection did [3].
Our study has several limitations. First, because we analyzed inpatient data from a single institution, the results were greatly influenced by regional circumstances and hospitalization policies, making it difficult to generalize the results. Second, the drugs that could be used during the epidemic period for each variant were limited and the criteria for drug use also changed, making it difficult to compare the effects of drugs on each variant directly. Additionally, because the characteristics of the patients admitted to the BMC at each time period were significantly different owing to the aforementioned circumstances, it was impossible to match and compare them accurately. Furthermore, since we used a time-based classification method rather than individual test results, some patients might have been infected with a different variant than the one assigned to their time period. Given these potential sources of error and the aforementioned heterogeneity of hospitalized patients across different time periods, caution is warranted when directly applying the observed results of this study to estimate the disease risk or drug effectiveness for each variant.
Nevertheless, this study has several implications. Based on real-world data, we analyzed the actual drug utilization status and clinical outcomes for the period dominated by each variant. In addition, it was possible to measure the impact of changes in drug use standards and hospitalization policies. Generally, the Omicron variant is known to have the lowest risk of progressing to severe disease, but in this study, the Omicron variant showed a higher risk of severe progression following the Delta variant. This is presumed to be due to the policy shift to hospitalize only patients with risk factors for disease progression, as mentioned earlier. Additionally, while other studies have tracked deaths up to post-discharge, this study was only able to observe in-hospital deaths. To compensate for this, transfers to tertiary hospitals due to worsening of the disease were included in the composite outcome. Nevertheless, the overall clinical outcomes may have been underestimated due to missing results, such as deaths among patients treated at home. Moreover, it is presumed that the policy of hospitalizing most confirmed cases in South Korea by the end of 2021 contributed to lowering the rates of severe progression and death, as it allowed for close observation and prompt action. Future studies are needed that use data on patients infected with the same variant virus but treated with different therapeutics, or the same therapeutic used on patients infected with different variant viruses. These studies should compare the real-world effectiveness and side effects of treatments while controlling for confounders through propensity score matching. We also hope that future research will include variant-specific comparisons based on individual test results to enhance the accuracy of the findings.
Conclusion
Regdanvimab was primarily used during the ancestral strain period, regdanvimab plus corticosteroids during the Delta variant period, and remdesivir during the Omicron variant period. The death or disease aggravation rate was highest during the Delta variant period, followed by the Omicron variant and the ancestral strain periods.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
WHO. WHO COVID-19 dashboard. https://data.who.int/dashboards/covid19/casesAccessed on 25 Mar 2024.
Our World in Data. COVID-19 Data explorer. https://ourworldindata.org/explorers/coronavirus-data-explorerAccessed on 25 Mar 2024.
CDC. SARS-CoV-2 Variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. Accessed on 25 Aug 2023.
Markov PV, Ghafari M, Beer M, Lythgoe K, Simmonds P, Stilianakis NI, Katzourakis A. The evolution of SARS-CoV-2. Nat Rev Microbiol. 2023;21(6):361–79.
Our World in Data. Statistics and research coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinationsAccessed on 25 Mar 2024.
Agarwal A, Hunt BJ, Stegemann M, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, Lytvyn L, Leo Y-S, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379.
Miyashita K, Hozumi H, Furuhashi K, Nakatani E, Inoue Y, Yasui H, Karayama M, Suzuki Y, Fujisawa T, Enomoto N. Changes in the characteristics and outcomes of COVID-19 patients from the early pandemic to the delta variant epidemic: a nationwide population-based study. Emerging Microbes & Infections. 2023;12(1):2155250.
NECA, KAMS. COVID-19 Living guideline https://www.neca.re.kr/lay1/bbs/S1T11C174/F/58/view.do?article_seq=9237&cpage=1&rows=10000&condition=&keyword=&show=&cat= Accessed on 25 Mar 2024.
US FDA. Emergency use authorizations for drugs and non-caccine biological products. https://www.fda.gov/drugs/emergency-preparedness-drugs/emergency-use-authorizations-drugs-and-non-vaccine-biological-productsAccessed 25 Mar 2024.
EMA. COVID-19 medicines. https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicinesAccessed 25 Mar 2024.
Syed YY. Regdanvimab: First approval. Drugs. 2021;81(18):2133–7.
VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE, Purcell LA, Kawaoka Y, Corti D, Fremont DH, Diamond MS. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28(3):490–5.
Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, Iwatsuki-Horimoto K, Chiba S, Halfmann P, Nagai H, et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N Engl J Med. 2022;386(10):995–8.
MFDS. Conditional marketing authorisation for Veklury(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=44455&srchFr=&srchTo=&srchWord=%EB%B2%A0%ED%81%B4%EB%A3%A8%EB%A6%AC&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1Accessed on 25 Mar 2024.
MFDS. Emergency use approval for Veklury(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=46086&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1Accessed on 25 Mar 2024.
MFDS. Conditional marketing authorisation for Regkirona(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=45029&srchFr=&srchTo=&srchWord=&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C1009&page=2Accessed on 25 Mar 2024.
Streinu-Cercel A, Săndulescu O, Preotescu L-L, Kim JY, Kim Y-S, Cheon S, Jang YR, Lee SJ, Kim SH, Chang I. Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019. In: Open forum infectious diseases. US: Oxford University Press; 2022. p. ofac053.
Park S, Je NK, Kim DW, Park M, Heo J. Effectiveness and Safety of Regdanvimab in Patients With Mild-To-Moderate COVID-19: A Retrospective Cohort Study. J Korean Med Sci. 2022;37(13):e102.
Kim T, Joo DH, Lee SW, Lee J, Lee SJ, Kang J. Real-World Efficacy of Regdanvimab on Clinical Outcomes in Patients with Mild to Moderate COVID-19. J Clin Med. 2022;11(5):1412.
Hong SI, Ryu BH, Hong KW, Bae IG, Cho OH. Real World Experience with Regdanvimab Treatment of Mild-to-Moderate Coronavirus Disease-19 in a COVID-19 Designated Hospital of Korea. Infect Chemother. 2022;54(1):114–24.
Lee JY, Lee JY, Ko J-H, Hyun M, Kim HA, Cho S, Lee YD, Song J, Shin S, Peck KR. Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease. Front Immunol. 2021;12(4998):772320.
KDCA. Guide to the use of COVID-19 treatment, 4–3rd edition. https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&tag=&act=view&list_no=718756#Accepted on 25 Mar 2024.
MFDS. Emergency use approval for Lagevrio(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=46243Accessed on 25 Mar 2024.
MFDS. Emergency use approval for Paxlovid(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=46032&srchFr=&srchTo=&srchWord=%ED%8C%8D%EC%8A%A4%EB%A1%9C%EB%B9%84%EB%93%9C&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1Accessed on 25 Mar 2024.
MFDS. Emergency use approval for Evusheld(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=46495&srchFr=&srchTo=&srchWord=%EC%9D%B4%EB%B6%80%EC%8B%A4%EB%93%9C&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1Accessed on 25 Mar 2024.
Offit PA. Bivalent Covid-19 Vaccines — A Cautionary Tale. N Engl J Med. 2023;388(6):481–3.
Wang Q, Bowen A, Valdez R, Gherasim C, Gordon A, Liu L, Ho DD. Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot. bioRxiv. 2022;2010:513349.
Tenforde MW, Self WH, Zhu Y, Naioti EA, Gaglani M, Ginde AA, Jensen K, Talbot HK, Casey JD, Mohr NM. Protection of mRNA vaccines against hospitalized COVID-19 in adults over the first year following authorization in the United States. Clin Infect Dis. 2023;76(3):ciac381.
Cai C, Peng Y, Shen E, Huang Q, Chen Y, Liu P, Guo C, Feng Z, Gao L, Zhang X. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther. 2021;29(9):2794–805.
Noh HJ, Song JH, Ham SY, Park Y, Won HK, Kim SJ, Chung KB, Kim CK, Ahn YM, Lee BJ, Kang HR. Clinical outcomes of mild to moderate coronavirus disease 2019 patients treated with Regdanvimab in delta-variant outbreak: Retrospective cohort study. Medicine (Baltimore). 2023;102(45):e35987.
Ayodele O, Ren K, Zhao J, Signorovitch J, Jonsson Funk M, Zhu J, Bao Y, Gondek K, Keenan H. Real-world treatment patterns and clinical outcomes for inpatients with COVID-19 in the US from September 2020 to February 2021. PLoS ONE. 2021;16(12):e0261707.
Kim SB, Ryoo S, Huh K, Joo EJ, Kim YJ, Choi WS, Kim YJ, Yoon YK, Heo JY, Seo YB, et al. Revised Korean Society of Infectious Diseases/National Evidence-based Healthcarea Collaborating Agency Guidelines on the Treatment of Patients with COVID-19. Infect Chemother. 2021;53(1):166–219.
Gandhi RT, Lynch JB, Del Rio C. Mild or Moderate Covid-19. N Engl J Med. 2020;383(18):1757–66.
MFDS. Official approval of Regkirona(R). https://www.mfds.go.kr/brd/m_99/view.do?seq=45778&srchFr=&srchTo=&srchWord=%EB%A0%89%ED%82%A4%EB%A1%9C%EB%82%98&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1Accessed on 25 Mar 2024.
EMA. EMA issues advice on use of regdanvimab for treating COVID-19. https://www.ema.europa.eu/en/news/ema-issues-advice-use-regdanvimab-treating-covid-19Accessed on 25 Mar 2024.
Ryu BH, Hong SI, Lim SJ, Cho Y, Hwang C, Kang H, Kim SH, Wi YM, Hong KW, Bae IG, Cho OH. Clinical Features of Adult COVID-19 Patients without Risk Factors before and after the Nationwide SARS-CoV-2 B.1.617.2 (Delta)-variant Outbreak in Korea: Experience from Gyeongsangnam-do. J Korean Med Sci. 2021;36(49):e341.
Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, Mallipattu SK, Skopicki HA, Singer AJ, Duong TQ. Continued In-Hospital Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Receptor Blocker Use in Hypertensive COVID-19 Patients Is Associated With Positive Clinical Outcome. J Infect Dis. 2020;222(8):1256–64.
Fosbøl EL, Butt JH, Østergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA. 2020;324(2):168–77.
Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, Eckhardt C, Bikdeli B, Platt J, Nalbandian A. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):1–9.
Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, Longhurst CA, Criqui MH, Messer K. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. Am J Cardiol. 2020;136:149–55.
Rivera-Caravaca JM, Núñez-Gil IJ, Vivas D, Viana-Llamas MC, Uribarri A, Becerra-Muñoz VM, Trabattoni D, Fernández Rozas I, Feltes G, López-Pais J, et al. Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID-19. Eur J Clin Invest. 2021;51(1):e13436.
Fröhlich GM, Jeschke E, Eichler U, Thiele H, Alhariri L, Reinthaler M, Kastrati A, Leistner DM, Skurk C, Landmesser U, Günster C. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021;110(7):1041–50.
Osborne TF, Veigulis ZP, Arreola DM, Mahajan SM, Röösli E, Curtin CM. Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration. PLoS ONE. 2021;16(2):e0246825.
Chow JH, Khanna AK, Kethireddy S, Yamane D, Levine A, Jackson AM, McCurdy MT, Tabatabai A, Kumar G, Park P, et al. Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019. Anesth Analg. 2021;132(4):930–41.
Pastor-Nieto M, Checa-Díaz P, González-Muñoz P, Martín-Fuentes A, Vergara-Sánchez A, Sánchez-Herreros C, Jiménez-Blázquez E, Cabana-Navia R, Martínez-Mariscal J, Cobo-Rodríguez P. Prior treatment with immunosuppressants among COVID-19 inpatients at one hospital in Spain. J Eur Acad Dermatol Venereol. 2020;34(12):e760–2.
Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–8.
WHO. Clinical management of COVID-19: Living guideline, 18 August 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.2Accessed on 25 Mar 2024.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed on 15 Aug 2023.
Ryu D-K, Kang B, Noh H, Woo S-J, Lee M-H, Nuijten PM, Kim J-I, Seo J-M, Kim C, Kim M, et al. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–6.
KDCA. Guide to the use of COVID-19 treatment, 4–1st edition. https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=718425Accessed on 25 Mar 2024.
Shi HJ, Yang J, Eom JS, Ko J-H, Peck KR, Kim UJ, Jung SI, Kim S, Seok H, Hyun M, et al. Clinical characteristics and risk factors for mortality in critical COVID-19 patients aged 50 years or younger during Omicron wave in Korea: comparison with patients older than 50 years of age. J Korean Med Sci. 2023;38(28):e217.
Kim JM, Yoo M-G, Bae SJ, Kim J, Lee H. Effectiveness of Paxlovid, an oral antiviral drug, against the Omicron BA.5 variant in Korea: severe progression and death between July and November 2022. J Korean Med Sci. 2023;38(27):e211.
Evans A, Qi C, Adebayo JO, Underwood J, Coulson J, Bailey R, Lyons R, Edwards A, Cooper A, John G. Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: a retrospective cohort study. J Infect. 2023;86(4):352–60.
KDCA. COVID-19 Vaccination for the Restoration of Daily Life Begins Now. February 26, 2021. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=712528&cg_code=&act=view&nPage=245Accessed on 25 Mar 2024.
KDCA. 70% of the Entire Population Vaccinated, Laying the Groundwork for a Phased Return to Normalcy. October, 23, 2021. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=717336&cg_code=&act=view&nPage=176Accessed on 25 Mar 2024.
Sánchez FJM, Martínez-Sellés M, García JMM, Guillén SM, Rodríguez-Artalejo F, Ruiz-Galiana J, Cantón R, Ramos PDL, García-Botella A, García-Lledó A. Insights for COVID-19 in 2023. Rev Esp Quimioter. 2023;36(2):114.
Xue L, Jing S, Zhang K, Milne R, Wang H. Infectivity versus fatality of SARS-CoV-2 mutations and influenza. Int J Infect Dis. 2022;121:195–202.
Choe YJ, Choi EH, Choi JW, Eun BW, Eun LY, Kim Y-J, Kim YH, Kim YA, Kim Y-K, Kwak JH, et al. Change in severity and clinical manifestation of MIS-C Over SARS-CoV-2 variant outbreaks in Korea. J Korean Med Sci. 2023;38(30):e225.
Lin L, Liu Y, Tang X, He D. The disease severity and clinical outcomes of the SARS-CoV-2 variants of concern. Front Pub Health. 2021;9:775224.
KDCA. Medical and Epidemic Response Plans Following the Phased Recovery from COVID-19. November 30, 2021. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=717703&cg_code=&act=view&nPage=165Accessed on 25 Mar 2024.
Acknowledgements
The authors thank Editage (www.editage.co.kr) for English language editing.
Funding
This research was supported by a Korea National Enterprise for Clinical Trials (KONECT) grant funded by the Korean Government (No. HE21C0006030021).
Author information
Authors and Affiliations
Contributions
J.Heo and SS.Park involved in conceptualization, literature search and references, data analysis, manuscript writing, review and editing. DW.Kim, M.Park and NK.Je involved in data collection and follow-up. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board (IRB) of BMC approved this study (IRB No. 2022–04-001) and waived the requirement for informed consent. The patient data used in this study were anonymized after extraction from the EMRs; therefore, they did not contain any personally identifiable information.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, S., Je, N.K., Kim, D.W. et al. Current status and clinical outcomes of pharmacotherapies according to SARS-CoV-2 mutations in patients with mild-to-moderate COVID-19: a retrospective single center study. BMC Infect Dis 24, 871 (2024). https://doi.org/10.1186/s12879-024-09765-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09765-4