Abstract
Background
The emergence and wide spread of carbapenemase-producing Enterobacteriaceae (CPE) poses a growing threat to global public health. However, clinically derived carbapenemase-producing Citrobacter causing multiple infections has rarely been investigated. Here we first report the isolation and comparative genomics of two blaNDM-5 carrying Citrobacter freundii (C. freundii) isolates from a patient with bloodstream and urinary tract infections.
Results
Antimicrobial susceptibility testing showed that both blaNDM-5 carrying C. freundii isolates were multidrug-resistant. Positive modified carbapenem inactivation method (mCIM) and EDTA-carbapenem inactivation method (eCIM) results suggested metallo-carbapenemase production. PCR and sequencing confirmed that both metallo-carbapenemase producers were blaNDM-5 positive. Genotyping and comparative genomics analyses revealed that both isolates exhibited a high level of genetic similarity. Plasmid analysis confirmed that the blaNDM-5 resistance gene is located on IncX3 plasmid with a length of 46,161 bp, and could successfully be transferred to the recipient Escherichia coli EC600 strain. A conserved structure sequence (ISAba125-IS5-blaNDM-5-trpF-IS26-umuD-ISKox3) was found in the upstream and downstream of the blaNDM-5 gene.
Conclusions
The data presented in this study showed that the conjugative blaNDM-5 plasmid possesses a certain ability to horizontal transfer. The dissemination of NDM-5-producing C. freundii isolates should be of close concern in future clinical surveillance. To our knowledge, this is the first study to characterize C. freundii strains carrying the blaNDM-5 gene from one single patient with multiple infections.
Similar content being viewed by others
Background
The emergence and wide spread of carbapenem-resistant Enterobacteriaceae (CRE) has become a major threat to global public health [1]. Resistant mechanism of CRE to carbapenems is mainly due to the production of carbapenemases, which are enzymes able to recognize almost all hydrolyzable β-lactams, including carbapenems [2]. New Delhi metallo-β-lactamase (NDM) is one of the main types of carbapenemases, and are resilient against inhibition by commercially available β-lactamase inhibitors, including avibactam, clavulanate, sulbactam, and tazobactam [3]. Since the first detection of blaNDM-1, 48 variants of NDM enzymes (NDM-1 to NDM-16a, 16b, and NMD-17 to NDM-48, except NDM-32) have been identified worldwide (https://www.ncbi.nlm.nih.gov/pathogens/refgene/#NDM, accessed 29 December 2022). Among them, NDM-5 has raised extensive concerns for increased resistance to carbapenems and expanded-spectrum cephalosporins, since first determined in a multidrug-resistant Escherichia coli ST648 isolate in the United Kingdom in 2011 [4].
Citrobacter freundii, belonging to the genus Citrobacter of the family Enterobacteriaceae, is rarely the causative pathogen of infections but can cause a wide spectrum of opportunistic nosocomial infections including respiratory tract, urinary tract, and bloodstream [5]. Additionally, it can lead to neonatal meningitis and brain abscesses, which are associated with high mortality rates [6]. The emergence of multidrug resistant C. freundii strains, particularly those that produce carbapenemase enzymes, has posed challenges for infection treatment and has become an increasing global public health concern. This is especially true for immunocompromised patients, who heavily rely on antibiotics [7].
In this study, we presented the results of complete-genome sequencing and comparative genomic characterization of two NDM-5 producing C. freundii strains isolated from a patient with concurrent bloodstream and urinary tract infections.
Results
Antimicrobial resistance profiles of both C. freundii isolates
Antimicrobial susceptibility testing of both C. freundii isolates revealed high MIC values for different drugs and different susceptibility-resistance levels depending on the drug tested. However, both isolates exhibited similar phenotypic antibiotic susceptibility, demonstrating a multi-drug resistant (MDR) characteristic. Both C. freundii isolates exhibited resistance to amoxicillin/clavulanic acid, piperacillin/tazobactam, ceftazidime, ceftriaxone, cefepime, cefotaxime, ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole, and gentamicin as shown in Table 1. In contrast, isolates remained susceptible to amikacin, aztreonam, fosfomycin and tigecycline (Table 1). Intermediate resistance was exhibited when isolates were cultured in the presence of imipenem, meropenem and polymixin B (Table 1).
Phenotype and genotype detection revealed the mechanism of carbapenems non-susceptibility
Positive modified carbapenem inactivation method (mCIM) and EDTA-carbapenem inactivation method (eCIM) results suggested metallo-carbapenemase production of both C. freundii isolates. Then, PCR and sequencing were used to study the molecular determinant, and the results demonstrated that both metallo-carbapenemase producers were blaNDM-5 positive, thus confirming their intermediate resistance phenotype towards imipenem and meropenem. Furthermore, in silico analysis showed that both blaNDM-5 carrying isolates carried additional resistance genes conferring resistance to β-lactams (blaTEM-1B, blaOXA-1, blaCMY-48, blaDHA-1), this genetic profile correlated with their phenotypic resistance to ceftazidime, ceftriaxone, cefepime, and cefotaxime. Moreover, they carried resistance genes for aminoglycosides (aac(6')-Ib-cr, aac(3)-IId, aadA1), which correlated with phenotypic resistance to gentamicin. The presence of the qnrB4 gene indicated resistance to quinolones, which was in line with their resistance to ciprofloxacin and levofloxacin. Furthermore, the isolates carried resistance genes for trimethoprim/sulfamethoxazole (dfrA1, sul1, sul2), which were associated with their resistance to trimethoprim/sulfamethoxazole. The presence of amphenicol resistance genes (catA2, catB3), a tetracycline resistance gene tet(D), and a macrolides resistance gene mph(A) correlated with their resistance to ciprofloxacin, tetracycline, and azithromycin, respectively. Although the presence of the rifamycin resistance gene ARR-3 contributed to both isolates having MIC values exceeding 128 mg/L for rifamycin, the absence of a defined breakpoint prevented the determination of the resistance phenotype with certainty.
High degree of genetic similarity of both NDM-5-producing C. freundii isolates
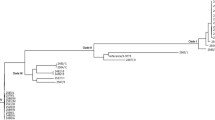
An average nucleotide identity blast (ANIb) analysis, which measures the nucleotide-level genomic similarity between the coding regions of two genomes, was performed. The analysis revealed that the two isolates exhibited a remarkable similarity of over 99% (Supplementary Fig. 1). Additionally, when core single nucleotide polymorphisms (SNPs) were calculated, only five base differences were detected between the two isolates. MLST typing revealed that both NDM-5-producing C. freundii isolates belonged to the sequence typing ST 22. Single-nucleotide polymorphism (SNP)-based phylogenetic tree for both isolates and other 78 NDM-producing strains indicated that DY2007 and DY2010 showed high degree of similarity, and were closely related to various strains from different countries, including Myanmar, USA, France, Australia, Malaysia, Germany, China, and Singapore (Fig. 1, Table S1).
Characterization of both bla NDM-5 carrying plasmids
S1-PFGE and Southern blotting revealed that both C. freundii isolates contained a ~ 50 kb plasmid harbouring the blaNDM-5 gene (Fig. 2). Plasmid replicons analysis indicated that both pNDM-5 were IncX3 type with a length of 46,161 bp. The results of the conjugation assay showed the blaNDM-5 gene of both isolates could successfully be transferred to the recipient Escherichia coli EC600 strain. The results were further confirmed by PCR using blaNDM-5 specific primers and sequencing.
blaNDM-5 gene location analysis. A S1-PFGE of both blaNDM-5 carrying C. freundii isolates DY2007 and DY2010. Salmonella enterica serotype H9812 was used as molecular marker. B Corresponding Southern blotting analysis using blaNDM-5-specific probe. A and B was cropped from different gels. Full-length blots/gels are presented in Supplementary Fig. 2
Whole-genome sequencing analysis of both bla NDM-5 carrying C. freundii isolates
The genomic characteristics of both blaNDM-5 carrying C. freundii isolates were shown in Table S2. DY2007 contained a circular chromosome and two plasmids with a genome size of 5,253,532 bp, while DY2010 consisted of a circular chromosome and three plasmids with a genome size of 5,260,876 bp. The average GC content of both genomes was 51.6%. The complete sequence of blaNDM-5 carrying pNDM-5 was covered using combinatorial PCR and standard Sanger sequencing to accomplish sequence integrality of contigs. It was found that both isolates harbored an identical pNDM-5 plasmid of 46,161 bp length, with a GC content of 46.65% and 65 predicted coding sequences (Fig. 3A). The plasmid carried multiple coding genes, including IncX plasmid conjugal transfer associated genes, antibiotic resistance genes, stability related genes, functional protein coding genes, mobile element associated genes, and other hypothetical genes. A search of the nr/nt database revealed a 100% identity to Escherichia coli strain WCHEC020031 plasmid pNDM5_020031 (GenBank accession number: CP033399.1) at 100% coverage, 100% identity to Klebsiella pneumoniae strain 19110124 plasmid p19110124-3 (GenBank accession number: CP064177.1) at 100% coverage, and 99.98% identity to Escherichia coli strain L53 plasmid pL53-4 (GenBank accession number: CP034737.1) at 99% coverage. Furthermore, a conserved structure sequence (ISAba125-IS5-blaNDM-5-trpF-IS26-umuD-ISKox3) was found in the upstream and downstream of blaNDM-5 (Fig. 3B).
Genomic analyses of pNDM-5 plasmid. A Comparison of the pNDM-5 plasmid sequence identified in isolates DY2007 and DY2010 with Escherichia coli strain WCHEC020031 plasmid pNDM5_020031 (GenBank accession number: CP033399.1), Klebsiella pneumoniae strain 19,110,124 plasmid p19110124-3 (GenBank accession number: CP064177.1), and Escherichia coli strain L53 plasmid pL53-4 (GenBank accession number: CP034737.1). The figure was plotted using BRIG, and the blaNDM-5 gene was highlighted in red. B Genetic environment of blaNDM-5 on pNDM-5 and related plasmids. Open reading frames were indicated as arrows. Shared areas with highly similar sequences were drawn by lake green. Conjugal transfer associated genes were shown by brown; blaNDM-5 gene were indicated by red arrows; functional protein coding genes were colored by green; other antibiotic resistance genes were colored by blue
Discussion
Pathogenic carbapenemase-producing Citrobacter freundii has been individually isolated from bloodstream, feces, open pus, and urine samples [6,7,8,9]. Carbapenem-resistant genes frequently found in C. freundii were blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP [5,6,7, 10]. In this study, we reported the complete-genome sequencing and comparative genomic characterization of two blaNDM-5 carrying C. freundii isolates from an inpatient with concurrent bloodstream and urinary tract infection. To the best of our knowledge, this is the first study to characterize C. freundii strains carrying the blaNDM-5 gene from one single patient with multiple infections.
Antibiotic susceptibility testing indicated the multidrug-resistant characteristic of both isolates, with resistant to classical β-lactams, aminoglycosides, macrolides, quinolones, and sulfonamides, and intermediate to polypeptide antibiotics and carbapenems, the resistant phenotype of which was consistent with resistant genotype (Table 1).
Phylogenetic analysis determined the high homology of both ST 22 isolates, which further highlighted the potential threat of systemic infections caused by this multidrug-resistant (MDR) strain. The multidrug-resistant blaNDM-5 carrying C. freundii isolates have been reported previously from South Korea and Nigeria [8, 9].
The New Delhi metallo-beta-lactamase NDM-5 was first reported in a multidrug-resistant Escherichia coli ST648 isolate, recovered from a patient in the United Kingdom [4]. Substitutions at positions 88 (Val → Leu) and 154 (Met → Leu) distinguished NDM-5 from NDM-1 and enhanced its hydrolytic activity toward carbapenems [11]. Since then, dissemination of NDM-5-producing Enterobacteriaceae has been reported worldwide in medical units and environment [12, 13]. Except for C. freundii, the emergence of the blaNDM-5 gene had been reported in Escherichia coli, Klebsiella pneumoniae, Klebsiella aerogenes, Klebsiella oxytoca, Proteus mirabilis, and Morganella morganii [12, 14,15,16,17,18].
Compared to vertical transmission, horizontal transfer seems to be more threatening for antimicrobial resistance. In this study, two isolates both successfully transferred blaNDM-5 and carbapenem non-susceptible phenotype to the recipient strain E. coli EC600, which confirmed the horizontal gene transfer characteristic of NDM-5. In recent years, IncX3 plasmids harboring blaNDM variants have increasingly been characterized worldwide. It has also been proved that IncX3 plasmids plays an important role in the dissemination of blaNDM-5 gene in Enterobacteriaceae [12]. Tian et al. observed that horizontal gene transfer (HGT) of blaNDM-5 among distinct Enterobacteriaceae species was mostly mediated by IncX3 plasmids [16]. A previous study found that blaNDM-5 might spread among humans and the environment via IncX3 plasmids in an intensive vegetable farming area in eastern China [19]. Consistently, blaNDM-5 was located on an IncX3 plasmid (~ 50 kb) in this study. However, blaNDM-5 was additionally detected on IncF and IncI1 plasmids [20, 21], highlighting the significant compatibility and transmission hazard associated with blaNDM-5.
In order to further understand the evolutionary relationship of blaNDM-5 harboring C. freundii isolates, we downloaded the nucleotide sequences of 78 NDM-producing strains from NCBI and analyzed their homology with both isolates in this study. The results revealed that 17 strains from around the world were closely related to both isolates, suggesting parallel evolution of these isolates. In plasmids, genes are typically associated with mobile genetic elements such as transposons (Tn) and insertion sequences (IS). According to the whole-genome sequencing analysis of the pNDM-5 plasmids, the comparison of genetic context flanking blaNDM-5 in both isolates was mostly identical to published plasmids, i.e., ISAba125-IS5-blaNDM-5-trpF-IS26-umuD-ISKox3. The blaNDM-5 genetic structure is widespread in Enterobacteriaceae for blaNDM horizontal transfer and has been reported in blaNDM-5 and blaNDM-9 transmission [22]. Characterization of the blaNDM-5 genetic contents revealed that it was flanked by multi-insertional sequences. Of these, ISAba125 was conservative in blaNDM-5-positive isolates. It is consistent with the discovery that ISAba125 (intact or truncated) upstream of blaNDM is common in blaNDM genetic settings [23], indicating its’ important role in blaNDM transmission. NDM, a highly prevalent plasmid-borne metallo-β-lactamase, has been identified in various species of Enterobacteriaceae worldwide. Its frequent co-occurrence with ISAba125 suggests a potential origin from Acinetobacter spp., a bacterium where this association is commonly observed [24]. Tn125, a composite transposon based on ISAba125, has been reported as one of the genetic elements implicated in the dissemination of blaNDM. However, in Enterobacteriaceae, Tn125 exhibits interruptions or truncations, leading to diverse genetic contexts for blaNDM [3].
Whole-genome sequencing (WGS) plays a pivotal role in clinical cases involving systemic infections. It offers a comprehensive perspective on the complete genome of the pathogen, allowing for meticulous analysis of genetic variations, resistance mechanisms, and the identification of potential virulence factors. WGS enhances our understanding of the pathogenesis of systemic infections, aids in tracing transmission routes, and assists in formulating suitable treatment strategies [25,26,27]. This study bears significance in the ongoing efforts to incorporate WGS into routine clinical diagnostic pipelines.
Conclusions
In this study, we sequenced and characterized the comparative genomes of two blaNDM-5 carrying C. freundii isolates from an inpatient with multiple infections, and found different antimicrobial resistant genes in a transferable IncX3-type plasmid. The dissemination of this MDR isolate should be of close concern in future clinical surveillance.
Materials and methods
Case presentation and bacterial isolates
Two C. freundii isolates were collected from a 62 years old female bladder cancer inpatient with multiple infections of the Affiliated Dongyang Hospital of Wenzhou Medical University (Wenzhou, China) in 2020. One isolate (DY2007) was first recovered from bloodstream on January 21 and the other one (DY2010) was subsequently obtained from urinary tract on February 12. The patient presented with lower back pain, accompanied by fever and chills, and was admitted on January 20, 2020. She received intravenous administration of 2g of ceftriaxone-sulbactam every 8 h for antimicrobial treatment until February 1. Additionally, on January 23, based on the strain's antimicrobial susceptibility testing results, amikacin injection was added to the treatment regimen at a dosage of 0.2g every 12 h. This treatment continued until February 1, when the patient's symptoms improved, and she requested discharge. Subsequently, on February 9, the patient was readmitted due to lower back pain and received the aforementioned amikacin treatment until February 17, when the patient made a complete recovery and was subsequently discharged. The strains were isolated using sheep blood agar cultured overnight at 37 ◦C and were initially identified using MALDI-TOF MS (BioMérieux, France). The isolates were stored in 30% glycerol at -80 ◦C until further analysis.
Antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs) of 17 antibiotics, including amoxicillin/clavulanic acid, piperacillin/tazobactam, ceftazidime, ceftriaxone, cefepime, cefotaxime, ciprofloxacin, levofloxacin, imipenem, meropenem, trimethoprim/sulfamethoxazole, amikacin, gentamicin, aztreonam, fosfomycin, tigecycline, and polymixin B, were determined by the VITEK 2 system with AST-GN13 card and the agar dilution method. The breakpoint of tigecycline was interpreted according to the recommendations of the Food and Drug Administration (FDA) [28], and the breakpoint of other antibiotics were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) 2021 guidelines [29].
Carbapenemases detection and molecular mechanisms identification
The mCIM and eCIM method were used to determine carbapenemase production according to the CLSI guidelines. Carbapenemase genes (blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48) were identified by PCR amplification as in our previous publication [30]. Positive amplification products were then sequenced for verification and subtype typing.
Sequence typing and genetic relationship analyses
Multilocus sequence typing (MLST) analysis of both C. freundii isolates was undertaken by amplifying seven housekeeping genes (aspC, clpX, fadD, mdh, arcA, dnaG, and lysP). The sequence type was assigned by allelic profile comparison using the pubMLST database (https://pubmlst.org/cfreundii/). A single-nucleotide polymorphism (SNP)-based phylogenetic tree, including the genome of both C. freundii isolates sequenced in this study and 78 additional genomes of NDM-producing strains downloaded from the NCBI GenBank database (Table S1) [31], was constructed. To do so, a core SNPs matrix was calculated by comparing such genomes using the kSNP version 3 (https://sourceforge.net/projects/ksnp/files/) [32] and used to generate a maximum likelihood tree using iTOL version 5 (https://itol.embl.de/) [33].
Plasmid characterization and conjugation assay
Plasmid sizes of the strains were determined using the S1 nuclease pulsed-field gel electrophoresis (S1-PFGE) method. The location of the blaNDM-5 gene was investigated by Southern blotting with a specific digoxigenin-labelled blaNDM-5 probe using the DIGHigh Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics, Germany) [10]. Replicon types of plasmid incompatibility (Inc) groups were identified by multiplex PCR as previously described [10]. The plasmid conjugation and transformation methods were performed to verify the transferability of the NDM-bearing plasmid with E. coli 600 as a recipient strain. The transconjugants were then screened on BHI agar plates supplemented with 2 mg/L meropenem and were identified by MALDI-TOF MS. NDM-bearing recipient strain was confirmed by PCR and sequencing.
Whole genome sequencing
Genomic DNA of both NDM-5 producing C. freundii isolates was extracted using the QIAmp DNA Mini Kit (Qiagen, Germany). A Qubit Fluorometer (Thermo scientific, USA) was then used to determine the concentration and purity of DNA. Sequencing libraries were prepared using the Illumina Nextera XT Kit and sequenced using Illumina HiSeq 4000-PE150 platform (Illumina, USA). Raw sequencing data of both isolates were assembled using SOAP de novo software [34] and were deposited in GenBank under the following accession numbers: JAJDSQ000000000, and CP086287-CP086290, respectively. Antimicrobial resistance genes and plasmid replicon types were matched to the Center for Genomic Epidemiology (http://www.genomicepidemiology.org/) Resfinder and Plasmid finder databases. The gaps were covered using combinatorial PCR to accomplish sequence integrality of contigs. The RAST server (http://rast.nmpdr.org/) was used to annotate the bacterial genomes, and the ISFinder database (https://www-is.biotoul.fr/) was used to identify IS elements and transposons. Multiple plasmid alignment was conducted and plotted between the blaNDM-5-harboring plasmid (named pNDM-5) and the reference plasmid using the BLAST Ring Image Generator (BRIG) [35]. Easyfig 2.2.3 was used to analyze the genetic environment surrounding the blaNDM-5 resistance gene [36].
Average nucleotide identity blast analysis
ANIb analysis was conducted using PyANI (https://github.com/widdowquinn/pyani). The analysis included the following genomes: strain DY2007, DY2010, and five reference strains of the Citrobacter genus, namely Citrobacter koseri ATCC BAA895, Citrobacter rodentium ATCC51459, Citrobacter braakii ATCC51113, Citrobacter youngae ATCC29220, and Citrobacter freundii ATCC8090. Pairwise ANIb data for each strain were clustered and visualized using a heatmap. All aforementioned software was utilized with their default settings.
Availability of data and materials
The datasets presented in this study can be found in NCBI repository (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA766039).
Abbreviations
- CPE:
-
Carbapenemase-producing Enterobacteriaceae
- C. freundii :
-
Citrobacter freundii
- mCIM:
-
Modified carbapenem inactivation method
- eCIM:
-
EDTA-carbapenem inactivation method
- MLST:
-
Multilocus sequence typing
- SNP:
-
Single-nucleotide polymorphism
- CRE:
-
Carbapenem-resistant Enterobacteriaceae
- NDM:
-
New Delhi metallo-β-lactamase
- MICs:
-
Minimum inhibitory concentrations
- FDA:
-
Food and Drug Administration
- CLSI:
-
Clinical and Laboratory Standards Institute
- S1-PFGE:
-
S1 nuclease pulsed-field gel electrophoresis
- BRIG:
-
BLAST ring image generator
- ANIb:
-
Average nucleotide identity blast
References
Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front Microbiol. 2019;10:80.
HammoudiHalat D, Ayoub Moubareck C. The Current Burden of Carbapenemases: Review of Significant Properties and Dissemination among Gram-Negative Bacteria. Antibiotics (Basel). 2020;9(4):186.
Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM Metallo-beta-Lactamases and Their Bacterial Producers in Health Care Settings. Clin Microbiol Rev. 2019;32(2):e00115-18.
Hornsey M, Phee L, Wareham DW. A novel variant, NDM-5, of the New Delhi metallo-beta-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother. 2011;55(12):5952–4.
Lalaoui R, Djukovic A, Bakour S, Hadjadj L, Sanz J, Salavert M, et al. Genomic characterization of Citrobacter freundii strains coproducing OXA-48 and VIM-1 carbapenemase enzymes isolated in leukemic patient in Spain. Antimicrob Resist Infect Control. 2019;8:167.
Yang L, Li P, Liang B, Hu X, Li J, Xie J, et al. Multidrug-resistant Citrobacter freundii ST139 co-producing NDM-1 and CMY-152 from China. Sci Rep. 2018;8(1):10653.
Chi X, Guo J, Zhou Y, Xiao T, Xu H, Lv T, et al. Complete-Genome Sequencing and Comparative Genomic Characterization of an IMP-4 Producing Citrobacter freundii Isolate from Patient with Diarrhea. Infect Drug Resist. 2020;13:1057–65.
Yoon EJ, Kang DY, Yang JW, Kim D, Lee H, Lee KJ, et al. New Delhi Metallo-Beta-Lactamase-Producing Enterobacteriaceae in South Korea Between 2010 and 2015. Front Microbiol. 2018;9:571.
Olowo-Okere A, Ibrahim YKE, Olayinka BO, Ehinmidu JO, Mohammed Y, Nabti LZ, et al. Phenotypic and genotypic characterization of clinical carbapenem-resistant Enterobacteriaceae isolates from Sokoto, northwest Nigeria. New Microbes New Infect. 2020;37:100727.
Xu H, Wang X, Yu X, Zhang J, Guo L, Huang C, et al. First detection and genomics analysis of KPC-2-producing Citrobacter isolates from river sediments. Environ Pollut. 2018;235:931–7.
Sun P, Xia W, Liu G, Huang X, Tang C, Liu C, et al. Characterization Of bla NDM-5-Positive Escherichia coli Prevalent In A University Hospital In Eastern China. Infect Drug Resist. 2019;12:3029–38.
Zhang F, Xie L, Wang X, Han L, Guo X, Ni Y, et al. Further Spread of bla NDM-5 in Enterobacteriaceae via IncX3 Plasmids in Shanghai. China Front Microbiol. 2016;7:424.
Zhao Q, Berglund B, Zou H, Zhou Z, Xia H, Zhao L, et al. Dissemination of blaNDM-5 via IncX3 plasmids in carbapenem-resistant Enterobacteriaceae among humans and in the environment in an intensive vegetable cultivation area in eastern China. Environ Pollut. 2021;273:116370.
Ramadan H, Gupta SK, Sharma P, Ahmed M, Hiott LM, Barrett JB, et al. Circulation of emerging NDM-5-producing Escherichia coli among humans and dogs in Egypt. Zoonoses Public Health. 2020;67(3):324–9.
Zhao W, Li S, Schwarz S, Li A, Yao H, Du XD. Detection of a NDM-5-producing Klebsiella pneumoniae sequence type 340 (CG258) high-risk clone in swine. Vet Microbiol. 2021;262:109218.
Tian D, Wang B, Zhang H, Pan F, Wang C, Shi Y, et al. Dissemination of the bla(NDM-5) Gene via IncX3-Type Plasmid among Enterobacteriaceae in Children. mSphere. 2020;5(1):e00699-19.
Paskova V, Medvecky M, Skalova A, Chudejova K, Bitar I, Jakubu V, et al. Characterization of NDM-Encoding Plasmids From Enterobacteriaceae Recovered From Czech Hospitals. Front Microbiol. 2018;9:1549.
Guo X, Rao Y, Guo L, Xu H, Lv T, Yu X, et al. Detection and Genomic Characterization of a Morganella morganii Isolate From China That Produces NDM-5. Front Microbiol. 2019;10:1156.
Zhao Q, Berglund B, Zou H, Zhou Z, Xia H, Zhao L, et al. Dissemination of bla(NDM-5) via IncX3 plasmids in carbapenem-resistant Enterobacteriaceae among humans and in the environment in an intensive vegetable cultivation area in eastern China. Environ Pollut. 2021;273:116370.
Hirabayashi A, Yanagisawa H, Takahashi H, Yahara K, Boeing P, Wolfenden B, et al. On-Site Genomic Epidemiological Analysis of Antimicrobial-Resistant Bacteria in Cambodia With Portable Laboratory Equipment. Front Microbiol. 2021;12:675463.
Dong H, Li Y, Cheng J, Xia Z, Liu W, Yan T, et al. Genomic Epidemiology Insights on NDM-Producing Pathogens Revealed the Pivotal Role of Plasmids on bla(NDM) Transmission. Microbiol Spectr. 2022;10(2):e0215621.
Zou H, Han J, Zhao L, Wang D, Guan Y, Wu T, et al. The shared NDM-positive strains in the hospital and connecting aquatic environments. Sci Total Environ. 2023;860:160404.
Huang Y, Ma X, Zeng S, Fu L, Xu H, Li X. Emergence of a Salmonella Rissen ST469 clinical isolate carrying bla (NDM-13) in China. Front Cell Infect Microbiol. 2022;12:936649.
Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int. 2014;2014:249856.
Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun. 2021;12(1):4188.
Wesevich A, Sutton G, Ruffin F, Park LP, Fouts DE, Fowler VG Jr, et al. Newly Named Klebsiella aerogenes (formerly Enterobacter aerogenes) Is Associated with Poor Clinical Outcomes Relative to Other Enterobacter Species in Patients with Bloodstream Infection. J Clin Microbiol. 2020;58(9):e00582-20.
Satlin MJ, Chen L, Gomez-Simmonds A, Marino J, Weston G, Bhowmick T, et al. Impact of a Rapid Molecular Test for Klebsiella pneumoniae Carbapenemase and Ceftazidime-Avibactam Use on Outcomes After Bacteremia Caused by Carbapenem-Resistant Enterobacterales. Clin Infect Dis. 2022;75(12):2066–75.
Stein GE, Craig WA. Tigecycline: a critical analysis. Clin Infect Dis. 2006;43(4):518–24.
Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. J Clin Microbiol. 2021;59(12):e0021321.
Tian X, Zheng X, Sun Y, Fang R, Zhang S, Zhang X, et al. Molecular Mechanisms and Epidemiology of Carbapenem-Resistant Escherichia coli Isolated from Chinese Patients During 2002–2017. Infect Drug Resist. 2020;13:501–12.
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Ostell J, Pruitt KD, et al. GenBank. Nucleic Acids Res. 2018;46(D1):D41–7.
Gardner SN, Slezak T, Hall BG. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics. 2015;31(17):2877–8.
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6.
Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1(1):18.
Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402.
Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–10.
Acknowledgements
We are grateful to the reviewers who helped to improve this paper.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82102457), the Zhejiang Provincial Natural Science Foundation of China (grant number LQ22H200004), the Zhejiang Provincial Science and Technology Plan Project of China (grant number 2023RC046), the Planned Science and Technology Project of Wenzhou (grant number Y20210110), Start-up Funding for Talent Research Program in the First Affiliated Hospital of Wenzhou Medical University (grant number 2019QD011) and Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province (no. 2022E10022).
Author information
Authors and Affiliations
Contributions
JZY, LLJ, and YLL conducted the experiments, analyzed the data, and wrote the manuscript. HX, and YSL validated the methodology and data. TLZ, BWZ, MFW, and ZYW designed, supervised and revised the study. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Issuing number: 2022-R049). Informed consent was waived by Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University due to the study’s observational nature mainly focused on bacteria and did no interventions to patients, additionally, patient information was anonymized and de-identified during data recording. All experiments were performed in compliance with the relevant laws and institutional guidelines in accordance with the ethical standards of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Heatmap and dendrogram of ANIb values of DY2007, DY2010 and 5 reference strains of genus Citrobacter. ANIb, average nucleotide identity blast. Supplementary Figure 2. Full-length gels and blots figure that was used to crop in Fig. 2.
Additional file 2: Table S1.
Information of DY2007, DY2010 and other 78 NDM-producing C. freundii strains used for phylogenetic tree construction.
Additional file 3: Table S2.
List of information for both genomes that were sequenced in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, J., Jin, L., Li, Y. et al. Complete-genome sequencing and comparative genomic characterization of blaNDM-5 carrying Citrobacter freundii isolates from a patient with multiple infections. BMC Genomics 24, 506 (2023). https://doi.org/10.1186/s12864-023-09579-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09579-9