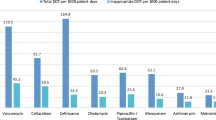
Abstract
Background
Bacterial infections (BIs) are widespread in ICUs. The aims of this study were to assess compliance with antibiotic recommendations and factors associated with non-compliance.
Methods
We conducted an observational study in eight French Paediatric and Neonatal ICUs with an antimicrobial stewardship programme (ASP) organised once a week for the most part. All children receiving antibiotics for a suspected or proven BI were evaluated. Newborns < 72 h old, neonates < 37 weeks, age ≥ 18 years and children under surgical antimicrobial prophylaxis were excluded.
Results
139 suspected (or proven) BI episodes in 134 children were prospectively included during six separate time-periods over one year. The final diagnosis was 26.6% with no BI, 40.3% presumed (i.e., not documented) BI and 35.3% documented BI. Non-compliance with antibiotic recommendations occurred in 51.1%. The main reasons for non-compliance were inappropriate choice of antimicrobials (27.3%), duration of one or more antimicrobials (26.3%) and length of antibiotic therapy (18.0%). In multivariate analyses, the main independent risk factors for non-compliance were prescribing ≥ 2 antibiotics (OR 4.06, 95%CI 1.69–9.74, p = 0.0017), duration of broad-spectrum antibiotic therapy ≥ 4 days (OR 2.59, 95%CI 1.16–5.78, p = 0.0199), neurologic compromise at ICU admission (OR 3.41, 95%CI 1.04–11.20, p = 0.0431), suspected catheter-related bacteraemia (ORs 3.70 and 5.42, 95%CIs 1.32 to 15.07, p < 0.02), a BI site classified as “other” (ORs 3.29 and 15.88, 95%CIs 1.16 to 104.76, p < 0.03), sepsis with ≥ 2 organ dysfunctions (OR 4.21, 95%CI 1.42–12.55, p = 0.0098), late-onset ventilator-associated pneumonia (OR 6.30, 95%CI 1.15–34.44, p = 0.0338) and ≥ 1 risk factor for extended-spectrum β-lactamase-producing Enterobacteriaceae (OR 2.56, 95%CI 1.07–6.14, p = 0.0353). Main independent factors for compliance were using antibiotic therapy protocols (OR 0.42, 95%CI 0.19–0.92, p = 0.0313), respiratory failure at ICU admission (OR 0.36, 95%CI 0.14–0.90, p = 0.0281) and aspiration pneumonia (OR 0.37, 95%CI 0.14–0.99, p = 0.0486).
Conclusions
Half of antibiotic prescriptions remain non-compliant with guidelines. Intensivists should reassess on a day-to-day basis the benefit of using several antimicrobials or any broad-spectrum antibiotics and stop antibiotics that are no longer indicated. Developing consensus about treating specific illnesses and using department protocols seem necessary to reduce non-compliance. A daily ASP could also improve compliance in these situations.
Trial Registration
ClinicalTrials.gov: number NCT04642560. The date of first trial registration was 24/11/2020.
Similar content being viewed by others
Background
Bacterial infections (BIs) are widespread in Paediatric and Neonatal Intensive Care Units (ICUs) and 30–61% of neonates and children hospitalised in ICUs receive antibiotic treatments [1, 2]. For suspected sepsis or septic shock, the Surviving Sepsis Campaign (SSC) recommends the early administration of empiric broad-spectrum antibiotic therapy with one or more antimicrobials to cover all likely pathogens, appropriate routine microbiologic cultures (including blood) before starting antibiotic therapy if doing so causes no substantial delay in starting antimicrobials as well as early de-escalation of antimicrobials based on culture results, susceptibility results and clinical improvement [3, 4]. The choice of antimicrobials, number of antibiotic doses in 24 h, daily dose and duration are determined by local epidemiology, patient characteristics (age, patient history, allergies, multidrug-resistant [MDR] status), BI characteristics (infection site(s), community or hospital-acquired infection, microbiological results) as well as clinical and biological evolution in line with published guidelines. Their recommendations are numerous: French (SPILF, GPIP, SFAR, SRLF, HAS) [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21], European (ESCMID) [22, 23] and American (IDSA) [24,25,26,27,28] guidelines. Long courses of antibiotic therapy and broad-spectrum antimicrobials increase the length of hospitalisation and are associated with changes in the microbiome [29], emergence of multidrug resistant organisms [30, 31] and antibiotic-associated adverse events (toxicity, overdose, allergy) [32]. On the other hand, too short antibiotic therapy can expose the patient to a risk of BI recurrence. In 2017 and 2021, the Société de Pathologie Infectieuse de Langue Française (SPILF or French Language Society for Infectious Diseases) and Groupe de Pathologies Infectieuses en Pédiatrie (GPIP or French Group for Paediatric Infectious Diseases) published French recommendations for the shortest treatment durations for BIs [5, 6]. To our knowledge, compliance with these recommendations has never been evaluated.
In this context, we assessed compliance with recommendations for antibiotic prescriptions, and factors associated with non-compliance for children hospitalised in ICU and receiving systemic antibiotics for an episode of suspected or proven BI.
Methods
Study design, setting and participants
We conducted an observational, prospective, multicentre study in eight French Paediatric and Neonatal ICUs (Appendix 1) during six separate time-periods between June 2020 and May 2021. After an inclusion period testing the feasibility of data collection at the Coordinator Centre (Toulouse) from June to August 2020, we then arbitrarily chose a priori five weeks each spread two months apart for multicentre inclusion over the course of one year. All participating ICUs had the possibility of an audit with an infectious disease specialist over the telephone and most had an antimicrobial stewardship programme (ASP) organised in once weekly multidisciplinary (intensivists, microbiologists and paediatric infectious disease specialists) staff meetings with prospective audit and feedback. Intensivists were the only prescribers. This study was supported by the Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP or French-Speaking Group for Paediatric Intensive and Emergency Care). All methods were performed in accordance with relevant guidelines and regulations. In accordance with French Ethics and Regulatory Law (Public Health Code), this trial is covered by reference methodology MR-004 from the French Data Protection Commission (CNIL). It was approved by Toulouse University Hospital and is registered on its Study Data Register under number RnIPH2019-79 and on the ClinicalTrials.gov website under number NCT04642560. The date of first trial registration on ClinicalTrials.gov was 24/11/2020.
During the study periods, all consecutive neonates and children hospitalised in ICUs and receiving systemic (intravascular, intramuscular or oral) antibiotic treatment for a suspected or proven community-acquired or nosocomial BI were assessed for eligibility. Antibiotics had to be initiated in ICUs during the study periods or no more than 24 h prior to ICU admission occurring over the study periods. We called this episode “first suspected or proven BI episode” to distinguish it from a possible BI recurrence at a later stage. Exclusion criteria were: newborns < 72 h old; neonates < 37 weeks post-menstrual age; age ≥ 18 years; children under surgical antimicrobial prophylaxis; and children previously included in an ongoing interventional study. Informed verbal consent was obtained from the parents or legal guardians of the patient prior to study enrolment.
Data collection
Data on patient characteristics and the first suspected or proven BI episode (characteristics, organ dysfunction scores, antibiotic therapy and concomitant therapeutics other than antibiotics) were prospectively collected on a daily basis by medical study site investigators so long as the patient was receiving antibiotics in hospital for the first suspected or proven BI episode (ICU and general paediatric ward if antibiotic therapy was not completed in the ICU) (Appendix 2). D0 was the day of antibiotic therapy initiation. Additional data on the length of ICU and hospital stay, mortality, outpatient antibiotic therapy (if this was the case) and recurrence of BI occurring within 28 days following D0 were registered upon hospital discharge. Antibiotics used for any other infections during the 28 days following D0 and surgical antimicrobial prophylaxis were not taken into account.
The primary endpoint was the number of first episodes for which antibiotics were prescribed inappropriately on the basis of current recommendations (non-compliance) involving one or more of the following parameters: length of antibiotic therapy, duration of each antimicrobial treatment, choice of antimicrobials, number of antibiotic doses in 24 h, daily dose of antibiotic therapy and reassessment of antibiotic therapy at 72 h. Secondary endpoints were: number of first episodes with non-compliance for any of the parameters; length of antibiotic therapy for the first suspected or proven BI episode; duration of broad-spectrum antibiotic therapy for the first suspected or proven BI episode; and recurrence of BI within 28 days following D0.
Recurrence of infection was defined as the isolation of one or more of the initial causative bacteria from the same or another site at 48 h or more after cessation of antibiotics, combined with clinical signs or symptoms of infection or the need to prescribe a new antimicrobial therapy covering this pathogen [33]. Only recurrences during the same hospital stay as for the first BI episode and occurring within 28 days following D0 were taken into consideration.
Finally, we used two separate definitions to identify broad-spectrum antibiotics: the standard definition [34] and the 2019 AWaRe (Access, Watch, Reserve) classification (Watch and Reserve groups) [35, 36].
Analysis of compliance with antibiotic recommendations
For each first suspected or proven BI episode, the same paediatric infectious disease expert committee (CB and EG) analysed compliance with antibiotic recommendations for length of antibiotic therapy, duration of each antimicrobial treatment, choice of antimicrobials, number of antibiotic doses in 24 h, daily dose of antibiotic therapy and reassessment of antibiotic therapy at 72 h (Appendix 2 and 3). The two experts relied principally on the SPILF and GPIP French guidelines [5, 6] for the duration of antibiotic therapy and each antimicrobial treatment and on a combination of French, European and American guidelines [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] for the choice of antimicrobials, number of antibiotic doses in 24 h and daily dose of antibiotic therapy. Non-compliance (inappropriate antibiotic prescription based on the guidelines) was only determined if both experts agreed. To detect a high background rate of resistant pathogens in ICUs which would justify the use of initial broad-spectrum empiric therapy during the study period, we asked participating centres for their local microbiological data from the two years prior to the study period (2018 and 2019).
Statistical analysis
The results of descriptive statistics were presented as absolute frequencies (%) for qualitative variables and as medians (IQR) for continuous variables.
For all first suspected (or proven) BI episodes and also for only first confirmed (documented or not) BI episodes, we performed univariate analyses to assess factors that might be associated with non-compliance for each parameter and for all parameters combined. For independent qualitative variables, we used the χ² test or Fisher’s exact test while independent quantitative variables employed a two-sample t-test or a Wilcoxon-Mann-Whitney rank-based test. To convert quantitative variables into classes when necessary, we determined the thresholds using Youden’s index.
To establish the independent predictive factors for non-compliance, we then carried out multivariate analyses by stepwise logistic regression after selecting the independent qualitative variables associated with the dependent variable with p < 0.20. The association between variables was significant if p < 0.05. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the significant variables. When one or more centres demonstrated significance during the multivariate analysis, ORs were adjusted for each centre.
Additionally, to evaluate if the use of an ASP during a suspected (or proven) BI episode is a factor modifying the effect of independent variables on non-compliance, we conducted ASP-stratified univariate logistic regression analyses of all factors potentially associated with non-compliance with recommendations for all parameters and for each parameter (length of antibiotic therapy, duration of each antimicrobial treatment, choice of antimicrobials, daily dose of antibiotic therapy and reassessment of antibiotic therapy at 72 h). The ASP sub-group included all episodes that received auditing with an antimicrobial stewardship team for antibiotic duration and/or choice of antimicrobials. Stratified ORs and 95% CIs were calculated for all independent variables in both the ASP and non-ASP sub-groups and were compared with ORs and 95% CIs of all suspected (or proven) BI episodes.
All statistical analyses were performed using SAS software (version 9.4, Cary, NC, USA).
Results
Local microbiological data of participating intensive care units
Local ICU microbiological data from the two years prior to the study period (years 2018–2019) were available at 5 of the 8 centres (Appendix 4). Isolation frequencies for major pathogens was quite similar between the centres. Resistance rates to major antibiotics (intermediate or resistance categories) remained at a low background rate overall except for Enterobacterales resistance to third-generation cephalosporins in some centres.
Population characteristics
During the study periods, 868 children were hospitalised in the eight participating ICUs. One hundred thirty-nine first suspected (or proven) BI episodes occurring in 134 children were included. All children meeting the entire inclusion criteria and with no non-inclusion criteria were included. No one was missed and none of the children had legal guardians who refused consent. The median age was 0.8 (IQR 0.1–6.3) years and 20.1% of episodes affected neonates. Patient characteristics, infection characteristics and antibiotic therapy for first suspected (or proven) BI episodes, and outcomes are set out in Table 1. The most frequent initially suspected BI sites were respiratory (56.1%), catheter-related bacteraemia (20.1%) and intra-abdominal (9.4%). Final diagnosis was no BI for 26.6% of the episodes, presumed (i.e., not documented) BI for 40.3% and documented BI for 35.3%. Three episodes combined both presumed and documented BI. The median length of antibiotic therapy for the first suspected (or proven) BI episode was 7.1 (IQR 4.0-10.5) days. Recurrence occurred for only two episodes (1.4%).
Diagnosis of BI (documented or not) was confirmed for 102 of the 139 first suspected BI episodes (Table 2). The 2005 IPSC infection severity was as follows: 25.5% infection without sepsis; 52.0% sepsis (excluding severe sepsis and septic shock); 11.8% severe sepsis (excluding septic shock); and 10.8% septic shock. Among the 76 episodes of sepsis, severe sepsis or septic shock, respiratory dysfunction was the most common acute organ dysfunction related to the BI and found in 60.5% of the episodes. The most frequent BI sites ultimately identified were respiratory (57.8%), mainly community-acquired pneumonia (13.7%) and aspiration pneumonia (23.5%), catheter-related bacteraemia (18.6%) and intra-abdominal (10.8%).
Documented BIs concerned 49 first confirmed BI episodes. Microbiological data are presented in Table 3. The most prevalent causative bacteria encountered were Staphylococcus sp (34.7%), Enterococcus sp (16.3%) and Klebsiella sp (16.3%). Coagulase-negative Staphylococci (22.4%) were mostly methicillin-resistant (90.9% of the 11 episodes where coagulase-negative Staphylococci were isolated) while Staphylococci aureus (14.3%) were predominantly methicillin-sensitive (85.7% of the 7 episodes where Staphylococcus aureus was isolated). Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae were involved in only one of the 17 episodes in which Enterobacteriaceae were identified and no carbapenem-resistant pathogens were isolated.
Non-compliance with antibiotic recommendations
As a result of death occurring during antibiotic treatment for 6 episodes (antibiotic therapy not completed), compliance for all parameters combined could only be analysed for 133 episodes. Non-compliance with recommendations for all parameters occurred in 51.1% of cases. Results of non-compliance with recommendations for each parameter are detailed in Table 4. The main reasons for non-compliance were inappropriate choice of antimicrobials (27.3%), duration of each antimicrobial treatment (26.3%) and length of antibiotic therapy (18.0%). In most cases, this duration was longer than that recommended by infection guidelines.
Factors associated with Non-compliance with antibiotic recommendations
Results of univariate analyses to determine the significant factors associated with non-compliance with recommendations for all parameters are presented in Table 1 for all first suspected (or proven) BI episodes and in Table 2 for only first confirmed (documented or not) BI episodes. In multivariate analyses (Tables 5 and 6), independent risk factors of non-compliance for all parameters were the use of 2 or more antimicrobials per episode (OR 4.06, 95% CI 1.69–9.74, p = 0.0017) and the duration of broad-spectrum antibiotic therapy based on the standard definition of 4 days or more (OR 2.59, 95% CI 1.16–5.78, p = 0.0199) while independent protective factors of non-compliance (i.e., factors increasing compliance) for all parameters were respiratory failure as the reason for ICU admission (OR 0.36, 95% CI 0.14–0.90, p = 0.0281), using department protocol for antibiotic duration (OR 0.42, 95% CI 0.19–0.92, p = 0.0313) and aspiration pneumonia as BI site ultimately identified (OR 0.37, 95% CI 0.14–0.99, p = 0.0486).
Similarly, we performed univariate and multivariate analyses to establish the independent predictive factors of non-compliance with recommendations for each parameter. Results of multivariate analyses are set out in Tables 5 and 6. In addition to those mentioned above, other independent risk factors for non-compliance were: (1) neurologic compromise as the reason for ICU admission; (2) suspected catheter-related bacteraemia; (3) suspected or confirmed BI site classified as “other” (for which antibiotic therapy is often poorly defined by guidelines); (4) late-onset ventilator-associated pneumonia (VAP) or non-ventilator hospital-acquired pneumonia (HAP); (5) presence of ≥ 1 risk factor for ESBL Enterobacteriaceae; and (6) duration of broad-spectrum antibiotic therapy ≥ 3 days according to the AWaRe classification (Watch and Reserve antibiotics). For children with neurologic compromise at time of ICU admission, the length of antibiotic therapy was prolonged for all non-compliant episodes and concerned aspiration pneumonia in half of all cases, catheter-related bacteraemia and one episode of BI that was finally ruled out. For late-onset VAP, non-compliance regarding the choice of antimicrobials involved empiric therapy for all non-compliant episodes. Otherwise, the independent factors for compliance were respiratory failure at the onset of suspected BI and suspected respiratory BI site. Notably, patient severity during the first suspected (or proven) BI episode, evaluated by PELOD-2 and pSOFA scores, did not represent an independent factor for non-compliance with recommendations. Serious infection (sepsis, severe sepsis and septic shock), particularly with one or more organ dysfunctions, constituted an independent factor for compliance in terms of length and daily dose of antibiotic therapy while the presence of ≥ 2 organ dysfunctions related to infection was an independent risk factor for non-compliance regarding the duration of each antimicrobial treatment. For sepsis with ≥ 2 organ dysfunctions, the duration of one or more antimicrobials was prolonged for all non-compliant episodes. This extended duration concerned antimicrobials used for VAP and non-ventilator HAP for 3 episodes and catheter-related bacteraemia, primary bacteraemia, pneumonia with parapneumonic pleural effusion, BI site classified as “other” and a final diagnosis of no BI for one episode each.
Finally, the use of an ASP during a suspected (or proven) BI episode did not modify the effect of independent variables on non-compliance with recommendations for all parameters (Table 7) and for each compliance parameter (results not shown). Indeed, all 95% CIs for stratified ORs overlapped in the two sub-groups although some independent variables were significant only in the ASP sub-group.
Discussion
In this prospective observational multicentre study, we assessed compliance with recommendations for antibiotic prescriptions made by intensivists in a large population of children hospitalised in 8 French ICUs, most of which have an ASP (once weekly infection multidisciplinary staff meeting with audit and feedback). We also analysed the factors associated with non-compliance. Half of the prescriptions complied with guidelines. In the cases where recommendations were not followed, the main reasons for non-compliance were inappropriate choice of antimicrobial(s), inappropriate duration of one or more antimicrobials and inappropriate length of antibiotic therapy (most frequently prolonged duration for both). In multivariate analyses, we identified situations where intensivists were more attentive to recommendations: patients with respiratory failure, when a respiratory site was initially suspected, when aspiration pneumonia was the ultimately identified site and when antibiotic protocols were available in their ICU. Conversely, we highlighted contexts where risk of non-compliance with guidelines is likely to exist: prescribing at least two antibiotics, duration of broad-spectrum antibiotic therapy ≥ 3–4 days, neurologic compromise at time of ICU admission, suspicion of catheter-related bacteraemia, suspecting or confirming a BI site for which antibiotic therapy is often poorly defined by guidelines (classified as “other”), late-onset VAP, non-ventilator HAP and presence of ≥ 1 risk factor for ESBL Enterobacteriaceae. In sepsis patients, the presence of ≥ 2 organ dysfunctions related to infection represented an independent factor of non-compliance for duration of each antimicrobial treatment while one or more organ dysfunctions were an independent factor of compliance for length of antibiotic therapy.
A few studies have looked at antibiotic prescribing in paediatric ICUs. A prospective multicentre paediatric study of ventilator-associated lung disease showed 70% compliance before implementation of a local protocol and 76% afterwards [37]. Another study described inappropriate antibiotic prescribing in paediatric ICUs ranging from 16.7 to 61.9% depending on the evaluator and the period [38]. In ICU adults with sepsis, studies have demonstrated a comparable compliance rate between 47% and 58% [39, 40].
Lindberg et al. reported that non-compliance was independently associated with an increased risk of 30-day mortality corresponding to 1.86 (95% CI 1.34 to 2.58, p < 0.001) for partial compliance and 2.18 (95% CI 1.34 to 3.40, p < 0.001) for complete non-compliance [40]. In our study, we did not find a statistically significant difference in all-cause mortality between the compliant and non-compliant episodes (7.7% versus 5.9% respectively). However, mortality in our population was 10.8%, much lower than in critically ill adults and our study was not designed to evaluate the impact of non-compliance on mortality. Similarly, non-compliance was not associated with recurrence of bacterial infection in our work although the recurrence rate was only 1.4%.
Good compliance with recommendations for respiratory patients is consistent with clinical practice. Indeed, respiratory infections were mainly community-acquired in our study. Antibiotic therapy for these infections is well-defined by current guidelines for children [5, 6, 9] which allows doctors to better comply with recommendations.
The advantage of antibiotic protocols has already been identified. Protocols, based on current guidelines and updated regularly, are available 24/7 and enable harmonisation of prescriptions made by intensivists that can change from day to day. In adults, the implementation of computerised local antibiotic therapy protocols has been associated with reduced antibiotic exposure and mortality in adult ICUs [41].
To steer decisions about antibiotic therapy in ICUs, a European expert panel has created an “antibiotic care bundle” (ABC-Bundle) with evidence-based recommendations for antibiotic prescribing [42]. The six steps are: (1) provide rationale for antibiotic start; (2) perform appropriate microbiological sampling; (3) prescribe empiric antibiotic therapy according to guidelines (day 1); (4) review diagnosis; (5) assess de-escalation based on microbiological results (days 2–5); and (6) consider discontinuation of treatment in patients with negative culture results and clinical improvement (days 3–5). To mitigate the risk of antibiotic resistance emergence, recent European recommendations have defined antimicrobial de-escalation (ADE) as discontinuing one or more antimicrobials in the empiric combination therapy or replacing broad-spectrum antimicrobials with narrower spectrum agents [43]. In our study, ADE was evaluated by the duration of each antimicrobial treatment, reassessment of antibiotic therapy at 72 h and choice of antimicrobials (narrow antimicrobial therapy once pathogen identification and susceptibility testing results are available). Based on the DIANA study [44], we decided that ADE should take place within the first 3 days of initiation of empiric therapy to be appropriate. In the DIANA study in which 152 adult ICUs in 28 countries participated, ADE within the first 3 days of empiric therapy occurred in only 16% of patients while combination therapy was prescribed in half of the patients and infections were documented in 56% of the study population. Our results concerning inappropriate choice of antimicrobials and duration of one or more antimicrobials as the most frequent reasons for non-compliance and the number of antimicrobials used per episode and length of broad-spectrum antibiotic therapy to be representative of independent risk factors for non-compliance, show that ADE is essential in clinical practice. Recent recommendations on sepsis management in children have also emphasised that dual therapy is no longer recommended for children who are not immunocompromised and present no risk of carrying multidrug-resistant bacteria [4].
ADE is more frequently used in patients with a favourable course [45]. To analyse compliance with antibiotic recommendations, our paediatric infectious disease experts took into account patient improvement. Despite a favourable trajectory, ADE was often missing in patients with the most serious infections (sepsis with ≥ 2 organ dysfunctions). We hypothesise that intensivists probably delayed or did not achieve de-escalation due to the initial severity of the infection. However, de-escalation is safe for patients with favourable evolution. De Bus and colleagues reported no significant difference in 28-day mortality and infection relapse for ADE patients versus non-ADE patients in adult ICUs [44]. The 2021 SSC adult guidelines also suggested daily assessment for ADE and early ADE based on adequate clinical improvement for adults with sepsis or septic shock [3].
In patients with neurologic compromise at time of ICU admission, non-compliance in terms of length of antibiotic therapy was due to aspiration pneumonia in 50% of the patients while aspiration pneumonia represented a factor exhibiting good compliance for the entire study population. We assume that intensivists considered these patients to be at higher risk. A recent study, including 27,455 hospitalised children with neurologic impairment and pneumonia, showed that there were more systemic complications (acute respiratory failure, sepsis or ECMO) in neurologically impaired children with aspiration pneumonia [46].
Early administration of appropriate empiric therapy in patients with sepsis is crucial to reduce mortality [3]. In children with sepsis-associated organ dysfunction and septic shock, the 2020 SSC paediatric guidelines recommended empiric broad-spectrum therapy with one or more antimicrobials to cover all likely pathogens, taking into account local epidemiology, patient characteristics (age, patient history, allergies, MDR status) and suspected BI sites [4]. We found that late-onset VAP was an independent factor for non-compliance regarding the choice of antimicrobials and empiric therapy was inappropriate for all non-compliant episodes. Furthermore, Mangino and colleagues reported a low rate of appropriate empiric therapy (44%) for HAP in adult ICUs despite the implementation of multimodal educational activities to teach ICU staff about the guidelines. The presence of ≥ 1 risk factor for ESBL Enterobacteriaceae was another aetiology for inappropriate empiric therapy in our study. However, local microbiological data from participating ICUs showed a low rate of ESBL pathogens and ESBL Enterobacteriaceae was isolated in only 2.0% of BI episodes while children presented one or more risk factors for ESBL Enterobacteriaceae in 29.5% of episodes. Indications of probabilistic antibiotic therapy covering ESBL Enterobacteriaceae are mainly based on adult recommendations [10, 20] and should perhaps be adapted to paediatrics.
In French Paediatric and Neonatal ICUs, antibiotic prescriptions are issued by intensivists. They have access to a possible audit with an infectious disease specialist over the telephone (at their discretion) on weekdays and sometimes during weekends as well as an ASP (multidisciplinary staff meeting with intensivists, microbiologists and paediatric infectious disease specialists) occurring once a week at most. Previous studies have reported that ASPs decreased inappropriate prescriptions, antibiotic consumption and drug resistance [47, 48]. We could not demonstrate better compliance with the use of an ASP. In stratified analyses, the use of an ASP occurring once a week at most did not modify the effect of independent variables on non-compliance with recommendations. However, a once weekly ASP does not allow for recommendations to be given to all patients on antibiotics or for the daily “correction” of non-compliance concerning the duration of antimicrobials or re-evaluation at 72 h. Moreover, we only identified the presence or absence of non-compliance without quantifying the number of days for each instance of non-compliance on a daily basis (except for duration of antibiotics).
This study has several limitations. Firstly, we arbitrarily chose several separate weeks to include patients and not just one study period. This is explained by the very time-consuming nature of data collection and by the lack of funding for this study. These separate periods enabled each participating centre to organise their data collection. As with point prevalence studies, such data collection can potentially be a source of selection bias and could limit the generalisation of our results to the entire paediatric ICU population. However, consecutive patients were included during the study periods and since these weeks were spread over one year, we were able to take into account seasonal variations specific to paediatrics. Furthermore, at the time of the study, three centres did not have an ASP but all centres had access to audits with an infectious disease specialist over the telephone on weekdays and sometimes during weekends. For centres implementing an ASP, there was a once weekly multidisciplinary staff meeting, with advice given for antibiotic prescription. Finally, local ICU microbiology data from the two years prior to the study period were missing for three participating centres, which may have impacted analysis of compliance regarding the choice of antimicrobials by the paediatric infectious disease expert. However, the low MDR rate for the episodes included and in the local epidemiology of the other centres suggest a minimal influence.
Conclusions
In French Paediatric and Neonatal ICUs, most of which hold a once weekly ASP, half of antibiotic prescriptions remain non-compliant with guidelines. For respiratory illnesses with clear treatment guidelines or for which antibiotic protocols already exist at a given centre, antibiotic use by intensivists tended to be more compliant. This highlights the importance of developing consensus/guidelines about treating specific illnesses and antibiotic protocols based on current guidelines that are updated regularly for use by clinicians. The benefit of using several antimicrobials and broad-spectrum antibiotic therapy should be reassessed daily by intensivists. Based on microbiological results and patient evolution, intensivists must perform an early de-escalation and stop antibiotics that are no longer indicated. A daily ASP could improve compliance with guidelines in these non-compliance prone situations.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- ADE:
-
Antimicrobial De-Escalation
- ASP:
-
Antimicrobial Stewardship Programme
- BI:
-
Bacterial Infection
- CI:
-
Confidence Interval
- ESBL:
-
Extended-Spectrum β-Lactamase producing
- ESCMID:
-
European Society of Clinical Microbiology and Infectious Diseases
- GFRUP:
-
Groupe Francophone de Réanimation et d’Urgences Pédiatriques (French-Speaking Group for Paediatric Intensive and Emergency Care)
- GPIP:
-
Groupe de Pathologies Infectieuses en Pédiatrie (French Group for Paediatric Infectious Diseases)
- HAP:
-
Hospital-Acquired Pneumonia
- HAS:
-
Haute Autorité de Santé (French National Authority for Health)
- ICU:
-
Intensive Care Unit
- IDSA:
-
Infectious Diseases Society of America
- IPSC:
-
International Pediatric Sepsis Consensus Conference
- IQR:
-
Inter-Quartile Range
- MDR:
-
Multidrug-Resistant
- OR:
-
Odds Ratio
- PELOD-2:
-
Pediatric Logistic Organ Dysfunction-2
- pSOFA:
-
Pediatric Sequential Organ Failure Assessment
- SFAR:
-
Société Française d’Anesthésie Réanimation (French Society of Anaesthesia and Intensive Care Medicine)
- SPILF:
-
Société de Pathologie Infectieuse de Langue Française (French Language Society for Infectious Diseases)
- SRLF:
-
Société de Réanimation de Langue Française (French Language Society for Intensive Care Medicine)
- SSC:
-
Surviving Sepsis Campaign
- VAP:
-
Ventilator-Associated Pneumonia
References
Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a Pediatric Sequential Organ failure Assessment score and evaluation of the Sepsis-3 definitions in critically Ill Children. JAMA Pediatr. 2017;171:e172352.
Versporten A, Bielicki J, Drapier N, et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71:1106–17.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21:e52–106.
Wintenberger C, Guery B, Bonnet E, et al. Proposal for shorter antibiotic therapies. Med Mal Infect. 2017;47:92–141.
Gauzit R, Castan B, Bonnet E, et al. Anti-infectious treatment duration: the SPILF and GPIP French guidelines and recommendations. Infect Dis Now. 2021;51:114–39.
Timsit J-F, Baleine J, Bernard L, et al. Expert consensus-based clinical practice guidelines management of intravascular catheters in the intensive care unit. Ann Intensive Care. 2020;10:118.
Cohen R, Haas H, Lorrot M, et al. Antimicrobial treatment of ENT infections. Arch Pediatr. 2017;24:S9–16.
Cohen R, Angoulvant F, Biscardi S, et al. Antibiotic treatment of lower respiratory tract infections. Arch Pediatr. 2017;24:S17–21.
Leone M, Bouadma L, Bouhemad B, et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med. 2018;37:83–98.
Cohen R, Raymond J, Gendrel D. Antimicrobial treatment of diarrhea/acute gastroenteritis in children. Arch Pediatr. 2017;24:S26–9.
Montravers P, Dupont H, Leone M, et al. Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med. 2015;34:117–30.
Cohen R, Raymond J, Launay E, et al. Antimicrobial treatment of urinary tract infections in children. Arch Pediatr. 2017;24:S22–5.
Hoen B, Varon E, Debroucker T, et al. Management of acute community-acquired bacterial meningitis (excluding newborns). Short text. Med Mal Infect. 2019;49:367–98.
Cohen R, Raymond J, Hees L, et al. Bacterial meningitis antibiotic treatment. Arch Pediatr. 2017;24:S42–5.
Gillet Y, Lorrot M, Cohena R, et al. Antibiotic treatment of skin and soft tissue infections. Arch Pediatr. 2017;24:S30–5.
Haute Autorité de santé. [Management of common bacterial skin infections]. J Med Vasc. 2019;44:274–84.
Lorrot M, Gillet Y, Le Gras C, et al. Antibiotic therapy of bone and joint infections in children: proposals of the French Pediatric Infectious Disease Group. Arch Pediatr. 2017;24:S36–41.
Haute Autorité de Santé. Prise en charge du nouveau-né à risque d’infection néonatale bactérienne précoce (≥ 34 SA). 2017. http://www.societe-francaise-neonatalogie.fr/2017/02/27/recommandationsss/.
Haute Autorité de Santé. Antibiothérapie des infections à entérobactéries et à Pseudomonas aeruginosa chez l’adulte: place des carbapénèmes et de leurs alternatives. 2019. https://www.has-sante.fr/upload/docs/application/pdf/2019-06/recommandations_infections_enterobacteries.pdf.
Bretonnière C, Leone M, Milési C, et al. Strategies to reduce curative antibiotic therapy in intensive care units (adult and pediatric). Intensive Care Med. 2015;41:1181–96.
Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious diseases (ESCMID) and Asociación Latinoamericana Del Tórax (ALAT). Eur Respir J. 2017;50. https://doi.org/10.1183/13993003.00582-2017.
van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37–62.
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.
Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect. 2010;11:79–109.
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59:147–59.
Kimberlin D, Brady M, Jackson M et al. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed., 2015.
Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s clinical practice guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;64:e34–65.
Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12:82.
Murray MT, Beauchemin MP, Neu N, et al. Prior antibiotic use and acquisition of multidrug-resistant organisms in hospitalized children: a systematic review. Infect Control Hosp Epidemiol. 2019;40:1107–15.
Fanelli U, Chiné V, Pappalardo M, et al. Improving the quality of Hospital Antibiotic Use: impact on Multidrug-resistant bacterial infections in children. Front Pharmacol. 2020;11:745.
Principi N, Esposito S. Antibiotic-related adverse events in pediatrics: unique characteristics. Expert Opin Drug Saf. 2019;18:795–802.
Bouadma L, Luyt C-E, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–74.
Ory EM, Yow EM. The use and abuse of the broad spectrum antibiotics. JAMA. 1963;185:273–9.
Hsia Y, Lee BR, Versporten A, et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of pediatric survey data from 56 countries. Lancet Glob Health. 2019;7:e861–71.
WHO. World Health Organization. WHO releases the 2019 AWaRe Classification Antibiotics. https://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/.
Karsies T, Tarquinio K, Shein SL, et al. Compliance with an Antibiotic Guideline for Suspected Ventilator-Associated infection: the Ventilator-Associated INfection (VAIN2) study. Pediatr Crit Care Med. 2021;22:859–69.
Blinova E, Lau E, Bitnun A, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med. 2013;14:e280–288.
Bodí M, Rodríguez A, Solé-Violán J, et al. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41:1709–16.
Lindberg O, De Geer L, Chew MS. Nonadherence to antibiotic guidelines in patients admitted to ICU with sepsis is associated with increased mortality: a registry-based, retrospective cohort study. Eur J Anaesthesiol. 2020;37:113–20.
Nachtigall I, Tafelski S, Deja M, et al. Long-term effect of computer-assisted decision support for antibiotic treatment in critically ill patients: a prospective before/after cohort study. BMJ Open. 2014;4:e005370.
Mutters NT, De Angelis G, Restuccia G, et al. Use of evidence-based recommendations in an antibiotic care bundle for the intensive care unit. Int J Antimicrob Agents. 2018;51:65–70.
Tabah A, Bassetti M, Kollef MH, et al. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious diseases (ESCMID) critically Ill patients Study Group (ESGCIP). Intensive Care Med. 2020;46:245–65.
De Bus L, Depuydt P, Steen J, et al. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: the DIANA study. Intensive Care Med. 2020;46:1404–17.
De Waele JJ, Schouten J, Beovic B, et al. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46:236–44.
Thomson J, Hall M, Ambroggio L, et al. Aspiration and Non-aspiration Pneumonia in Hospitalized Children with neurologic impairment. Pediatrics. 2016;137:e20151612.
Donà D, Barbieri E, Daverio M, et al. Implementation and impact of pediatric antimicrobial stewardship programs: a systematic scoping review. Antimicrob Resist Infect Control. 2020;9:3.
Pallares C, Hernández-Gómez C, Appel TM, et al. Impact of antimicrobial stewardship programs on antibiotic consumption and antimicrobial resistance in four Colombian healthcare institutions. BMC Infect Dis. 2022;22:420.
Acknowledgements
We thank all physicians and nurses at the participating centres for their kind contribution to data collection.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RA and CBre designed the study. RA, AC, SB, DD, OB, BR, GJ, CBro, BT, NS, BB, GG, IP, PD, RW, LM and SD contributed to the acquisition of data. CBre and EG analysed compliance with antibiotic recommendations. CM and CF carried out all the statistical analyses. RA, CBre and EG analysed and discussed the results. RA and CBre wrote the paper. All authors critically reviewed the manuscript. All Authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Strict confidentiality was ensured at all times during data collection, storage and analysis. All methods were carried out in accordance with relevant guidelines and regulations. According to French Ethics and Regulatory Law (Public Health Code), studies based on the exploitation of usual care data should not be submitted to an Ethics Committee but must be declared or covered by reference methodology from the French Data Protection Commission (CNIL). Toulouse University Hospital signed a commitment of compliance with reference methodology MR-004 from the CNIL. After evaluation and validation by the data protection officer and according to General Data Protection Regulations, this study met all of the criteria and is registered on the Toulouse University Hospital Study Data Register (register number: RnIPH2019-79) and covered by MR-004 (CNIL number: 2206723 v 0). This study was approved by Toulouse University Hospital and we confirm that the ethics requirements were fully complied with in the above report. For clinical trials covered by reference methodology MR-004, informed written consent is not required. Informed verbal consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amadieu, R., Brehin, C., Chahine, A. et al. Compliance with antibiotic therapy guidelines in french paediatric intensive care units: a multicentre observational study. BMC Infect Dis 24, 582 (2024). https://doi.org/10.1186/s12879-024-09472-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09472-0