Abstract
Background and Aims
Data on the safety and effectiveness of tenofovir alafenamide (TAF) plus peginterferon-alpha (Peg-IFN-α) in children with chronic hepatitis B (CHB) are lacking. The current study aimed to present the characteristics of four pediatric CHB patients who obtained a functional cure by using TAF and Peg-IFN-α.
Methods
In this case series study initiated in May 2019, ten children who had no clinical symptoms or signs received response-guided (HBV DNA undetectable, hepatitis B e antigen [HBeAg] loss or seroconversion, and hepatitis B surface antigen [HBsAg] loss or seroconversion) and functional cure-targeted (HBsAg loss or seroconversion) TAF (25 mg/d, orally) plus Peg-IFN-α-2b (180 µg/1.73m2, subcutaneously, once weekly) in combination (9/10) or sequential (1/10) therapy. The safety and effectiveness of these treatments were monitored.
Results
As of April 2024, four out of ten children obtained a functional cure after a mean of 31.5 months of treatment, and the other six children are still undergoing treatment. These four cured children, aged 2, 4, 8, and 6 years, were all HBeAg-positive and had alanine aminotransferase levels of 80, 47, 114, and 40 U/L; HBV DNA levels of 71200000, 93000000, 8220, and 96700000 IU/mL; and HBsAg levels of 39442.8, 15431.2, 22, and 33013.1 IU/mL, respectively. During treatment, all the children (10/10) experienced mild or moderate adverse events, including flu-like symptoms, anorexia, fatigue, and cytopenia. Notably, growth retardation (8/10) was the most significant adverse event; and it occurred in three cured children (3/4) treated with combination therapy and was present to a low degree in the other cured child (1/4) treated with sequential therapy. Fortunately, all three cured children recovered to or exceeded the normal growth levels at 9 months posttreatment.
Conclusions
TAF plus Peg-IFN-α-2b therapy is potentially safe and effective for pediatric CHB patients, which may provide important insights for future clinical practice and study designs targeting functional cures for children with CHB.
Similar content being viewed by others
Introduction
Chronic hepatitis B virus (HBV) infection is a global public health burden that affects 257.5 million individuals worldwide [1]. Although perinatal transmission has significantly decreased since the implementation of maternal antiviral prophylaxis and infant vaccination [2, 3], almost 2 million new infections have occurred annually through perinatal and horizontal transmission in children aged 5 years or younger [4].
There is an unmet need in real-life clinical practice. On the one hand, many children continue to be at risk of progressive liver disease due to active hepatitis [5]; however, less attention has been given to this topic, and limited antiviral options have been approved for younger children with chronic hepatitis B (CHB). Moreover, most hepatologists have adopted a conservative attitude toward antiviral treatment for CHB children [4, 6], which has resulted in few concerns about functional cures. On the other hand, discrimination against chronic HBV infection is severe in China [7], and most families are eager to cure their children in an easy way so as not to interfere with their schooling and work.
In this case series study, we report four functionally cured CHB children treated with two first-line antivirals, tenofovir alafenamide (TAF, which theoretically does not need dosage adjustment because of its favorable safety profile and similar high genetic barrier to resistance compared with tenofovir disoproxil fumarate [TDF]) plus peginterferon-alpha-2b (Peg-IFN-α-2b, the only available Peg-IFN-α in our hospital and even in most areas of China), which has not been evaluated or reported previously in young CHB children.
Patients and methods
Patients
From May 2019 to August 2023, a total of 10 children (all of whom were less than 8 years old) visited and were diagnosed with treatment-naïve and asymptomatic hepatitis B e antigen (HBeAg)-positive CHB in our center; moreover, their parents strongly demanded a functional cure and could not accept the cumbersome oral antiviral drug (entecavir and TDF) dosing adjustments or frequent regular interferon injections. As of April 2024, four children aged 2, 4, 8, and 6 years who were enrolled from May 2019 to November 2021 and treated with TAF plus Peg-IFN-α-2b have achieved functional cure. Here, we mainly report the management process of four cured children.
Clinical procedures
After careful discussion by the expert group and approval by the Medical Management Department of the hospital, we decided to administer response-guided and functional cure-targeted TAF (25 mg/d, orally, once daily) plus Peg-IFN-α-2b (180 µg/1.73 m2, subcutaneously, once weekly) therapy to the 10 children. In detail, children with strong immune clearance features (high [abnormal] alanine aminotransferase [ALT] levels with or without relatively low levels of virological markers, i.e., HBV DNA, HBeAg, and hepatitis B surface antigen [HBsAg]) received initial combination therapy, and children with weak immune clearance features (low [normal] ALT levels with or without relatively high levels of virological markers [HBV DNA, HBeAg, and HBsAg]) received TAF monotherapy first and then received Peg-IFN-α-2b add-on therapy sequentially at specific time points in the future.
Definition of functional cure
The definition of a functional cure for CHB is seroclearance of HBsAg, i.e., loss of detectable serum HBsAg by the assays as described as following with or without seroconversion to hepatitis B surface antibody (HBsAb) [8]. Certainly, before or simultaneously with the seroclearance of HBsAg, HBV DNA and HBeAg should also become undetectable or undergo seroclearance.
Definition of response-guided treatment
Commonly, there are three virologic steps for the functional cure of HBeAg-positive CHB, i.e., undetectable HBV DNA, HBeAg loss or seroconversion, and HBsAg loss or seroconversion, although these steps may not occurr sequentially. Therefore, response-guided therapy and functional cure-targeted treatment are aimed at these three steps. In fact, prior to the start of this study, we did not specifically define this response-guided therapy quantitatively. Generally, on the basis of good adherence and tolerance, if a child’s HBV DNA level continues to decrease to an undetectable level, treatment will continue to achieve this goal. Similarly, if a child’s HBeAg continues to decrease to a negative level, treatment will continue to achieve this goal. Again, if a child’s HBsAg continues to decrease to a negative level, treatment will continue to achieve this goal. In contrast, if a child’s HBV DNA, HBeAg, or HBsAg do not exhibit an obvious decrease at two follow-up timepoints with an interval of 3 months, combination therapy will be discontinued, or only TAF will be retained to continue monotherapy.
Management of the side effects of hemocytopenia
During treatment, when the neutrophil count is ≤ 0.75 × 109/L, the platelet count is < 50 × 109/L, and/or the ALT level is > 5 times the upper limit of normal (ULN, 40 U/L), the interferon dose should be reduced; 1 to 2 weeks later, these parameters should be retested; if recovery occurs, increase to the original dose. When the neutrophil count is ≤ 0.5 × 109/L, the platelet count is < 25 × 109/L, and/or the ALT concentration is > 10 ULN, interferon should be suspended [6]. For patients with significantly decreased blood cell counts, granulocyte colony-stimulating factor or granulocyte macrophage colony-stimulating factor may be used; additionally, for patients with significantly increased ALT levels, hepatoprotective drugs (glutathione tablets) may be used [6].
Laboratory assessments and growth evaluations
Serum HBV markers were tested by using the Abbott ARCHITECT Alinity i Reagent Kit (Abbott Ireland Diagnostics Division, Finisklin Bussiness Park, Sligo, Ireland), with a lower limit of quantification [LLOQ] of 0.05 IU/mL for HBsAg (< 0.05 IU/mL indicating a negative result or HBsAg loss), a normal range of 0–10 mIU/mL for HBsAb (> 10 mIU/mL indicating a positive result), and a normal range of 0–0.18 IU/mL for HBeAg (< 0.18 IU/mL indicating a negative result or HBeAg loss). The serum HBV DNA levels (the LLOQ was 10 IU/mL) were measured by using an Abbott Real Time HBV Assay (Abbott Molecular Inc., Des Plaines, IL, USA). The upper limit of normal for ALT was defined as 40 U/L [9]. The growth conditions (weight and height) were referred to as “Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years” [10].
Results
Baseline characteristics
Table 1 presents the detailed characteristics of the four cured children before treatment initiation. The baseline characteristics of the other six children who were still undergoing treatment and follow-up testing are presented in Table 2. The four cured children, aged 2, 4, 8, and 6 years, were infected with HBV through mother-to-child transmission, were asymptomatic and HBeAg-positive, and had alanine aminotransferase levels of 80, 47, 114, and 40 U/L, HBV DNA levels of 71200,000, 93000,000, 8220, and 96700000 IU/mL, and HBsAg levels of 39442.8, 15431.2, 22, and 33013.1 IU/mL, respectively. Three children (Nos. 1–3, Table 1) with strong immune clearance features received initial TAF plus Peg-IFN-α-2b combination therapy, and one child (No. 4, Table 1) with weak immune clearance features received TAF and Peg-IFN-α-2b sequential therapy.
Effectiveness
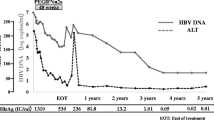
As of April 2024, four out of ten children obtained a functional cure after a mean of 31.5 months of treatment (ranging from 18 to 42 months), and the detailed viral dynamics are presented in Fig. 1. HBV DNA was undetectable in three children (Nos. 1–3) after 3 to 6 months of TAF and Peg-IFN-α-2b combination therapy. Notably, child No. 1 received TAF monotherapy for 15 to 24 months due to the infeasibility of regular follow-up due to the coronavirus disease 2019 (COVID-19) lockdown in his hometown. Unfortunately, child No. 4 was not able to undergo regular monitoring during the first 21 months of TAF monotherapy because of the COVID-19 lockdown in his hometown, and undetectable HBV DNA and HBeAg seroconversion were both detected at 21 months of treatment (Fig. 1). Moreover, HBeAg seroclearance was detected in the other three children (Nos. 1–3) after 24, 18, and 30 months of TAF and Peg-IFN-α-2b combination therapy; however, persistent HBeAg seroconversion was detected only in child No. 2 (Fig. 1). For the functional cure, it is interesting that child No. 3 had the lowest level of HBsAg (22 IU/mL) but the longest treatment duration. Notably, HBsAg seroconversion occurred in children Nos. 1, 2, and 3, with HBsAb levels of 991.1, 53.5, and 29.4 mIU/mL, respectively, at posttreatment month 9, and child No. 4 did not achieve HBsAg seroconversion but maintained the HBsAg seroclearance at posttreatment month 9.
Safety profiles
During treatment, all four cured children experienced mild or moderate adverse events, including flu-like symptoms, anorexia, and fatigue (Table 3), and laboratory abnormalities, including elevated ALT levels (Fig. 2), neutropenia (Fig. 3), and thrombopenia (Fig. 4). However, none of the children presented elevated total bilirubin or abnormal thyroid function parameters. Notably, children Nos.1–3, who received long-term initial combination therapy, exhibited growth retardation, and child No. 4, who received sequential therapy (only one year of Peg-IFN-α-2b treatment), was less affected by growth retardation (Figs. 5 and 6).
Infants’ growth during posttreatment follow-up
Interestingly, by 9 months post-treatment, all three cured children were catching up with or even exceeding the expected growth parameters (Figs. 5 and 6). Unexpectedly, child No. 3 grew 17 cm after 9 months of drug withdrawal (Fig. 6). Notably, although child No. 4, who received sequential therapy, had normal growth parameters during treatment, the baseline gap between the actual and expected growth parameters was obviously larger than that at treatment discontinuation, indicating that only one year of Peg-IFN-α-2b add-on treatment may still slightly influence a child’s growth (Figs. 5 and 6).
Discussion
Many studies have reported the safety and efficacy (effectiveness) of Peg-IFN-α therapy in CHB children [4, 11,12,13,14,15,16]; however, data on the use of Peg-IFN-α plus TAF combination therapy are limited or even lacking. This study is the first to demonstrate the generally favorable safety and effectiveness of TAF plus Peg-IFN-α-2b combination therapy in children with CHB, and reversible growth retardation is the most significant adverse event. These findings may provide important insight for future clinical practice and study designs targeting functional cures for children with CHB.
The functional cure rate of CHB in children is obviously different from that in adults. A previous study indicated that sequential combination treatment with lamivudine and interferon can lead to remarkable HBsAg loss in children with chronic HBV infection and immune-tolerant characteristics [17]. Recently, a randomized trial revealed that peg-interferon and TDF combination therapy followed by protocolized TDF withdrawal led to earlier but not greater HBsAg clearance [18]. Our recent study showed that, compared with those aged 7 years and older, children aged between 1 and 7 years with active CHB can attain a high rate of functional cure through antiviral therapy (nucleos[t]ide analog monotherapy or combination therapy with regular interferon-α), which suggests that early antiviral treatment is beneficial for children with CHB [19].
Currently, the first-line anti-HBV drugs used include entecavir, TDF, TAF, and interferon [6]. Previous studies have indicated that TAF is noninferior to TDF in adult CHB patients and has improved bone and renal effects [20, 21]. Furthermore, our previous studies demonstrated the favorable safety and effectiveness of short-term TAF therapy to prevent mother-to-child transmission in pregnant women with chronic HBV infection and high HBV DNA levels and long-term TAF therapy to treat pregnant women with active CHB [2, 3]. However, data on the safety and effectiveness of TAF in CHB patients (especially those younger than 6 years) are lacking, as are data on TAF and interferon combination therapy.
Notably, entecavir and TDF require dose adjustment for children, and regular interferon necessitates frequent injection, which results in strong rejection by parents. The TAF concentration is less than 1/10 of the TDF concentration and has a high genetic resistance barrier similar to that of TDF [22, 23]. Moreover, a previous study indicated that antiviral monotherapy with Peg-IFN α-2a in children with CHB is well tolerated and effective [24]. Currently, Peg-IFN-α-2b (PegBeron) is the only available Peg-IFN-α in China [25]. Therefore, after providing written informed consent, the expert group decided to administer the TAF plus Peg-IFN-α-2b combination or sequential therapy to meet their parents’ needs.
During long-term treatment, growth retardation was the most significant adverse event. We speculate that this is mainly caused by long-term Peg-IFN-α-2b injection rather than TAF. Fortunately, this adverse event was reversible after 9 months of treatment discontinuation. Notably, the reversible nature of the growth retardation that occurred in children Nos. 1–3 was impressive, especially for child No. 3. During the 9 months posttreatment, child No. 3’s growth was so remarkable that his family, neighbors, teachers, and physicians were all astonished. These findings may greatly alleviate concerns in future clinical practice and research.
Initial combination and sequential therapies are two common treatment strategies for functional cure. In this study, Child No. 4, who received sequential therapy (shorter Peg-IFN-α-2b treatment duration), exhibited less Peg-IFN-α-2b–induced growth retardation, which may provide critical insight into management strategy selection or study design for the future treatment of CHB. Therefore, TAF monotherapy can be used first to achieve the first goal of undetectable HBV DNA. TAF monotherapy or TAF plus Peg-IFN-α-2b combination therapy can then be selected based on the decreasing trend in HBeAg to achieve the second goal of HBeAg loss or seroconversion. Finally, TAF plus Peg-IFN-α-2b combination therapy or even Peg-IFN-α-2b monotherapy can eventually be selected based on the decreasing trend in HBsAg to achieve the final goal of HBsAg loss or seroconversion.
Currently, the achievement of a functional cure in children with CHB does not have a fixed mode or standard of care. Response-guided and functional cure-targeted therapy may be an important mode of treatment, as mentioned above. This means that the treatment plan needs to be adjusted according to the response during treatment, and whether the drug should be stopped if “a standard or prespecified course is completed and there is an obvious response but no functional cure” has become a problem worth considering. In this study, we reported only four children who were cured; the other six children who responded favorably to treatment have not yet been cured, and it is not even possible to predict whether or when they will be cured in the future. Therefore, on the basis of safety, "determining the functional cure goal without determining a clear course of treatment" has become an important strategy for the functional cure of CHB patients in China, including children, which can also be interpreted as a “response-guided and functional cure-targeted strategy”, as described in the Methods section of this study.
In conclusion, although this study had a small sample size, the findings clearly demonstrated the potential for a functional cure of children with CHB treated with TAF plus Peg-IFN-α-2b, as well as the reversibility of growth retardation, which may provide important clues and may change clinical practice or thinking in the future.
Availability of data and materials
Availability of data and materials. All data generated or analyzed during this study are included in this published article.
Abbreviations
- ALT:
-
Alanine aminotransferase
- CHB:
-
Chronic hepatitis B
- HBeAg:
-
Hepatitis B e antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- LLOQ:
-
Lower limit of quantification
- Peg-IFN-α-2b:
-
Peginterferon-alpha-2b
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
References
Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol. 2023;8(10):879–907.
Zeng QL, Yu ZJ, Ji F, Li GM, Zhang GF, Xu JH, et al. Tenofovir Alafenamide to Prevent Perinatal Hepatitis B Transmission: A Multicenter, Prospective. Observational Study Clin Infect Dis. 2021;73(9):e3324–32.
Zeng QL, Zhang HX, Zhang JY, Huang S, Li WZ, Li GM, et al. Tenofovir Alafenamide for Pregnant Chinese Women With Active Chronic Hepatitis B: A Multicenter Prospective Study. Clin Gastroenterol Hepatol. 2022;20(12):2826–37 e2829.
Li M, Li Q, Qu J, Yang H, Lv T, Kong Y, Zhang H. The effectiveness of combination therapy with interferon and nucleoside analogs in pediatric patients with chronic hepatitis B: a systematic review and meta-analysis. Hepatol Int. 2023;17(1):52–62.
Ling SC, Lin HS, Murray KF, Rosenthal P, Mogul D, Rodriguez-Baez N, et al. Chronic Hepatitis Is Common and Often Untreated Among Children with Hepatitis B Infection in the United States and Canada. J Pediatr. 2021;237:24-33 e12.
You H, Wang FS, Li TS, Xu XY, Sun YM, Nan YM, et al. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J Clin Transl Hepatol. 2023;11(6):1425–42.
Yang T, Wu MC. Discrimination against hepatitis B carriers in China. Lancet. 2011;378(9796):1059.
Yip TC, Lok AS. How Do We Determine Whether a Functional Cure for HBV Infection Has Been Achieved? Clin Gastroenterol Hepatol. 2020;18(3):548–50.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Li H, Ji CY, Zong XN, Zhang YQ. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. 2009;47(7):487–92.
Ruiz-Moreno M, Rua MJ, Molina J, Moraleda G, Moreno A, Garcia-Aguado J, Carreno V. Prospective, randomized controlled trial of interferon-alpha in children with chronic hepatitis B. Hepatology. 1991;13(6):1035–9.
Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, et al. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114(5):988–95.
Kobak GE, MacKenzie T, Sokol RJ, Narkewicz MR. Interferon treatment for chronic hepatitis B: enhanced response in children 5 years old or younger. J Pediatr. 2004;145(3):340–5.
Wirth S, Zhang H, Hardikar W, Schwarz KB, Sokal E, Yang W, et al. Efficacy and Safety of Peginterferon Alfa-2a (40KD) in Children With Chronic Hepatitis B: The PEG-B-ACTIVE Study. Hepatology. 2018;68(5):1681–94.
Rosenthal P, Ling SC, Belle SH, Murray KF, Rodriguez-Baez N, Schwarzenberg SJ, et al. Combination of Entecavir/Peginterferon Alfa-2a in Children With Hepatitis B e Antigen-Positive Immune Tolerant Chronic Hepatitis B Virus Infection. Hepatology. 2019;69(6):2326–37.
Komatsu H, Inui A, Yoshio S, Kanto T, Umetsu S, Tsunoda T, Fujisawa T. High Dose of Pegylated Interferon for the Treatment of Chronic Hepatitis B in Children Infected With Genotype C. JPGN Rep. 2020;1(2):e005.
Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu W, et al. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study. J Hepatol. 2018;68(6):1123–8.
Terrault NA, Lok AS, Wahed AS, Ghany MG, Perrillo RP, Fried MW, et al. Randomized Trial of Tenofovir With or Without Peginterferon Alfa Followed by Protocolized Treatment Withdrawal in Adults With Chronic Hepatitis B. Am J Gastroenterol. 2023;118(7):1214–25.
Zhang M, Li J, Xu Z, Fan P, Dong Y, Wang F, et al. Functional cure is associated with younger age in children undergoing antiviral treatment for active chronic hepatitis B. Hepatol Int. 2024;18(2):435–48.
Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206.
Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–95.
Cathcart AL, Chan HL, Bhardwaj N, Liu Y, Marcellin P, Pan CQ, et al. No Resistance to Tenofovir Alafenamide Detected through 96 Weeks of Treatment in Patients with Chronic Hepatitis B Infection. Antimicrob Agents Chemother. 2018; 62(10).
De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol. 2016;119:1–7.
Hu Y, Ye YZ, Ye LJ, Wang XH, Yu H. Efficacy and safety of interferon alpha-2b versus pegylated interferon alpha-2a monotherapy in children with chronic hepatitis B: a real-life cohort study from Shanghai. China World J Pediatr. 2019;15(6):595–600.
Zeng QL, Yu ZJ, Shang J, Xu GH, Sun CY, Liu N, et al. Short-term Peginterferon-Induced High Functional Cure Rate in Inactive Chronic Hepatitis B Virus Carriers With Low Surface Antigen Levels. Open Forum Infect Dis. 2020;7(6):ofaa208.
Acknowledgements
The authors sincerely thank the children and their families for their cooperation in the on‐treatment and follow‐up evaluations.
Funding
National Natural Science Foundation of China (82270629, 82100177); Henan Provincial Science Fund for Distinguished Young Scholars, China (232300421011); Health Science and Technology Innovation Fund for Distinguished Young Scholars, Health Commission of Henan Province, China (YXKC2020024), Program for Science & Technology Innovation Talents in Universities of Henan Province, China (24HASTIT064); Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University, China (QNCXTD2023014), and Young and Middle-aged Academic Leaders of Health Commission of Henan Province, China (HNSWJW-2023029). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
Q.L.Z., F.S.W., and Z.J.Y. contributed to the conception and design of this study. Q.L.Z., R.Y.C., X.Y.L., and Y.J. P. contributed to the data collection and interpretation. S.H. and W.Z.L. contributed to the statistical analysis. Q.-L. Z. contributed to the drafting and revision of this manuscript. F.S.W. and Z.J.Y. contributed to the critical revision of the manuscript for important intellectual content. All authors approved the final version of this manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from the parents before the initiation of the treatment. This study was approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University.
Consent for publication
Written informed consent for publication was obtained from all the parents of the children.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, QL., Chen, RY., Lv, XY. et al. Functional cure induced by tenofovir alafenamide plus peginterferon-alpha-2b in young children with chronic hepatitis B: a case series study. BMC Infect Dis 24, 830 (2024). https://doi.org/10.1186/s12879-024-09723-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09723-0