Abstract
Background
Bats are renowned for harboring a high viral diversity, their characteristics contribute to emerging infectious diseases. However, environmental and anthropic factors also play a significant role in the emergence of zoonotic viruses. Metagenomic is an important tool for investigating the virome of bats and discovering new viruses.
Results
Twenty-four families of virus were detected in lung samples by sequencing and bioinfomatic analysis, the largest amount of reads was focused on the Retroviridae and contigs assembled to Desmodus rotundus endogenous retrovirus, which was feasible to acquire complete sequences. The reads were also abundant for phages.
Conclusion
This lung virome of D. rotundus contributes valuable information regarding the viral diversity found in bats, which is useful for understanding the drivers of viral cycles and their ecology in this species. The identification and taxonomic categorization of viruses hosted by bats carry epidemiological significance due to the potential for viral adaptation to other animals and humans, which can have severe repercussions for public health. Furthermore, the characterization of endogenized viruses helps to understanding the host genome and the evolution of the species.
Similar content being viewed by others
Introduction
Chiropterans represent the second most diverse mammalian order in the world after rodents [1]. They are renowned for harboring a high viral diversity among mammals, their migratory ability, longevity and unique immunology contribute to emerging infectious diseases. However, environmental and anthropic factors also play a significant role in the emergence of zoonotic viruses [2]. Deforestation, environmental disasters, climatic changes, urbanization and human economic and cultural activities increase the contact with the viral diversity of bats and the occurrence of many outbreaks caused by families such as Paramyxoviridae, Filoviridae, Rhabdoviridae, and Coronaviridae, including the recent caused by SARS-CoV-2 in 2020 [3, 4].
Amazon forest has a greater richness of bat species, Brazil exhibits a substantial portion of the recorded, with a total of 181 species distributed across 68 genera and 9 families, according to the latest list provided by the Brazilian Society for Chiropteran Studies [5], mainly related to Phyllostomidae, Vespertilionidae and Molossidae, they are important to polinization, seed dispertion and insect predation [6, 7]. The state of Pará, has the most records of species in North of Brazil, Desmodus rotundus is commonly used in research due to their role as reservoirs of Lyssavirus rabies in Amazon region [8, 9]. Environmental disturbances have modified the interactions and virome composition of this specie, hematophagous bats have a feeding dynamic that facilitates the transmission of viruses to wild or domestic animals, as well as directly to humans, particularly those residing in communities around rivers and forests. Extractive activities, agriculture and agribusiness in Amazon cause habitat and bat colony destruction, leaving local populations more susceptible to spillover [4].
The decreasing costs of Next-Generation Sequencing (NGS) combined with the need for research on emerging pathogens has resulted in a focused effort to discover viruses in bats. This has led to the identification of entire clades of viruses, with metagenomics providing a broader perspective and allowing for the detection of new microorganisms beyond the main disease-causing agents [10]. In recent years, numerous viruses have been discovered in bats using NGS, with around 30% of bat-related viral sequences in GenBank obtained through metagenomics, primarily consisting of RNA viruses. RNA viruses are prone to mutation, which increases their pathogenicity and severity of the resulting effects. Urine, saliva, and feces samples are commonly used in studies as they have a lower impact on bat populations and represent possible transmission routes. However, relying solely on these samples may lead to the failure to detect viruses associated with specific tissues or environmental interference in the results [11].
Monitoring the virome in animals that are important reservoirs assists in identifying viruses with pathogenic potential. Knowing about viruses hosted by bats in the Amazon region is a crucial measure for virological surveillance and may serve as a preliminary step in detecting potential zoonotic viruses in circulation. Studies that utilize NGS for this purpose have the capacity to provide valuable insights that can help mitigate the impact of future epidemics or pandemics [9, 11].
Currently, studies involving samples from bats inhabiting Amazonian regions have already identified numerous viral families related to different taxa, such as insect, plant, protozoan, vertebrate viruses, and a substantial quantity of bacteriophages, through metagenomic analyses [12, 13]. Families that harbor zoonotic viruses are always highlighted, however, bats also play a role as hosts of retroviruses. Thus, detecting Endogenous Retroviruses (ERVs) can contribute to understanding evolutionary aspects of species, gaining insights into the potential development of cancer, neurological diseases, and autoimmune conditions, as observed in other mammals ERVs [14, 15].
The purpose of this investigation was to detect RNA viruses in the lung samples from brazilian D. rotundus bats. Moreover, to perform a comparative analysis of the identified viruses with those present in genomic sequence databases from humans and/or other animals and taxonomically classify them using phylogenetic analysis.
Results
Sequencing and bioinformatic analyses
We obtained 840,125,868 raw reads sequences, and after the reduction steps, 188,423,522 reads were used for de novo assembly. Considering the relative amount, the pools from Melgaço had the greatest reduction (Fig. 1). The taxonomic classification programs estimated 69,252,530 microbial reads, with the lowest amount being for viruses, with 294,124 (Fig. 2). The alignment resulted in considerable viral diversity at different taxonomic levels when visualized in MEGAN. Reads corresponding to 24 families and other unclassified viruses can be observed in the heat map (Fig. 3). The number of contigs obtained by each assembler and the taxon classification were available in the Tables S1 to S4 (see Additional file 1).
Raw reads counts and counts after software steps. A and B illustrates the amount of reads before and after the Fastp filtration, host genome mapping with Bowtie2 for removal, and removal of ribosomal RNA using SortMeRNA to facilitate the matching of reads with viral sequences. The Melgaço pools showed the lowest quantity of reads
The heat map represents the distribution of reads for viral families. The figure displays the viral families that were detected and reads that could not be classified into families, such as Caudovirales. The quantity of reads is shown using a color gradient ranging from red, indicating the highest quantity, to blue for families with the lowest quantity. Myoviridae, Podoviridae, and Siphoviridae, which are among the eight phage families detected, as well as Mimiviridae that infects protists, had a significant number of reads. The Melgaço 1 sample exhibited the highest read counts for Siphoviridae and Myoviridae, as depicted by the red rectangles in the bottom right corner of the heatmap. On the other hand, RNA viruses were predominantly represented by the Retroviridae family across all samples
Overview of virome
Many reads and contigs matched phages. In total, eight phage families were detected: Herelleviridae, Salasmaviridae, Achermamviridae, Inoviridae, Phycodnaviridae, Siphoviridae, Podoviridae, Myoviridae, and unclassified Caudovirales, all with double-stranded DNA (dsDNA). All samples had the highest number of reads corresponding to Myoviridae, Podoviridae, and Siphoviridae, mainly in Melgaço 1 pool. This sample also showed the highest number of reads for Mimiviridae, which infects protists. Few families were related to infecting plants, fungi, and protozoa, such as Mimiviridae, Partiviridae, Pithoviridae, also with dsDNA genome, and Mitoviridae with single-stranded RNA (ssRNA).
Nine vertebrate viral families were identified, with dsDNA viruses predominating, related to Iridoviridae, Poxviridae, Herpesviridae, Marseilleviridae, and Polydnavirida. It was detected that only two families of single-stranded DNA (ssDNA) viruses exist, Parvoviridae and Anelloviridae. Bornaviridae, Nairoviridae, Paramyxoviridae, Retroviridae, and a group of unclassified Picornavirales were the single-stranded RNA (ssRNA) virus families identified. Some of them are able to infect invertebrates too.
Despite the significant number of reads, the marjority of families had very short contigs, which could represent low presence of these organisms or identity in the alignment. Therefore, we did not consider the species-level attributions reliable for most of them.
Even though the sequencing focused on RNA viruses, numerous families corresponding to DNA genomes were detected. This could be attributed to the imprecision of total RNA extraction kits, which were not entirely reliable or capable of detecting all transcripts. However, RNA viruses were strongly represented by Retroviridae, especially for the Betaretrovirus genus with 7,444 reads and 1,682 contigs and Gammaretrovirus with 22,796 reads and 6,592 contigs. Despite the abundance of Gammaretrovirus, the assemblers generated short contigs with an average length of 150 nt and low similarity to sequences in the database. On the other hand, Betaretrovirus obtained larger contigs with 95-99% similarity.
Viral characterization and phylogenetic relationships of selected virus
During the inspection of the Betaretrovirus contigs, homologous sequences to the Desmodus rotundus endogenous retrovirus (DrERV) were discovered, with identity ranging from 95% to 99%. To obtain complete sequences of DrERV from all pools, the reference genome acquired in a previous study with D.rotundus from different locations in the Amazon (accession number MH648003) was used. De novo mapping was performed against the reads from each sample pool. The study sequences had an average length of 8,586 nt, which falls within the expected range for the Betaretrovirus genome length. The average GC content of the sequences was 49%.
The Gag gene had an average length of 2,186 nt, Pro had 836 nt, Pol had 2,387 nt, and Env had 1,070 nt. The GC content of each coding region ranged from 45.7% to 52.11%. Among the sequences, Gag and Pro had intact Open Reading Frames (ORFs), while Pol had some stop codons and Env was the coding region that exhibited the most divergence between positions 7,463 to 8,279, which was an expected result since the reference sequences also displayed this variability (Fig. 4). Another alignment showing the gaps and diversity regions in the Env gene in more details (Additional file 2).
Alignment of the study sequences with DrERV genome (KP175581) from NCBI database. The reference sequence is represented by yellow bars, indicating the presence of Gag, Pro, Pol, and Env genes. The green bars represent the corresponding genes detected in the bat sequences from this study. Among these genes, the Env gene exhibited the highest amino acid divergence, observed in all sequences. Each sequence is identified by the location of origin and the year of collection
In the phylogenetic inference, 18 DrERV sequences were aligned with various Betaretrovirus strains accessible in the NCBI, with information available in Table S5 [see Additional file 1]. The Gag, Pol, and Env coding regions were used to generate three trees. The 18 sequences formed a monophyletic clade with other bat DrERV sequences, while the Squirrel Monkey Retrovirus (SMRV) sequences positioned themselves in a nearby branch, formed a paraphyletic group with primate Betaretroviruses (Fig. 5).
Phylogenetic inference performed based on the coding regions Gag, Pol, and Env. The trees show sequences of Betaretroviruses from bats and primates that were obtained from NCBI. The numbers at each main node in the tree correspond to bootstrap values in percentage (1000 replicates). The scale bar corresponds to the nucleotide divergence per site between sequences. The sequences from this study formed a monophyletic clade with other bat DrERV sequences from the database, as indicated by the underlined blue color. Meanwhile, the SMRV sequences clustered in a nearby branch, forming a paraphyletic group with non-human primate Betaretroviruses in all trees, highlighted in red, indicating a certain phylogenetic correlation
Discussion
Bats serve as the primary natural host for a diverse range of mammalian viruses and play a crucial role in transmitting numerous emerging or re-emerge virus to humans and other animals in their natural habitats. Traditional virological methods identified many bat-borne viruses worldwide, which took multiple research laboratories and several decades to identify. However, with the introduction of metagenomic analysis utilizing NGS and high-throughput screening, the discovery of new bat viruses has greatly accelerated [2].
While feces, urine, oral swabs, and blood are commonly used for analysis, studies involving tissue samples are not as prevalent. The type of sample can affect the amount of information obtained, with fecal and intestinal samples typically producing more reads [16, 17].
The viruses infecting vertebrates stand out in metagenomic studies. Consistent with our analyses, families of DNA viruses, such as Anellovirida and Parvoviridae, were identified in fecal and saliva samples from D. rotundus in microhabitats of the Amazon region in French Guiana, with abundance varying across sampling locations. RNA viruses were less represented. This variation in the presence of DNA and RNA families is associated with the types of samples and the RNA depletion protocols using commercial kits [12].
A substantial number of reads and contigs in this study were linked to phages of Myoviridae, Siphoviridae, and Podoviridae families. It has been observed in other studies involving D. rotundus, but these DNA families are also frequently reported in non-hematophagous bats such as Molossus, Myotis, and Rhinolophus [12, 18, 19]. The prevalence of these families likely reflects a common state of phage infection in bat bacterial microecosystems across all studied regions and may indicate active replication within the hosts.
Identification of the RNA virome was the main focus, as viruses with simple genome can easily cross species barriers due to their high mutation rates. Thus, the occurrence of emerging viruses from bats demonstrates a high incidence of morbidity and mortality [11]. But the viral characterization could also be affected by rRNA depletion using laboratory kits [16]. Therefore, the removal of host rRNA and other organisms in the laboratory enabled the detection of more RNA taxa and improved sequencing depth for virus detection. This technique would be an option to ensure that more RNA viruses were detected in this study.
The lung is used in isolation in a few studies and is important because it is involved in the exchange of pathogens through the airborne route. Although, analyses of this organ suggesting their potential significance in detecting viruses associated with specific tissues, such in Retroviridae and Nairoviridae families [17, 20]. The present study identified 9 out of 24 viral families as being of vertebrate origin, mainly Retroviridae. This quantity is slightly lower than that reported in other metagenomic studies using bat tissues, it could be attributed to the choice of a single organ for analysis, while other studies have utilized multiple tissues to create the pools [17, 18].
The hematophagous diet of bats facilitates exposure to blood-borne viruses that can lead to an increase in the invasion of endogenous retroviral and non-retroviral elements in their genome. However, the diversity of endogenous retroviral elements is not exclusive to hematophagous animals. Several endogenized Betaretroviruses were detected in Megachiroptera and Microchiroptera, which have an insectivorous and frugivorous feeding habit. During the evolution of species, exogenous retroviruses infect germ cells and leave behind elements known as ERVs. The use of high-throughput sequencing techniques has rapidly increased the number of identified ERVs, with approximately 51 different bat species having been reported to carry ERVs [15]. In this study, Gammaretrovirus reads outnumbered Betaretrovirus, but all assembled contigs had low identity with corresponding sequences, preventing any inference in this genus.
The DrERV was first detected in samples of D. rotundus from Mexico and Germany, with sequences exhibiting intact ORFs for Gag and Pro genes, while Pol and Env exhibited several stop codons that resulted in truncated products [21]. The DrERV sequences from this study also presented stop codons in the Pol and Env genes, a characteristic of endogenous retroviruses, as most are unable to generate infectious particles. Moreover, both exhibited variability among the sequences. The Gag and Pro genes had a similar length to the reference sequence used in the mapping, while Pol and Env were slightly shorter with 2,186 and 1,070 nt, respectively, compared to other described sequence in brazilian vampire bats, with 2,418 and 1,770 nt [22].
Carollia perspicillata, also from the Phyllostomidae family, share a 75% ORF global nucleotide similarity with DrERV. However, such similarity did not occur with other Phyllostomidae, such as Diphylla ecaudata and Artibeus jamaicensis, suggesting that the insertion of DrERV occurred through independent infections after species divergence. Nonetheless, further investigations with a larger sample size are needed to represent the diversity of species found in this group. The DrERV sequences showed some similarity with the neotropical primate SMRV. In the first evidence of DrERV, the reads were mapped against the SMRV genome, and then phylogenetic inference was performed for the complete genome assembly [21]. At this study, the Gag, Pol and Env trees also formed a cluster with SMRV.
An analysis of thousands of ERVs in mammalian genomes demonstrates that bats play an important role as hosts of retroviruses and are involved in frequent events of cross-species transmission. Therefore, the presence of ERVs can aid in understanding evolutionary issues of the species that carry them. The discovery of endogenous or exogenous retroviruses in bats also raises the possibility of the existence of infectious retroviruses for humans hosted by these animals that are yet to be discovered [15].
The identification of Adenovirus, Parvovirus and Paramyxovirus is commonly described in studies with bats, some with brazilian bats [23,24,25]. In our analysis, even the presence of reads belonging to these families, along with the small contigs, did not aid in further analysis of the possible sequences carried by the D. rotundus in this areas.
Publications depicting viral diversity in Vespertilionidae, Rhinolophidae, and Pteropodidae have brought public health benefits by discovering the source of outbreaks. Bats from various places in Asia have shown bat-CoVs phylogenetically related to SARS-CoV-2, the causative agent of COVID-19 [11, 26]. Phyllostomid bats have interesting virome data, studies are focused on D. rotundus, as they are the main wildlife reservoirs of Lyssavirus rabies in the Americas. In the latest update of "The Database of Bat-associated Viruses”, 281 viral sequences are related to D. rotundus. Moreover, in the region search, 767 sequences are from different bat species in Brazil, a number much higher than in other South American countries [27].
The samples were collected from municipalities that can only be accessed by river transportation and are among the areas with the lowest Human Development Index (HDI) in Brazil, according to the United Nations Development Program. Melgaço has the lowest HDI, while others are associated with inappropriate management of solid waste, which causes damage to the environment and public health. This factor contributes to hypotheses about the virome characteristics harbored by circulating bats in the region since the contamination and transmission of viruses and other associated microorganisms may be favored in this scenario, as we can see from the readings referring to phages in this work [28, 29].
The traditional economy based on nature-extracted products necessitates entering forested areas, which puts the population at risk of attacks by hematophagous bats. Additionally, due to the low HDI and limited access to sanitation and quality housing despite the expansion of municipalities for extractive activities, locals are at an increased risk of contracting zoonotic diseases from wildlife. Recent studies have detected viruses belonging to the Alphacoronavirus, Orthoparamyxovirus, Dependoparvovirus, Mastadenovirus, and Betaretrovirus in Brazilian D. rotundus. However, it is crucial to emphasize that the majority of studies are conducted using samples from the state of São Paulo, and even though comprising half of the researches conducted in the country, they do not provide a comprehensive characterization of the virome carried by Brazilian bats [9, 23,24,25, 30, 31].
Despite the absence of families known to cause infections in mammals in this study, the identification of reads associated with other viral families is crucial for virological surveillance. Surveillance focused on wild animals in regions with environmental imbalances is necessary to comprehend the risk of contact between hosts of zoonotic viruses, domestic animals and humans [32]. This metagenomic study may initiate the understanding of the viral diversity carried by D. rotundus in the northern region of Brazil.
Conclusion
These data are important for comparative analyses of bat virome obtained between lung and other types of samples. It was possible to correlate the presence of a common endogenous retrovirus in all tested samples, which may serve as a basis to aid in understanding the host genome. Phylogenetic analyses record that Betaretroviruses, such as DrERV, are present in neotropical bats and are a source of understanding the evolution of bats and other mammals, as well as may be related to other intrinsic factors of the species that still need to be studied.
Methods
Sites and sample collection
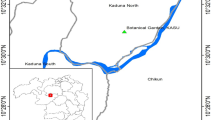
Lung samples of 78 D. rotundus were collected between 2018-2021 and send to Evandro Chagas Institute (IEC) for routine surveillance and detection of Lyssavirus rabies. All these samples were negative for this virus and were grouped in 18 pools taking a fragment of lung organized by year and collection locate. Bats were captured in Melgaço, Afuá, Anajás, Breves, Bagre, Gurupá, and Soure, which are located in Marajó, the largest fluvial-maritime island in the world, situated in northern Brazil (Fig. 6). The utilization of bats was approved by the Animal Use Ethics Committee of IEC, certificate number 06/2022, and adhered to the prescribed research protocols in accordance with the ARRIVE guidelines [33].
Localization and organization of the pools. A The figure displays the map of Brazil, and the red area is a part of Pará state. B The right-hand side illustrates an approximate map of the Marajó Island region, with colored areas indicating the municipalities where bats were collected for analysis. Although the Marajó Island region encompasses a total of 16 municipalities, only 7 were sampled for this study. C The pie chart illustrates the number of pools, each containing fragments from 3 to 5 bat lungs
RNA preparation, cDNA synthesis and sequencing
Total RNA was extracted using TRIzol™ Plus RNA Purification Kit following owing manufacturer’s recommendations. RNA concentration was measured using Qubit® RNA HS Assay Kit in Qubit® 2.0 Flurometer (Invitrogen) and RNA integrity was assessed using the RNA Agilent RNA 6000 Pico Kit of the Bioanalyzer 2100 system (Agilent Technologies). For the synthesis of complementary DNA (cDNA), the SuperScript VILO MasterMix (Invitrogen) was used for the first strand, while the NEBNext mRNA Second Strand Synthesis Module (New England Biolabs) was used for the second strand. Then, cDNA was purified using AMPure beads. Sequencing libraries were generated using Nextera XT DNA Library Preparation Kit for Illumina®, following manufacturer’s recommendations. The library quantification was performed with the Qubit® dsDNA HS Assay kit, and the fragment sizes were analyzed using the High Sensitivity DNA Analysis Kit on the Bioanalyzer 2100 (Agilent Technologies). Following this, sequencing was performed on an Illumina® NextSeq 500 platform using the NextSeq 500/550 High Output v2.5 (300 cycles) kit and paired-end reads were generated.
Bioinformatic analysis
The quality of the raw reads was evaluated using Fastp [34], which filtered out short reads of low quality and undetermined bases with a threshold of 20 and a length criterion of 60%. The reads were then mapped against the host genome (SM294091V2 Feb. 2018) and removed using Bowtie2 [35]. Ribosomal RNA was removed using SortMeRNA v.2.1 [36], and the quality of the resulting reads was re-evaluated using Fastp. The reads were aligned using DIAMOND [37] to compare with the viral non-redundant (NR) sequence database from the National Center for Biotechnology Information (NCBI) [38] and the taxonomic classification were evaluated using MEGAN v.6.23.2 [39]. The Kraken program was also used for taxonomic classification and Pavian was utilized for interactive data table, heatmaps and flow diagrams [40, 41]. De novo assembly was performed using SPAdes and MEGAHIT with k-mers size of 21, 33, 55, 77; and 21, 31, 41, 51, 61, 71, 81, 91 and 99 [42, 43]. The Quast program was used to generate the metrics of assemblers. The obtained sequences were realigned using DIAMOND and the contigs were inspected in MEGAN using the lowest common ancestor (LCA) assignment parameter with a maximum value of \(10^{-10}\) to verify their classification. The resulting alignment was imported into GeneMarkS for gene prediction and InterProScan was used to determine the functional domains of the proteins [44, 45].
Phylogenetic analysis
Reference genomes or nucleotide sequences of previously identified viruses from Betaretrovirus genus were downloaded from GenBank [38]. Alignments of nucleotide sequences with the study sequences were subjected to Mafft v.7 and manually inspected for corrections using the Geneious v.9.1.8 [46]. The aligned dataset was analyzed to identify the phylogenetic signal and determine the best nucleotide substitution model. Phylogenetic trees were constructed using Maximum Likelihood (ML) with IQ-TREE v.1.6.12 [47, 48]. Additionally, the bootstrap test was conducted with 1000 replicates and visualization was performed using FigTree v.1.4.4, employing the midpoint rooting methodology [49].
Availability of data and materials
The raw data of Brazilian bats have been submitted to the National Center for Biotechnology Information under BioProject PRJNA985242, BioSamples SAMN35848906 to SAMN35848923, SRA SRR25239926 to SRR25239943, and all sequences under accession numbers OR344913 to OR344930.
References
Burgin CJ, Colella JP, Kahn PL, Upham NS. How many species of mammals are there? J Appl Phys. 2018;99(1):1–14. https://doi.org/10.1093/jmammal/gyx147.
Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. Bat-borne virus diversity, spillover and emergence. Nat Rev Microbiol. 2020;18(8):461–71. https://doi.org/10.1038/s41579-020-0394-z.
Nunes H, Rocha FL, Cordeiro-Estrela P. Bats in urban areas of Brazil: roosts, food resources and parasites in disturbed environments. Urban Ecosystems. 2016;20(4):953–69. https://doi.org/10.1007/s11252-016-0632-3.
Ellwanger JH, Kulmann-Leal B, Kaminsk VL, Valverde-Villegas JM, Veiga ABGD, Spilki FR, et al. Beyond diversity loss and climate change: impacts of Amazon deforestation on infectious diseases and public health. An Acad Bras Ciências. 2020;92(1). https://doi.org/10.1590/0001-3765202020191375.
Garbino GST, Gregorin R, Lima IP, Loureiro L, Moras L, Moratelli R, et al. Updated checklist of Brazilian bats (SBEQ): versão 2020. 2022. https://www.sbeq.net/lista-de-especies. Accessed 1 Dec 2023
Bernard E, da Cunha Tavares V, Sampaio E. Compilação atualizada das espécies de morcegos (Chiroptera) para a Amazônia Brasileira. Biota Neotropica. 2011;11(1):35–46. https://doi.org/10.1590/s1676-06032011000100003.
Delgado-Jaramillo M, Aguiar LMS, Machado RB, Bernard E. Assessing the distribution of a species-rich group in a continental-sized megadiverse country: Bats in Brazil. Divers Distrib. 2020;26(5):632–43. https://doi.org/10.1111/ddi.13043.
Sodré MM, da Gama AR, de Almeida MF. Updated list of bat species positive for rabies in Brazil. Rev Inst Med Trop São Paulo. 2010;52(2):75–81. https://doi.org/10.1590/s0036-46652010000200003.
Wallau GL, Barbier E, Tomazatos A, Schmidt-Chanasit J, Bernard E. The Virome of Bats Inhabiting Brazilian Biomes: Knowledge Gaps and Biases towards Zoonotic Viruses. Microbiol Spectr. 2023;11(1). https://doi.org/10.1128/spectrum.04077-22.
Harvey E, Holmes EC. Diversity and evolution of the animal virome. Nat Rev Microbiol. 2022;20(6):321–34. https://doi.org/10.1038/s41579-021-00665-x.
Brussel KV, Holmes EC. Zoonotic disease and virome diversity in bats. Curr Opin Virol. 2022;52:192–202. https://doi.org/10.1016/j.coviro.2021.12.008.
Salmier A, Tirera S, de Thoisy B, Franc A, Darcissac E, Donato D, et al. Virome analysis of two sympatric bat species (Desmodus rotundus and Molossus molossus) in French Guiana. PLoS ONE. 2017;12(11):0186943. https://doi.org/10.1371/journal.pone.0186943.
Finoketti F, dos Santos RN, Campos AAS, Zani ALdS, Barboza CM, Fernandes MES, et al. Detection of adenovirus, papillomavirus and parvovirus in Brazilian bats of the species Artibeus lituratus and Sturnira lilium. Arch Virol. 2019;164(4):1015–1025. https://doi.org/10.1007/s00705-018-04129-1.
Mager DL, Stoye JP. Mammalian Endogenous Retroviruses. Microbiol Spectr. 2015;3(1). https://doi.org/10.1128/microbiolspec.mdna3-0009-2014.
Hayward JA, Tachedjian G. Retroviruses of Bats: a Threat Waiting in the Wings? mBio. 2021;12(5). https://doi.org/10.1128/mbio.01941-21.
Bergner LM, Orton RJ, da Silva Filipe A, Shaw AE, Becker DJ, Tello C, et al. Using noninvasive metagenomics to characterize viral communities from wildlife. Mol Ecol Resour. 2018;19(1):128–43. https://doi.org/10.1111/1755-0998.12946.
Hardmeier I, Aeberhard N, Qi W, Schoenbaechler K, Kraettli H, Hatt JM, et al. Metagenomic analysis of fecal and tissue samples from 18 endemic bat species in Switzerland revealed a diverse virus composition including potentially zoonotic viruses. PLoS ONE. 2021;16(6):0252534. https://doi.org/10.1371/journal.pone.0252534.
He B, Li Z, Yang F, Zheng J, Feng Y, Guo H, et al. Virome Profiling of Bats from Myanmar by Metagenomic Analysis of Tissue Samples Reveals More Novel Mammalian Viruses. PLoS ONE. 2013;8(4):61950. https://doi.org/10.1371/journal.pone.0061950.
Armero A, Li R, Bienes KM, Chen X, Li J, Xu S, et al. Myotis fimbriatus Virome, a Window to Virus Diversity and Evolution in the Genus Myotis. Viruses. 2022;14(9):1899. https://doi.org/10.3390/v14091899.
Baker KS, Leggett RM, Bexfield NH, Alston M, Daly G, Todd S, et al. Metagenomic study of the viruses of African straw-coloured fruit bats: Detection of a chiropteran poxvirus and isolation of a novel adenovirus. Virology. 2013;441(2):95–106. https://doi.org/10.1016/j.virol.2013.03.014.
Escalera-Zamudio M, Mendoza MLZ, Heeger F, Loza-Rubio E, Rojas-Anaya E, Méndez-Ojeda ML, et al. A Novel Endogenous Betaretrovirus in the Common Vampire Bat (Desmodus rotundus) Suggests Multiple Independent Infection and Cross-Species Transmission Events. J Virol. 2015;89(9):5180–4. https://doi.org/10.1128/jvi.03452-14.
Filho LCF, Barata RR, Cardoso JF, de Vasconcelos Massafra JM, da Silva Lemos P, Casseb LMN, et al. Complete Endogenous Retrovirus Genome Sequence from a Brazilian Vampire Bat (Desmodus rotundus). Microbiol Resour Announc. 2019;8(2). https://doi.org/10.1128/mra.01497-18.
de Sales Lima FE, Cibulski SP, Elesbao F, Junior PC, de Carvalho Ruthner Batista HB, Roehe PM, et al. First detection of adenovirus in the vampire bat (Desmodus rotundus) in Brazil. Virus Genes. 2013;47(2):378–381. https://doi.org/10.1007/s11262-013-0947-6.
de Souza W, Dennis T, Fumagalli M, Araujo J, Sabino-Santos G, Maia F, et al. Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity. Viruses. 2018;10(4):143. https://doi.org/10.3390/v10040143.
de Souza WM, Fumagalli MJ, Carrera JP, de Araujo J, Cardoso JF, de Carvalho C, et al. Paramyxoviruses from neotropical bats suggest a novel genus and nephrotropism. Infect Genet Evol. 2021;95: 105041. https://doi.org/10.1016/j.meegid.2021.105041.
Zhou H, Ji J, Chen X, Bi Y, Li J, Wang Q, et al. Identification of novel bat coronaviruses sheds light on the evolutionary origins of SARS-CoV-2 and related viruses. Cell. 2021;184(17):4380–439114. https://doi.org/10.1016/j.cell.2021.06.008.
Chen L, Liu B, Yang J, Jin Q. DBatVir: the database of bat-associated viruses. Database. 2014;2014. https://doi.org/10.1093/database/bau021.
Pinto D G CMA, A MML. The Brazilian Municipal Human Development Index. Brasίlia: Instituto de Pesquisa Econômica Aplicada (Ipea); 2013.
Crispim DL, Rodrigues RSS, de Abreu Vieira AS, de Oliveira Silveira RNP, Fernandes LL. Espacialização da cobertura do serviço de saneamento básico e do índice de desenvolvimento humano dos municípios do Marajó, Pará. Rev Verde Agroecologia Desenvolvimento Sustentável. 2016;11(4):112. https://doi.org/10.18378/rvads.v11i4.4507.
Asano KM, Hora AS, Scheffer KC, Fahl WO, Iamamoto K, Mori E, et al. Alphacoronavirus in urban Molossidae and Phyllostomidae bats, Brazil. Virol J. 2016;13(1). https://doi.org/10.1186/s12985-016-0569-4.
Filho LCF, Barata RR, Cardoso JF, de Vasconcelos Massafra JM, da Silva Lemos P, Casseb LMN, et al. Metagenomic Analysis of Samples from Three Bat Species Collected in the Amazon Rain Forest. Microbiol Resour Announc. 2019;8(2). https://doi.org/10.1128/mra.01422-18.
Levinson J, Bogich TL, Olival KJ, Epstein JH, Johnson CK, Karesh W, et al. Targeting Surveillance for Zoonotic Virus Discovery. Emerg Infect Dis. 2013;19(5):743–7. https://doi.org/10.3201/eid1905.121042.
du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):3000410. https://doi.org/10.1371/journal.pbio.3000410.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):884–90. https://doi.org/10.1093/bioinformatics/bty560.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. https://doi.org/10.1038/nmeth.1923.
Kopylova E, Noé L, Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics. 2012;28(24):3211–7. https://doi.org/10.1093/bioinformatics/bts611.
Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2014;12(1):59–60. https://doi.org/10.1038/nmeth.3176.
NCBI. National Center for Biotechnology Information. 2023. https://www.ncbi.nlm.nih.gov/nucleotide/. Accessed 14 Jun 2022.
Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17(3):377–86. https://doi.org/10.1101/gr.5969107.
Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3). https://doi.org/10.1186/gb-2014-15-3-r46.
Breitwieser FP, Salzberg SL. Pavian: interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics. 2019;36(4):1303–4. https://doi.org/10.1093/bioinformatics/btz715.
Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27(5):824–34. https://doi.org/10.1101/gr.213959.116.
Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31(10):1674–6. https://doi.org/10.1093/bioinformatics/btv033.
Besemer J, Borodovsky M. GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res. 2005;33(Web Server):451–4. https://doi.org/10.1093/nar/gki487.
Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–40. https://doi.org/10.1093/bioinformatics/btu031.
Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 2013;30(4):772–80. https://doi.org/10.1093/molbev/mst010.
Myung IJ. Tutorial on maximum likelihood estimation. J Math Psychol. 2003;47(1):90–100. https://doi.org/10.1016/s0022-2496(02)00028-7.
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol Biol Evol. 2014;32(1):268–74. https://doi.org/10.1093/molbev/msu300.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–91. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x.
Acknowledgements
This work was supported by Biological Sciences Institute (ICB) of the Federal University of Pará (UFPA) and the Evandro Chagas Institute, Health and Environment Surveillance Secretariat, Ministry of Health, Brazil.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) Grant number “314522/2021-02” to ACRC. The manuscript payment was funded by PROPESP/UFPA(PAPQ).
Author information
Authors and Affiliations
Contributions
The project was conceived by N.K.A., S.P.S., and A.C.R.C. The responsibility of receiving and storing the samples was assigned to T.F.S.B.C., F.A.S.P., and T.C.A.S.C. The molecular biology and bioinformatics aspects were carried out by S.P.S., C.F.A and N.K.A. The manuscript was written by N.K.A., S.P.S. and A.C.R.C. providing manuscript review. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The project was submitted to the Animal Use Ethics Committee under certificate number 06/2022, register 01/2022 and follows the ethical principles for animal experimentation and adhered to the prescribed research protocols in accordance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Albuquerque, N.K., Silva, S.P., Aragão, C.F. et al. Virome analysis of Desmodus rotundus tissue samples from the Amazon region. BMC Genomics 25, 34 (2024). https://doi.org/10.1186/s12864-023-09950-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09950-w