Abstract
Background
Sphaerophysa kotschyana Boiss. is naturally distributed in overly salty regions. The key to the completion of the life cycles of S. kotschyana in harsh saline soils may be hidden in changes of its osmo-protectants, but there is currently no information about the interaction between osmotic adjustment and water relations in adaptation to saline conditions. The aim of this article was to determine growth, relative growth rate (RGR), relative water content (RWC), osmotic potential (ΨΠ), photosynthetic efficiency (Fv/Fm), thiobarbituric acid-reactive substances (TBARS) and osmo-protectant contents [proline (Pro), choline (Cho) and glycine betaine (GB)] in S. kotschyana leaves and roots exposed to 0, 150 or 300 mM NaCl for 7 and 14 d (days).
Results
The results clearly showed that the reductions in growth, RWC, Fv/Fm, RGR and ΨΠ were more pronounced at 300 mM, especially after 14 d. In the same group, the highest increase in TBARS was recorded in roots (126%) and leaves (31%). The induction at 150 mM was not as high. Therefore, roots appear to be the most vulnerable part of this plant. Moreover, S. kotschyana was able to withstand short-term low salinity.
Conclusions
The osmo-protectant accumulation in S. kotschyana as a salinity acclimation or adaptation was sufficient for toleration of low salt concentration (150 mM). In contrast, the plants exposed to the highest NaCl concentration (300 mM) were not able to maintain the ability to prevent water loss because of further decrease in root/shoot ratio of fresh weight (FW) and dry weight (DW), RWC and RGR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
About one-fifth of irrigated world agricultural lands are adversely affected by salinity, leading to induction of a wide range of perturbations at cellular and whole-plant levels (Belkheiri and Mulas, 2011). Salt stress may provoke (i) osmotic or water-deficit effect which cause reduction of water and nutrient uptake and (ii) ion-excess effect resulting from altered K+/Na+ ratios and/or accumulation of toxic levels of Na+ and Cl¯. Salt stress-induced oxidative stress results from excess reactive oxygen species (ROS) formation (Munns and Tester, 2008) which damages membrane lipids, proteins and nucleic acids (Mittler, 2002). In the cellular adaptive responses of salt-tolerant plants, a major factor of salt tolerance mechanisms is not only acceleration of ROS scavenging systems (Chinnusamy et al., 2005), but also the ability of plant cells to adjust osmotically and to accumulate organic solutes known as compatible solutes. Compatible solutes referred to as osmo-protectants are thought to provide (i) positive effects on cellular components and membrane integrity (ii) maintenance of subcellular structure and cellular turgor and (iii) increase in the osmotic potential of the cell in plants subjected to stress conditions (Sakamoto and Murata, 2002; Ashraf and Foolad, 2007). These osmo-protectant solutes include proline (Pro) and, quaternary ammonium compounds such as glycine betaine (GB) (Belkheiri and Mulas, 2011).
Pro is believed to play adaptive roles in plant stress tolerance. Pro has been proposed to act as (i) a compatible osmolyte, (ii) a molecular chaperone stabilizing the structure of proteins, (iii) a mechanism to store carbon and nitrogen, (iv) to balance cell redox status and (v) a part of stress signal influencing adaptive responses (Ashraf and Foolad, 2007; Verbruggen and Hermans 2008). Among the compatible solutes, GB is a small organic metabolite abundant mainly in the chloroplast, that stabilizes the activities of enzymes/protein complexes and maintains the integrity of membranes against the damaging effects of various environmental stresses (Sakamoto and Murata, 2002). The relationship between GB content and increasing in salinity, however, is controversial. For example, GB accumulates in response to stress in many plants, including Nicotiana tabacum and Atriplex halimus (Banu et al., 2009; Belkheiri and Mulas, 2011). In contrast, contradictory data has been obtained in Trifolium alexandrinum and Salicornia dolichostachya by different researchers (Varshney et al., 1988; Katschnig et al., 2013). Therefore, GB-induced defensive responses under salt stress have still been a matter of some confusion and debate. On the other hand, choline (Cho), was synthesized by GB in the chloroplast, has a vital role as the precursor for phosphatidyl choline, a dominant constituent of membrane phospholipids in eukaryotes (Sakamoto and Murata, 2002). In the literature, accumulation of these compatible solutes has been extensively investigated under stress conditions, but not interaction between these osmo-protectants. Hence, the interplays among Pro, Cho and GB accumulations need to be assessed, especially in halophytes having natural defense systems against salinity.
The Salt Lake (Tuz Gölü, Turkey), the habitat of Sphaerophysa kotschyana (Fabaceae), features a unique ecosystem with its natural attractive environments and habitats for biota. Although salinity level changes with seasonal fluctuations, this lake water is extremely saline with a salt content of 32%. The lake bottom is covered with a 1 to 30 cm thick salt layer, which has given rise to a local salt industry providing 55% of all Turkish salt (Dengiz and Baskan, 2009). S. kotschyana is naturally distributed in overly salty regions and is well equipped to survive and complete their life cycles in saline soils (Duran et al., 2010). It has been documented in previous studies that halophytic species have developed protective or compensatory mechanisms to resist salt stress, but there is no information about tolerance and/or avoidance defined by osmotic adjustment and tighter control of water relations in the S. kotschyana under salinity. Interestingly, how S. kotschyana can survive to the harsh salt conditions is not well understood. The answer to this question may be hidden in the change of its osmo-protectants under salt stress, as well as in antioxidant tolerance mechanisms. Nevertheless, in roots and leaves of S. kotschyana, no details are known about osmotic adjustment and water relations in tolerance processes in relation to changes of these osmo-protectants.
Rather than examining the most commonly studied osmolytes individually, we focused on the unifying features associated with the accumulation of Pro, Cho and GB. Also, the present work was conducted in order to assess to defense strategies produced by leaves and roots of S. kotschyana against salt stress. With this aim, the study was undertaken to comparatively to investigate the role of the accumulation of osmo-protectants such as Pro, Cho and GB on enhancing salt tolerance. Furthermore, these parameters were correlated with some physiological and biochemical parameters including growth, relative growth rate (RGR), leaf relative water content (RWC), osmotic potential (ΨΠ), chlorophyll fluorescence (Fv/Fm) and thiobarbituric acid-reactive substances (TBARS) concentration in leaves and roots of S. kotschyana exposed to 0, 150 and 300 mM NaCl for 7 and 14 d.
Methods
Plant material and experimental design
Sphaerophysa kotschyana Boiss. plant specimens and seeds were collected in July 2010 from around Yavsan Saltworks (Salt Lake), Konya (lat. 42°90′336“ long. 36°51′649” at an altitude of 910 m), Turkey. Taxonomic identification of the plant material was confirmed. The voucher specimen has been deposited at the Herbarium of the Department of Biology, Selcuk University, Konya, Turkey (Voucher No: EY2150). After seeds collection, immature seeds and those attacked by insects were removed and the healthy seeds were stored at 4°C.
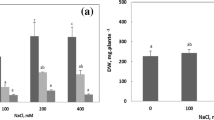
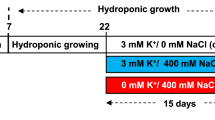
Sphaerophysa kotschyana seeds were sterilized with 5% sodium hypochlorite for 10 min and were washed thoroughly with deionized water. Then, seeds were sown into the pots filled with perlite and were grown under controlled conditions (light/dark regime of 16/8 hours (h) at 23°C, 70% relative humidity and 350 μmol m-2 s-1 photosynthetic photon flux density). For determination of the range of salt concentration applied to S. kotschyana, 0 (control), 150, 300 and 400 mM NaCl was used in the pre-treatment experiment. The data obtained from it suggested that 150 and 300 mM were chosen for stress-induced oxidative damage and the plants subjected to 400 mM NaCl greatly damaged and very few of them could survive against stress treatment. Seedlings were grown for 50 d in a full-strength Hoagland’s solution (Hoagland and Arnon, 1950). On day 50 of normal growth (applied to seedling with five-leaf), a stress treatment was initiated by giving Hoagland’s solution containing 0, 150 and 300 mM NaCl. Plants were harvested on the 7 and 14 d of salt treatment and then stored at -80°C until further analyses.
Growth and osmo-protectant accumulation of S. kotschyana under salinity
Six random plants for each group were used for measuring changes on the growth parameter. They were separated to shoot and root fractions on 7 and 14 d of stress and measured with the ruler for length. Shoot and root fresh weights (FW) of seedlings were weighed. After the samples were dried at 70°C for 72 h, dry weights (DW) were measured to calculate the relative growth rate (RGR). RGR values were calculated according to the following formula by Hunt et al. (2002):
where DW1 = dry weight (g) at t1; DW2 = dry weight (g) at t2, t1; initial harvest and t2;final harvest.
After the stress exposure period, six leaves were weighted and their FW was recorded. The samples were kept in ultrapure water for 8 h and then the turgid leaves (TW) were measured again. After oven drying at 75°C for 72 h, DW was reported. RWC was calculated by the formula given by Smart and Bingham (1974):
After harvest on 7 and 14 d of stress treatment, chlorophyll fluorescence parameters (PSII maximum efficiency, Fv/Fm) of leaves were measured by Plant Efficiency Analyze of Hansatech, UK. This parameter provided an estimate of the maximum photochemical efficiency of the photosystem II (PSII).
Samples were homogenized by a glass rod. After centrifugation (12000 × g) for 10 min, the extraction was directly used for ΨΠ determination. ΨΠ was measured by Vapro Vapor pressure Osmometer 5600. The data was collected from six samples per replicate. ΨΠ was converted to MPa according to Santa-Cruz et al. (2002) by multiplying coefficient of 2.408 × 10-3.
Pro content was measured according to Bates et al. (1973). The samples were homogenized in 3% sulphosalicylic acid and homogenate was filtered through filter paper. After addition of acid ninhydrin and glacial acetic acid, the mixture was heated at 100°C. The mixture was extracted with toluene and the absorbance of the toluene fraction aspired from liquid phase was measured at 520 nm.
Cho level was determined according to Grieve and Grattan (1983). The dry sample material was suspended in 1 ml of distilled water and then was centrifuged at 5000 × g for 5 min. The supernatants were diluted 1:1 with 0.2 M potassium phosphate buffer (pH 6.8). After cooling the mixtures, 20 μl of cold KI-I2 reagent was added. The samples were stored at 0-4°C for 16 h and then centrifuged at 10000 × g for 15 min. Supernatant was removed and periodide crystals dissolved in 900 μl of 1,2-dichloroethane. After vortexing and incubating for 2–2.5 h at room temperature, the absorbance was recorded at 365 nm. GB content was performed according to Grieve and Grattan (1983) with minor modifications. To obtain the supernatant for GB analysis, the procedures were the same as described as for Cho content except that the supernatants diluted 1:1 with 2 N H2SO4. A calibration curve with reference standards of GB (5–500 μg ml-1) was determined.
Determination of thiobarbituric acid-reactive substances (TBARS)
The level of lipid peroxidation was determined by thiobarbituric acid-reactive substances (TBARS) according to Madhava Rao and Sresty (2000). TBARS was calculated from the absorbance at 532 nm and measurements were corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. The concentration of TBARS was calculated using an extinction coefficient of 155 mM-1 cm-1.
Statistical analysis
The experiments were repeated twice independently, and each data point was the mean of six replicates. All data obtained were subjected to a one-way analysis of variance (one way ANOVA, SPSS Statistics 20). Tukey’s post-test was used to compare the treatment groups. Comparisons with p < 0.05 were considered significantly different. In all the figures, the error bars represent standard errors of the means.
Results
Except for 7 d of 150 mM-treated plants, at which growth did not change significantly, salinity caused a decrease in the shoot and root length of S. kotschyana when compared with the control group. 300 mM NaCl had a significant negative effect on the growth of S. kotschyana, especially at 14 d (Table 1). As evidenced by phenotypic change of S. kotschyana, decreased length of seedlings (Figure 1A) and visible symptoms of leaf burns (Figure 1B) was more acute at 300 mM than at 150 mM NaCl. In addition, while after 14 d of stress, shoot length decreased by 25% in 300 mM NaCl-treated plants, the reduction in root length was 29% at the same day of stress exposure. As well as root and shoot length, at 14 d, 150 and 300 mM NaCl significantly decreased FW by 18% and 41% (root) and 23% and 41% (shoot), respectively. On the other hand, root/shoot ratio of dried plants (DW) increased in 150 mM-treated plants but decreased at 300 mM at 7 d as compared to the control (Table 2).
All the salt treatments caused a significant reduction in RGR in comparison to the control group (Figure 2). Further decline (49%) in RGR of shoots was observed in S. kotschyana treated with 300 mM NaCl for 14 d. Similarly, the reduction in RGR of roots was more pronounced at 300 mM NaCl especially for 14 d, at which it attained 71% of control.
As is evident from Figure 3, RWC was close to the control group after 7 d of stress, but it decreased at 14 d. RWC was 10% and 17% lower than in the control group at 150 and 300 mM NaCl at 14 d, respectively.
As is clear from Table 1, Fv/Fm did not change at 150 mM NaCl throughout the experiment and PS-II efficiency was preserved. However, at 300 mM NaCl, Fv/Fm decreased after 14 d of stress (7%).
ΨΠ in leaves and roots showed the same changes at both sampling days and salt concentrations (Figure 4). The ΨΠ in leaves decreased after 7 d exposure to 150 and 300 mM. ΨΠ in leaves dropped from -1.1 (control levels) to -1.6 MPa after 14 d of 300 mM NaCl. Similarly, 300 mM NaCl caused more reduction in root ΨΠ (-1.2 MPa) than that of control (-0.9 MPa) at 14d. However, the reductions were more pronounced in leaves than in roots. For example, 300 mM NaCl yielded a larger ΨΠ reduction (46%) in S. kotschyana leaves at 14 d when compared to the ΨΠ of roots (33%).
Pro, Cho and GB contents in leaves and roots increased with salinity during the experimental period (Figure 5). Even greater contents of the osmo-protectants were observed in 300 mM-treated leaves at 14 d (Figure 5A, C, E). Under 150 mM, Pro in roots was close to control level at 7d (Figure 5B). On the other hand, except this group, 150 and 300 mM NaCl increased Pro relative to control group, more so after 14 d exposure to 300 mM (274% and 247%, leaves and roots, respectively). Cho accumulation showed a significant increase in response to salinity in both leaves and roots. At 150 mM NaCl, Cho increased 1.9 and 3 fold in leaves after 7 and 14 d of the beginning of the salinity treatments as compared with the control, respectively (Figure 5C). Also, Cho content increased in roots of stress-treated S. kotschyana, but this induction was as high as that in leaves (Figure 5D). For example, when NaCl stress became more severe, the increase in leaves (315%) was much greater than in root (62%) at 14 d. On the other hand, GB in leaves markedly increased in response to 150 and 300 mM NaCl and accumulated to significantly higher levels at the higher salt concentration (Figure 5E). GB levels in roots did not change with salinity at 7 d (Figure 5F). In contrast, GB concentration showed a dramatic increase in response to 300 mM. The highest induction in roots (84%) was obtained at 14 d.
Effects on proline (Pro, μmol g-1FW), choline (Cho, μmol g-1DW) and glycine betaine (GB, μmol g-1DW) in S. kotschyana leaves (A, C, E) and roots (B, D, F) exposed to 0 (Control), 150 mM and 300 mM NaCl for 7 and 14 d are shown. Vertical bars indicate ± SE and values sharing a common letter are not significantly different at P > 0.05.
Except in leaves of 150 mM-treated plants at 7 d, TBARS in leaves and roots increased with salinity as compared to control group (Figure 6A, B). Moreover, 300 mM NaCl induced the maximum rate of lipid peroxidation in membranes and caused an increase in damage by 31% in leaves at 14 d when compared to the control. On the other hand, the highest increase in TBARS in roots was 126% at the same days of stress.
Discussion
Growth of S. kotschyana was significantly reduced with NaCl treatments in a dose-dependent manner, which is in agreement with different studies (Athar et al., 2009; Noreen et al., 2010). This change might be due to toxic effects of Na+ and Cl¯ and unbalancing of nutrient uptake. A previous study carried out with cotton (Meloni et al., 2003) also showed that shoot growth was more inhibited by NaCl than that of root. In our experiment, while the reduction percentages of root lengths were parallel to the results of previous studies, changes in FW and DW were in conflict. On the other hand, increased root/shoot ratio appears to be an adaptation to salinity, resulting in a more efficient water and nutrient uptake under stress (Gorham et al., 1985), supporting our findings, especially in 150 mM NaCl-treated plants.
Information on the relationship between salt tolerance and RWC, RGR or ΨΠ would be useful to use these parameters as selection criteria in water regulations. After 14 d of stress in S. kotschyana, the decrease in RWC is in confirmation of results reported by Sairam et al. (2002). The decrease in RWC resulted a loss of turgor and then limited water availability for cell extension processes (Katerji et al., 2003). In the present study, the inhibition in RGR was stronger in 300 mM NaCl-treated roots of S. kotschyana, demonstrating results of FW and DW. This result was well in agreement with those of Kim et al. (2004) and Lacerda et al. (2005). 300 mM might inhibit the growth rate of S. kotschyana due to the osmotic and ionic effects of salinity in line with the findings of Sun et al. (2009). This allows soil water uptake and sustains the turgor potential. In S. kotschyana exposed to salinity, the increase in osmolytes was compatible with the reduction in ΨΠ.
Fv/Fm is used as chlorophyll fluorescence parameter, closely correlated to photochemical efficiency of PSII and is a sensitive indicator of photosynthetic performance in plants (Kalaji et al., 2010). This parameter is thought to play a vital role in the process of photoinhibition when plants are exposed to drought and salt stresses (Maxwell and Johnson, 2000). Fv/Fm is close to 0.83 for most plants grown without stress (Bjorkman and Demmig 1987). Values lower than 0.83 suggest that plants are growing under stress and that PSII reaction centers are damaged which, in turn, is connected with reduced effectiveness of electron transport (Basu et al., 1998) when plants are grown under salinity. Similar to this, Fv/Fm showed a significantly decrease after 14 d exposure to 300 mM NaCl. However, 150 mM NaCl did not change the Fv/Fm of S. kotschyana. It is reported by Sakamoto and Murata (2002) that the protection of PSII complexes from oxidative damage might be connected with increased GB content under salt stress.
Pro maintains NAD+/NADH ratios necessary for respiratory and photosynthetic processes, enhances photosystem II-mediated photochemical activity in thylakoid membranes and prevents the photoinhibitory loss of photochemical activity under stress conditions (Kavi Kishor et al., 2005). In our experiment, a dramatic increase in Pro content in the plants exposed to the highest NaCl concentration could contribute to the lack of notable change in photosynthetic efficiency. Supporting findings come from other plants such as sugar beet (Ghoulam et al., 2002) where salt stress results in extensive Pro accumulation. Also, it has been reported in salt treated Anacardium occidentale that Pro accumulation is higher in leaves than roots (Alvarez-Pizarro et al., 2009) which is in accordance to the results obtained in this experiment. Recent experimental evidence has demonstrated that plant species able to synthesize GB may also accumulate other organic compatible solutes, such as Pro (Martinez et al., 2005). GB has been shown in vitro (i) to stabilize membranes of the oxygen evolving photosystem II complex and (ii) to accumulate in the cytoplasm to balance the accumulation of solutes and ions in the vacuole during osmotic adjustment (Papageorgiou and Murata, 1995). There was a significant positive correlation among Pro, GB contents and the extent of tolerance to oxidative in S. kotschyana. However, it seems to be that Pro had more contribution than GB regarding osmotic adjustment. Another contributor to osmotic adjustment in salt stressed-S. kotschyana was Cho. Cho is synthesized from GB in the chloroplast. Like Pro and GB, Cho increased with salinity treatments in S. kotschyana as suggested by Varshney et al. (1988). Therefore, the induced contents of these osmo-protectants were compatible with each other under salinity. Also, due to the increase in Pro, GB and Cho contents in leaves of 150 mM-treated plants, accumulation of these osmo-protectants might be sufficient for protection of the photosynthetic apparatus and water status against oxidative damage.
Increases in TBARS have been reported in salt stress-treated plants (Sudhakar et al., 2001; Sairam et al., 2002). This increase is related to the amount of stress and is well correlated with lipid membrane damage. Roots of S. kotschyana seemed to be more affected by the NaCl-induced oxidative stress, as evidenced by a considerable increase in TBARS, as well as by the further decrease in RGR. Additionally, roots had a lower induction in osmo-protectant accumulation than did leaves.
Conclusions
This is the first report to provide insights into the mechanisms of stress tolerance and to compare water relations, osmotic adjustment and photosynthetic alterations in response to salinity of S. kotschyana. The results clearly showed that roots would appear to be the most vulnerable part of this plant, as they were directly exposed to the salt. Thus, in summary, the degree of osmotic adjustment in leaves was greater than roots with the increase in NaCl concentration. Any increase in osmotic adjustment under saline conditions might likely be a result of increase in the ions in the vacuole. Our results support the idea that leaves and roots of S. kotschyana were well-adapted to saline environments, as suggested by maintenance of a high root/shoot ratio, lower increase in TBARS accumulation, lower decline in RGR, plant growth parameters and ΨΠ especially on 7 d. Therefore, S. kotschyana could withstand both short-term salinity and low salt concentration. Therefore, the amounts of osmo-protectants in S. kotschyana’s response to salinity acclimation or adaptation were sufficient to for tolerance of low salt concentration (150 mM). In contrast, in the plants exposed to 300 mM NaCl, despite the increase in osmolyte accumulation, was not able to maintain the ability to prevent water loss and became oxidatively damaged as demonstrated by a further decrease in growth, root/shoot ratio, RGR, RWC and a decline in antioxidant enzymes activity.
Abbreviations
- Cho:
-
Choline
- GB:
-
Glycine betaine
- Pro:
-
Proline
- RGR:
-
Relative growth rate
- RWC:
-
Relative water content
- TBARS:
-
Thiobarbituric acid-reactive substances.
References
Alvarez-Pizarro JC, Gomes-Filho E, Lacerda CF, Alencar NLM, Prisco JT: Salt-induced changes on H+-ATPase activity, sterol and phospholipid content and lipid peroxidation of root plasma membrane from dwarf-cashew ( Anacardium occidentale L.) seedlings. Plant Growth Regul 2009, 59: 125–135. 10.1007/s10725-009-9395-7
Ashraf M, Foolad MR: Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environ Exp Bot 2007, 59: 206–216. 10.1016/j.envexpbot.2005.12.006
Athar H, Ashraf M, Wahid A, Jamil A: Inducing salt tolerance in canola ( Brassica napus L.) by exogenous application of glycinebetaine and proline: response at the initial growth stages. Pak J Bot 2009, 41: 1311–1319.
Banu M, Hoque M, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Murata N: Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 2009, 166: 146–156. 10.1016/j.jplph.2008.03.002
Basu PS, Sharma A, Sukumaran NP: Changes in net photosynthetic rate and chlorophyll fluorescence in potato leaves induced by water stress. Photosynthetica 1998, 35: 13–19. 10.1023/A:1006801311105
Bates L, Waldrenn R, Teare I: Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39: 205–207. 10.1007/BF00018060
Belkheiri O, Mulas M: The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ Exp Bot 2011, 86: 17–28.
Bjorkman O, Demmig B: Photon yield of O 2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170: 489–504. 10.1007/BF00402983
Chinnusamy V, Jagendorf A, Zhu J: Understanding and improving salt tolerance in plants. Crop Sci 2005, 45: 437–448. 10.2135/cropsci2005.0437
Dengiz O, Baskan O: Land quality assessment and sustainable land use in Salt Lake (Tuz Gölü) specially protected area. J Environ Monitor 2009, 148: 233–243.
Duran A, Martin E, Ozturk M, Cetin O, Dinc M, Ozdemir A: Morphological, karyological and ecological features of halophytic endemic Sphaerophysa kotschyana Boiss. (Fabaceae) in Turkey. Biogeosciences 2010, 3: 163–169.
Ghoulam C, Foursy A, Fares K: Effects of salt stress on growth inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 2002, 47: 39–50. 10.1016/S0098-8472(01)00109-5
Gorham J, Wyn Jones RG, McDonnell E: Some mechanisms of salt tolerance in crop plants. Plant Soil 1985, 89: 15–40. 10.1007/BF02182231
Grieve CM, Grattan SR: Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70: 303–307. 10.1007/BF02374789
Hoagland DR, Arnon DI: The water culture method for growing plants without soil. Calif AES Bull 1950, 347: 1–32.
Hunt R, Causton DR, Shipley B, Askew AP: A modern tool for classical plant growth analysis. Ann Bot 2002, 90: 485–488. 10.1093/aob/mcf214
Kalaji HM, Govindje E, Bosa K, Koscielniak J, Zuk-Golaszewska K: Effects of salt stress on photosystem II efficiency and CO 2 assimilation of two Syrian barley landraces. Environ Exp Bot 2010, 73: 64–72.
Katerji N, van Hoorn JW, Hamdy A, Mastrorilli M: Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. Agric Water Manage 2003, 62: 37–66. 10.1016/S0378-3774(03)00005-2
Katschnig D, Broekman R, Rozema J: Salt tolerance in the halophyte Salicornia dolichostachya Moss: growth, morphology and physiology. Environ Exp Bot 2013, 92: 32–42.
Kavi Kishor PB, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Sreenath R, Reddy KJ, Theriappan P, Sreenivasulu N: Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 2005, 88: 424–438.
Kim JY, Lee SC, Jung KH, An G, Kim SR: Characterization of a cold responsive gene, OsPTR1, isolated from the screening of β-glucuronidase (GUS)-gene trapped rice. J Plant Biol 2004, 47: 133–141. 10.1007/BF03030644
Lacerda CF, Cambraia J, Oliva MA, Ruiz HA: Changes in growth and insolute concentrations in sorghum leaves and roots during salt stress recovery. Environ Exp Bot 2005, 54: 69–76. 10.1016/j.envexpbot.2004.06.004
Madhava Rao KV, Sresty TVS: Antioxidative parameters in the seedlings of pigeon pea ( Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci 2000, 157: 113–128. 10.1016/S0168-9452(00)00273-9
Martinez JP, Kinet JM, Bajji M, Lutts S: NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J Exp Bot 2005, 419: 2421–2431.
Maxwell K, Johnson GN: Chlorophyll fluorescence: a practical guide. J Exp Bot 2000, 51: 659–668. 10.1093/jexbot/51.345.659
Meloni DA, Oliva MA, Martinez CA, Cambraia J: Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 2003, 49: 69–76. 10.1016/S0098-8472(02)00058-8
Mittler R: Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002, 7: 405–410. 10.1016/S1360-1385(02)02312-9
Munns R, Tester M: Mechanisms of salinity tolerance. Annu Rev Plant Biol 2008, 59: 651–681. 10.1146/annurev.arplant.59.032607.092911
Noreen Z, Ashraf M, Akram NA: Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip ( Brassica rapa L.). J Agron Crop Sci 2010, 196: 273–285.
Papageorgiou GC, Murata N: The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem complex. Photosynth Res 1995, 44: 243–252. 10.1007/BF00048597
Sairam RK, Veerabhadra RK, Srivastava GC: Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 2002, 163: 1037–1046. 10.1016/S0168-9452(02)00278-9
Sakamoto M, Murata N: The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 2002, 25: 163–171. 10.1046/j.0016-8025.2001.00790.x
Santa-Cruz A, Martinez-Rodriguez MM, Perez-Alfocea F, Romero-Aranda R, Bolarin CM: The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci 2002, 162: 825–831. 10.1016/S0168-9452(02)00030-4
Smart RE, Bingham GE: Rapid estimates of relative water content. Plant Physiol 1974, 53: 258–260. 10.1104/pp.53.2.258
Sudhakar C, Lakshmi A, Giridarakumar S: Changes in the antioxidant enzyme activities in two high yielding genotypes of mulberry ( Morus alba L.) under NaCl salinity. Plant Sci 2001, 161: 613–619. 10.1016/S0168-9452(01)00450-2
Sun J, Chen SL, Dai SX, Wang RG, Li NY, Shen X, Zhou XY, Lu KF, Zheng SJ, Hu ZM, Zhang ZK, Song J, Xu Y: NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 2009, 149: 1141–1153.
Varshney KA, Gangwar LP, Goel N: Choline and betaine accumulation in Trifolium alexandrinum L. during salt stress. Egypt J Bot 1988, 31: 81–86.
Verbruggen N, Hermans C: Proline accumulation in plants: a review. Amino Acids 2008, 35: 753–759. 10.1007/s00726-008-0061-6
Acknowledgements
Financial support for this work was provided by Selcuk University Scientific Research Projects Coordinating Office (Project number: 11401069).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EY, CO-K and MK designed the experiment. EY, CO-K and YD contributed extensively to the work presented in this paper and were also responsible for growing, sampling, data analysis/collection and interpretation of Sphaerophysa kotschyana seedlings. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yildiztugay, E., Ozfidan-Konakci, C., Kucukoduk, M. et al. Variations in osmotic adjustment and water relations of Sphaerophysa kotschyana: Glycine betaine, proline and choline accumulation in response to salinity. Bot Stud 55, 6 (2014). https://doi.org/10.1186/1999-3110-55-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1999-3110-55-6